INTRODUCTION
Currently, free radical scavenging is needed because it can inhibit free radical reactions in the human body that cause various dangerous diseases, such as cancer, neurodegenerative, and cardiovascular [1]. Besides that, free radical scavenging capacity was measured to represent the antioxidant ability of a compound by radical reaction 2,2-diphenyl-1-picrylhydrazyl (DPPH) [2]. One of the potential medicinal plants for free radical scavenging is the Adenostemma lavenia family Asteraceae, which is spread across the Asian countries and Pacific islands [3]. Empirically, A. lavenia has been used as a traditional medicine to cure pneumonia, fever, hepatitis, pulmonary embolism, edema, and skin wounds 4,5]. This plant is also reported to contain high levels of flavonoids, alkaloids, terpenoids, and essential oils that have potential as antioxidants [6].
In A. lavenia, terpenoid groups were major constituents with a relative abundance of 23.46%, followed by alkaloids and phenolics with 11.24% and 4.37%, respectively [7]. Supported by recent studies, the main compound in A. lavenia leaf is ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (11αOH-KA, kaurenoic acid group), which belongs to the diterpenoid [8]. 11αOH-KA is also found in other plants, such as Gochnatia decora and Pteris semipinnata [3]. Interestingly, this compound is known to have antioxidant, antiviral, anticancer, antitumor, and anti-inflammatory activities and can inhibit the SARS-CoV-2 virus by in-silico [9]. Nonetheless, each plant’s chemical content and biological activity depend on various factors, such as environmental conditions, season, fertilizer used, age, and geographical factors [7]. As supported by Singh et al. [10], biotic and abiotic factors influence the growth and production of plant secondary metabolites. Therefore, cultivation techniques can impact the chemical content and biological activity of plants. Unfortunately, no one has reported the effect of cultivation on the chemical content or biological activity of A. lavenia plants. According to Liang et al. [11], two important factors regulate plant metabolism: light intensity and nitrogen. Thus, different shade intensities and doses of nitrogen fertilizer were chosen for this research as a factor for cultivating A. lavenia because no one has reported this before.
The production of secondary metabolites depends on the biomass per plant and the total secondary metabolite content. Shading was chosen because it is a method that has been widely used for growing medicinal plants [12]. In some plants, shading at optimum intensities can affect medicinal plants’ biomass and secondary metabolites [13]. However, the compound present in plants responds differently to an increase in shade intensity. In addition, plant nutrition is essential in determining secondary metabolites and biological activity. Nitrogen is one of the main nutrients for increasing the process of photosynthesis so that it can increase growth, chlorophyll, and the synthesis of plant metabolites [14]. The synergism between shading and nitrogen is expected to affect the biosynthesis of secondary metabolites, one of which is terpenoids, which are produced via two pathways: mevalonic acid (MVA) in the cytoplasm and methylerythritol 4-phosphate (MEP) in the plastids [15].
In addition, profiles of other secondary metabolites based on the effect of differences in shade intensity and nitrogen fertilizer doses were also analyzed using liquid chromatography-mass spectrometry (LC-MS/MS). This technique is used because it is not limited by sample volatility and can be used to measure metabolites well, so it is appropriate for use in a metabolomics approach [16]. Therefore, this study aimed to determine the optimum metabolite profile, terpenoid production, 11αOH-KA, and free radical scavenging capacity of A. lavenia cultivated with variations in shading and different doses of nitrogen fertilizers.
MATERIALS AND METHODS
Cultivation of A. lavenia
Cultivation was carried out in the Biopharmaca Cultivation Conservation Unit Research Station, Tropical Biopharmaca Research Center, IPB University, Bogor, West Java, Indonesia (06°32′ 49.28? S, 106°42′ 59.00? E) from December 2021 to January 2022. The research used a nested plot design method, the main plot was given different shade intensities, namely 0 (control), 25%, 50%, and 75%, on a 6 × 4.5 m plot. Treatment of subplots with different doses of urea fertilizer, namely 0 kg ha−1 (N: 0 kg ha−1), 100 kg ha−1 (N: 45 kg ha−1), and 200 kg ha−1 (N: 90 kg ha−1). Each treatment consisted of 3 replications, with the number of each experimental unit being 30 plants, and the distance between plants was 30 × 30 cm. Based on the soil analysis results, the soil used was latosol type with a pH of 4.69, soil nitrogen content of 0.2%, organic carbon of 2.05 ppm, and phosphor of 8.0 ppm. Based on Indonesian Agency for Meteorological, climatological, and Geophysics (in Indonesian is Badan Meteorologi, Klimatologi, dan Geofisika) (BMKG) [17], from December 2021 to January 2022, maximum temperature is 25.0°C?25.2°C, relative humidity is 86.9%?90.0%, rainfall is 7.8?14.4 mm, and sunshine is 1.6?2.7 hours.
Before cultivation, A. lavenia was determined at the Herbarium Bandungense (in Indonesian is Faculteit Ilmu Pasti dan Ilmu Alam) (FIPIA) School of Life Sciences and Technology (in Indonesian is Sekolah Ilmu dan Teknologi Hayati) (SITH) Bandung Institute of Technology (in Indonesian is Institut Teknologi Bandung) (ITB). Voucher specimens were deposited at FIPIA, SITH, and ITB with the collection number code FIPIA-DEP32. The stem cuttings of A. lavenia with a length of 10 cm were transferred to land where the mineral content of the soil had been determined, shaded, and different nitrogen fertilizers according to the treatment. All parameters were given additional treatment, namely SP-36 100 kg ha−1, KCl 100 kg ha−1, and cow manure 20 tons ha−1. Harvesting was carried out 6 weeks after planting (WAP). Before harvesting, leaf area was observed with Image J software and chlorophyll with soil plant analysis development.
Sample preparation
All 36 experimental units of A. lavenia leaf from various treatments were taken, dried in an oven at 45°C, and crushed with a blender to a dried leaf. The dried leaf’s water, ash, and acid-insoluble ash content was determined by referring to the Association of Official Analytical Chemist (AOAC) standard method numbers 990.19 and 941.12 [18].
Terpenoid production
Analysis of total terpenoids refers to Lukowski et al. [19], using nerol as a standard. A 0.2 g A. lavenia dried leaf was added to 2.5 ml of cold methanol and incubated for 48 hours at room temperature. Afterward, the mixtures were centrifuged at 4,000 g for 15 minutes at room temperature. The 200 μl supernatant was transferred to a test tube, and then 1.5 ml of chloroform was added and homogenized with vortex. After homogenization, 100 μl of concentrated H2SO4 was added to the sample and incubated for 1.5–2 hours at room temperature. About 1.5 ml of methanol was added to the red brick precipitate and homogenized with a vortex until the precipitate dissolved again. The solutions were then analyzed with a ultraviolet (UV)-Vis spectrophotometer at 520 nm. Total terpenoids were determined based on the standard nerol curve at various concentrations (0.050–0.150 mg Nerol/μl methanol). Total terpenoid was quantified in nerol equivalent (NE) using linear regression y = 0.0005x–0.0026 (R2 = 0.99). The following formula determined terpenoid production:
Terpenoid production (μmol NE/plant) = Dried weight (g dried leaf/plant) × Total terpenoid content (μmol NE/g dried leaf).
11αOH-KA production
11αOH-KA was determined using the high-performance liquid chromatography method referred by Maeda et al. [8]. A 100 mg dried leaf of A. lavenia was added to 1.5 ml of distilled water, macerated for 12 hours, and centrifuged at 13,800 g to collect the supernatant. The supernatant and 0.5 ml of chloroform were homogenized with a vortex, and the chloroform phase was added by 0.4 ml dichloromethane. The mixture was evaporated until a precipitate was formed. The precipitate was dissolved with 0.1 ml ethanol. A total of 10 μl of the supernatant was injected into the C18-ODS column (4.6 × 50 mm: RP-18 GP, Kanto Kagaku, Tokyo, Japan) and detected using an UV at 254 nm. Solvent A was 0.01% formic acid in H2O and solvent B was 100% Acetonitril. In 0–1 minutes, the eluent composition is 10% solvent B; in 1–7 minutes, the composition is 35% solvent B; in 7–10 minutes, the composition is 47.5% solvent B; in 10–13.6 minutes, the composition is 99% solvent B; and in 13.6–18.0 minutes, the eluent composition returns to the beginning. 11αOH-KA were obtained from the standard curve of the 11αOH-KA compound at various concentrations (0–30 mM 11αOH-KA) using linear regression y = 4796.3x + 44234 (R2 = 0.99). Standard was obtained from the isolation results [8]. The following formula determines the production of 11αOH-KA:
11αOH-KA production (mM 11αOH-KA/plant) = Dried weight (g dried leaf/plant) × 11αOH-KA content (mM 11αOH-KA/g dried leaf).
Free radical scavenging capacity with the DPPH method
The free radical scavenging capacity was determined using the DPPH method using a procedure described by Batubara et al. [20], with modifications of ascorbic acid concentrations. A 100 μl of sample extract at a concentration of 5% was added with 100 μl of 0.124 mM DPPH into 96-well microplates. Then, incubated in a dark place at room temperature for 30 minutes. The absorbance was measured at a wavelength of 517 nm. Ascorbic acid was used as a standard at various concentrations (0.625–20 μg ascorbic acid/ml methanol). The results were quantified in equivalent ascorbic acid (AAE).
Separation and identification of metabolites with LC-MS/MS analysis
LC-MS/MS conditioning for separating and identifying metabolites must be optimized before the analysis stage. Methanol extract of A. lavenia leaves was analyzed by LC-MS/MS using the Vanquish Tandem Q Exactive Plus Orbitrap HRMS ThermoScientific system. The column conditions used were AccucoreTM Vanquish C18 column (1.5 m, 100 × 2.1 mm), column temperature 30°C, and flow rate 0.2 ml/minute for 23 minutes. The injection volume used was 2 μl with Mass spectrometry (MS) electrospray ionization system in positive ion mode with a spray voltage of 3800 V, capillary temperature of 320°C, and detection with the mobile phase: 0.1% formic acid in H2O (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B). In 0–1 minutes, the eluent composition is 5% phase B; in 1–17 minutes, the composition is 90%–5% phase A and 10%–95% phase B; in 17–18.5 minutes, the composition is 100% phase B; and in 18.5–23 minutes, the eluent composition returns to the beginning. The mass scan range was set to m/z 100–1,500 with ionization energies of 18, 35, and 53 eV.
The three sample replicates were corrected using a blank. The resulting data were processed using Compound Discoverer 3.2 software (Thermo Scientific, Germany) with an online database to identify detected metabolites, and the chosen m/z tolerance was 5 ppm or 0.05 m/z. Furthermore, confirmation of compound components based on MS2 fragmentation with the help of PubChem, ChemSpider, HMDB, and CFM-ID to predict compounds in samples.
Data analysis
The data obtained from each variable were statistically analyzed using ANOVA. This study used two treatment levels, so the statistical results were seen from the effect of each treatment (shading or nitrogen) and the combination/interaction treatments (shading*nitrogen). If p < 0.05 of three outcomes (shading, nitrogen, and shading*nitrogen), then followed by Duncan’s multiple range test, significant differences were defined at a 5% level (p < 0.05) using the SAS 9.0 version. The LC-MS/MS results were processed with the Thermo Scientific Xcalibur software and then processed with Origin software to obtain a chromatogram. Data analysis was also performed with a heatmap created using MetaboAnalyst 5.0.
RESULTS AND DISCUSSION
Effect of shading or nitrogen fertilizer on harvest variables and proximate analysis of A. lavenia
The analysis of variance showed no interaction between shading treatment and nitrogen fertilizer on chlorophyll A, chlorophyll B, total chlorophyll, and leaf area of A. lavenia. Shading did not significantly affect chlorophylls A, B, or total chlorophyll. However, compared to the control, the nitrogen dose significantly increased chlorophyll A and total chlorophyll by 10.57% and 8.74%, respectively. In contrast to chlorophyll, shading significantly increased leaf area by 44.27% of the control, while the dose of nitrogen had no significant. effect on the leaf area (Table 1).
Based on statistical analysis, shading or nitrogen had p < 0.05 for ash content but not for dried leaf weight at harvest and water content. Referring to Figure 1A and B, 25% shade or nitrogen 90 kg/ha tended to increase the dried weight of the harvested leaves but not significantly. Plants with shading had a range of water content ranging from 9.93% to 11.29% while applying nitrogen dose was around 10.64%?10.76%. Furthermore, the ash content is significantly affected by shade or nitrogen dose. The shade significantly increased ash content by 34.33% to the control, different from the addition of nitrogen dose, which decreased ash content by 8.58% of the control. The combination of shading*nitrogen had a p-value > 0.05 for the ash content, so there was no significant difference between shading and nitrogen dosage (Fig. 1C). Therefore, the ash content is better displayed in Figure 1A and B to show the significance level. In contrast to water and ash content, the analysis variance of the acid-insoluble ash content showed an interaction between shading and nitrogen fertilizer because the combination between shading*nitrogen had p < 0.05. Therefore, the data shown in the combination of shading and nitrogen dosage, the resulting acid-insoluble ash content ranged from 0.60% to 1.77% (Fig. 2).
Giving nitrogen increases the synthesis of chlorophyll because nitrogen is a constituent element of chlorophyll. It is related to the study’s results that the application of nitrogen increased the dried weight of the harvested leaves, which was in line with the increase in total chlorophyll (Table 1, Fig. 1). In addition, an increase in shade intensity also causes an increase in leaf area. It is a form of leaf morphological adaptation to maximize light capture in photosynthesis [21]. Providing 50% shade and 138 kg/ha nitrogen increased the leaf area index in Triticum aestivum L. compared to controls [22]. Increasing the leaf area also causes an increase in the photosynthesis rate because the light is more easily received, thereby, increasing the assimilate for the forming of secondary metabolites 22,23]. This photosynthetic activity can increase plant growth and is closely related to the increase in the dried weight of the harvest, affecting the increase in secondary metabolite production [2
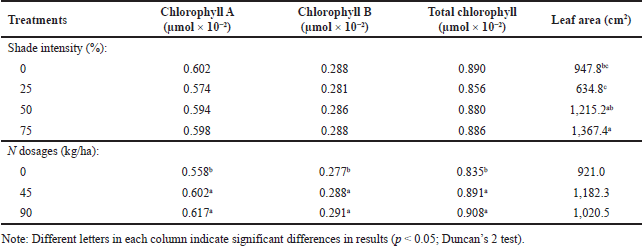 | Table 1. Chlorophyll A, chlorophyll B, total chlorophyll, and leaf area of A. lavenia. [Click here to view] |
Based on previous studies, the water content of dried leaf A. lavenia is 7.97% [25] and 9.34% [7], whereas the ash content of A. lavenia leaves was 16.92% [7]. The results obtained from the study were still below those from the literature. High ash content indicates a tendency for plants to contain relatively large amounts of minerals, such as silicates of sodium, phosphorus, potassium, phosphate, carbonate, and magnesium [26]. Acid insoluble ash content is a further analysis to determine how many minerals and foreign materials in these medicinal plants are not soluble in acids, such as silica, quartz, sand, and stone [27]. There still needs to be more information regarding the effect of shading and N fertilizer on ash content. Khalil et al. [28] have observed that increasing nitrogen content decreases ash content in wheat plants. This is because the conditions without nitrogen increased the elements of P and Mg by 5.69% and 11.48%, respectively, from giving nitrogen 75 kg/ha. These elements are obtained from the soil as a form of self-defense.
Determination of ash and acid-insoluble ash content is important to determine the quality of medicinal plants [29]. Increasing acid-insoluble ash content can be caused by impurities from sand or soil. The research about the acid-insoluble ash content of the medicinal plant A. lavenia has not been reported. Based on previous studies, the acid-insoluble ash content of the medicinal plant Microtrichia perotitii DC. Belonging to the Asteraceae family is 2.73%. The values obtained indicate that the medicinal plants are in good physiological condition and contain little contamination from soil and sand [30]. There still needs to be more information regarding the effect of shading and N fertilizer on acid-insoluble ash content because there is still no related research.
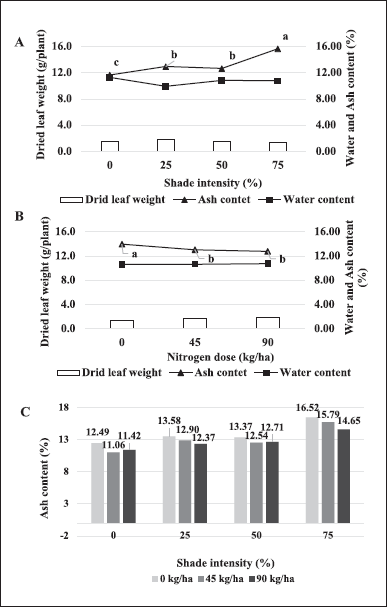 | Figure 1. The dried weight of harvested leaves, water content, and ash content in shading (A); different nitrogen dosages (B); and ash content with interaction of shading and nitrogen dose (C). [Click here to view] |
Terpenoids and 11αOH-KA production, and free radical scavenging capacity of A. lavenia
Determining the optimum treatment in this study is necessary to get the best results at the lowest cost so that it is more profitable [31]. The analysis variance results showed no interaction between shading and nitrogen dosage on terpenoids and 11αOH-KA production (p > 0.05 for shading*nitrogen interaction), but nitrogen dose significantly affected terpenoid and 11αOH-KA production (p < 0.05 for nitrogen). Optimum terpenoid production was given 25% shade or N dose of 45 kg/ha, with an increase in terpenoid production of 65.13% and 28.21%, respectively, of the control (Table 2). This treatment was chosen because, based on statistical analysis, the shading of 25% and 75% were not significantly different, it was indicated by the presence of the same notation for the two treatments. Likewise, the nitrogen doses of 45 and 90 kg/ha were not significantly different. Therefore, they increased terpenoid production compared to control. The shading results did not have a significant effect (p > 0.05), but 25% shading tended to increase the productivity of 11αOH-KA. The nitrogen dose significantly increases the production of 11αOH-KA. Giving N 45 kg/ha was more optimum than the other treatments, with an increase in the production of 11αOH-KA by 40.87% of the control (Table 2). Shading or dose of nitrogen treatments did not affect the free radical scavenging capacity, so the shade or nitrogen dose treatment had the same effect. The free radical scavenging capacity tends to be high in the no-shade and 50% shaded conditions but not significantly. This is supported by high total terpenoid content at 50% shade intensity (Fig. S1) and 11αOH-KA content at no shade conditions (Fig. S2). Terpenoids are one of the important phytochemicals for antioxidant and free radical scavenging effects [32].
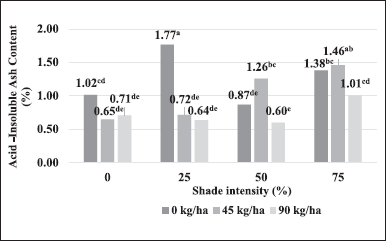 | Figure 2. Acid-insoluble ash content in shading and different doses of nitrogen. [Click here to view] |
The difference in the best treatment results for the total terpenoid and 11αOH-KA content and their productivity was due to the influence of the dried weight of the harvest per plant. The application of nitrogen at 90 kg/ha markedly increased the production of terpenoids and 11αOH-KA because an increase in elemental nitrogen increased photosynthetic activity [22]. Increasing photosynthesis will increase the availability of carbon substrates for the synthesis of isopentyl pyrophosphate precursors in the MVA and dimethyl pyrophosphate precursors in the MEP pathway to form terpenoids and their derivatives [33]. Therefore, this research was conducted to get better treatment for A. lavenia so this plant will be more stable and adaptive when further cultivated. This is to support the future needs of the drug and cosmetic industry in utilizing the medicinal plant of A. lavenia, because its production requires much biomass and is supported by high levels of secondary metabolites. No literature reports the productivity of terpenoids and 11αOH-KA, especially in A. lavenia.
11αOH-KA is part of the terpenoids that belong to the diterpenoid group and has been reported to have various biological activities, such as anti-melanogenesis, anti-inflammatory, and antioxidant [31]. Determination of free radical scavenging capacity is carried out to determine the extent to which a substance or compound fights or inhibits free radical damage in the body [34]. This is also possible because 11αOH-KA (diterpenoid) contains hydroxyl groups (−OH) and carbonyl (C?O), which can reduce the reduction of radical compounds from DPPH [35].
Putative identification of metabolites in A. lavenia leaves using LC-MS/MS
Twelve compounds were confirmed from the methanol extract of A. lavenia leaves using LC-MS/MS (Table 3). The compound groups identified were five terpenoid groups (diterpenoids and sesquiterpenoids), two alkaloid groups, one flavonoid (flavonol) group, one amino acid group, and three fatty acid groups. Base peak chromatogram patterns using the positive ionization mode for all extracts produced almost the same basic peaks for each treatment (Fig. 3). This is indicated by the few differences in the number of compounds in each treatment (Table 4).
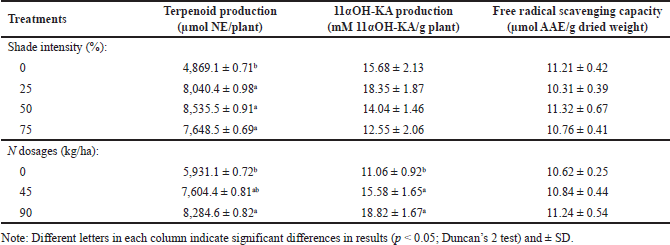 | Table 2. Production of terpenoids, 11αOH-KA, and free radical scavenging capacity of A. lavenia. [Click here to view |
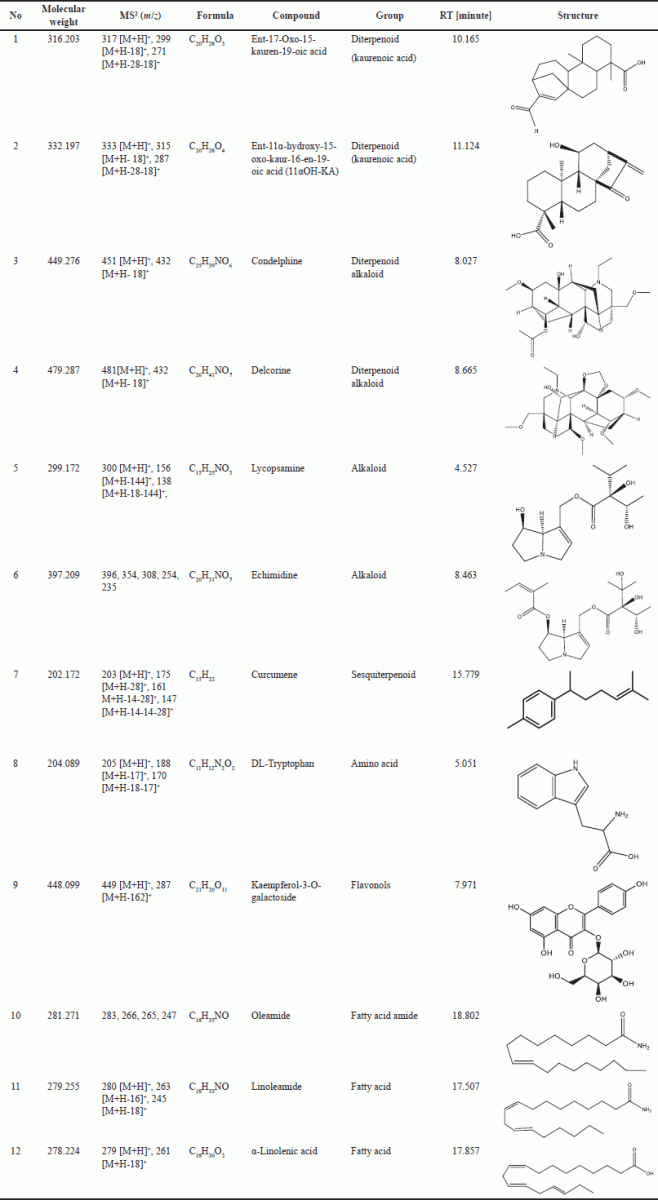 | Table 3. Profiling compounds of A. lavenia leaf extract. [Click here to view |
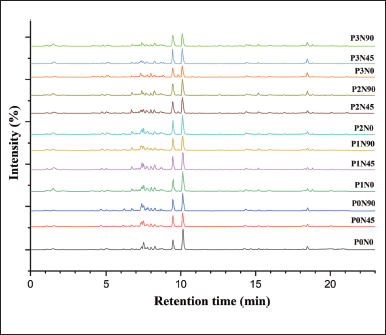 | Figure 3. The base peak of the chromatogram in the positive ionization mode of A. lavenia leaves replicate 1 [p = shade intensity (1 = 0%, 2 = 25%, 3 = 50%, and 4 = 75%), N = nitrogen dose (0, 45, and 90 kg/ha)]. [Click here to view] |
Terpenoids are the dominant metabolite in A. lavenia [7]. In this study, 5 compounds were identified, namely Ent-17-Oxo-15-kauren-19-oic acid (1), 11αOH-KA (2), condelphine (3), Delcorine (4), and curcumene (7). These compounds can be fragmented by releasing various functional groups. For example, in 11αOH-KA identified at m/z 333 [M+H]+ which fragmented into m/z 315 and 287 by releasing H2O [M+H-18]+ and CO[M+H-28-18]+ [8]. Condelphine and delcorine were identified at 451 [M+H] + and 481 [M+H] +, respectively. Both release H2O, so the fragmented are 432 [M+H-18]+ and 462 [M+H-18]+, respectively. Curcumene at m/z 203 [M+H]+ is fragmented by releasing C2H4 to become m/z 175 [M+H-28]+ and releasing CH2 at 161 [M+H-14-28]+ and 147 [M+H-14-14-28]+ [36]. The identified amino acids are DL-tryptophan (8) at m/z 205 [M+H]+, fragmented releasing NH3 at m/z 188 [M+H-17]+, and H2O at m/z 170 [M+H-18-17]+, respectively [37].
The alkaloid metabolites identified in this study are lycopsamine (5) and echimidine (6). Lycopsamine was identified at m/z 300 [M+H]+ and fragmented to 156 [M+H-144]+ by releasing C7H12O3. Echimidine was identified at 398 [M+H]+, fragmented into 175 [M+H-28]+ by releasing C7H13O5 and 161 [M+H-14-28]+ by releasing C5H8O2, respectively. The flavonoid group identified was Kaempferol-3-O-galactoside (9) with m/z 448 [M+H]+, and fragmentation was found at m/z 287 [M+H-62]+ by releasing C15H11O6 [38]. Furthermore, fatty acids found in this study were oleamide (10), linoleamide (11), and α-Linolenic acid (12). Oleamide at m/z 282 [M+H]+, has fragmentation of 263 [M+H-16]+ and 245 [M+H-18-16]+ which releases NH2 and H2O, respectively. Linoleamide at m/z 280 [M+H]+ results in fragmentation of m/z 263 [M+H-16]+ by releasing NH2 and m/z 245 [M+H-18]+ releasing H2O. α-Linolenic acid with m/z 279 [M+H]+ and fragmented to 261 [M+H-18]+ by releasing H2O [39].
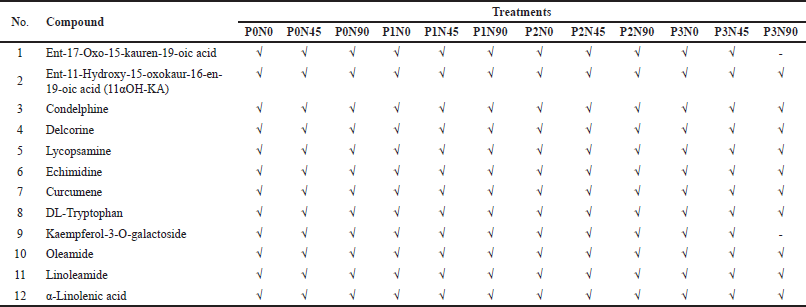 | Table 4. Confirmed compounds in A. lavenia leaf extract. [Click here to view] |
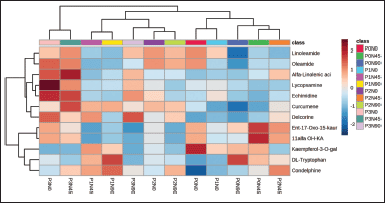 | Figure 4. Heatmap of the identified compound of A. lavenia [p = shading intensity (1 = 0%, 2 = 25%, 3 = 50%, and 4 = 75%), N = nitrogen dose (0, 45, and 90 kg/ha)]. [Click here to view] |
The peak area data of identified components and confirmed LC-MS/MS results were also analyzed using a heatmap (Fig. 4). The grouping of these compounds is based on the similarity of the constituent structures. For example, the compounds Ent-17-Oxo-15-kauren-19-oic acid and 11αOH-KA, the kaurenoic acid groups, are close. Based on heatmap analysis, the 11αOH-KA component increased under shaded conditions and N 45 kg/ha (P0N45). These results are related to the analysis of 11αOH-KA content that is optimum in conditions without shade or N 45 kg/ha (Fig. S2). The 75% shading treatment and 45 nitrogen dose (P3N45) had a more varied metabolite content than the other treatments. Treatment without shade and nitrogen dose (P0N0) increased kaempferol-3-O-galactoside compared to other treatments. This is because, under stress conditions, the plants increased the availability of acetyl-CoA and malonyl-CoA to produce naringenin, a precursor for kaempferol formation [40].
The 11αOH-KA compound was successfully isolated in A. lavenia. It was reported to exhibit antioxidant activity by activating NRF2-HO1 in B16F10 cells, anti-inflammatory by inhibiting Inducible Nitric Oxide Synthase (iNOS) through Lipopolysaccharide (LPS) induction in Rapid American Withdrawal (RAW) 264.7 cells, and anti-melanogenesis in mouse and human melanocyte cells [8]. In addition, based on previous study, several compounds with antioxidant activity have been reported, such as condelphine [41], echimidine [42], curcumene [1], DL-tryptophan [37], kaempferol-3-O-galactoside [35], oleamide [43], linoleamide [44], and α-linolenic acid [39]. Therefore, these compounds also have the potential for free radical scavenging.
CONCLUSION
Adenostemma lavenia is a potential medicinal plant for the world pharmaceutical industry. The optimum terpenoid and 11αOH-KA productions were obtained under 25% shade or nitrogen 45 kg/ha. The free radical scavenging capacity tended to increase at 50% shade or nitrogen 90 kg/ha. Evaluating the antioxidant of at least three or four techniques other than the DPPH method is necessary. In addition, other in vitro analyses are also needed, such as anti-diabetic, anti-inflammatory, and others. Twelve compounds have been confirmed, eight of which have been reported to have antioxidant activity, so it is potential as free radical scavenging. Based on the results of this study, A. lavenia has great potential in the pharmaceutical industry and research.
SUPPLEMENTARY MATERIALS
There is data in supplementary materials.
ACKNOWLEDGMENT
The research was supported by the bilateral exchange program directorate general of higher education/Japan Society for promoting the science joint research project 2022, ongoing no. 023.17.1. 690439/2022
AUTHORS’ CONTRIBUTIONS
All authors have made a significant contribution to this manuscript. Concept and design: IB, SAZ, and HT—data acquisition and analysis/interpretation: AES, RN, and TR—drafting manuscript: AES—critical revision of the manuscript: IB, SAZ, MF, MR—supervision and final approval: IB, SAZ, MF, and MR. The published version of the manuscript has been read and approved by all authors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Rohman A, Riyanto S, Hidayati NK. Antioxidant activity, total phenolics, and total flavonoid contents of mengkudu (Morinda citrifolia L.) leaves. Agritech. 2007;27(4):147–51.
2. de Oliveira S, de Souza GA, Eckert CR, Silva TA, Sobral ES, Fávero OA, et al. Evaluation of antiradical assays used in determining the antioxidant capacity of pure compounds and plant extracts. Química Nova. 2014;37(3):497–503. CrossRef
3. Batubara I, Astuti RI, Prastya ME, Ilmiawati A, Maeda M, Suzuki M, et al. The antiaging effect of active fractions and ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid isolated from Adenostemma lavenia (L.) o. Kuntze at the cellular level. J Antioxidants. 2020a;9(8):1–14. CrossRef
4. Chen JJ, Deng JS, Huang CC, Li PY, Liang YC, Chou CY, et al. P-coumaric-acid-containing Adenostemma lavenia ameliorates acute lung injury by activating AMPK/Nrf2/HO-1 signaling and improving the antioxidant response. Am J Chinn Med. 2019;47(7):1–24. CrossRef
5. Nurlela N, Badrunanto, Ilmiawati A, Nurcholis W, Takemori H, Batubara I. The medicinal potential of plants from the Adenostemma genus. J Appl Pharm Sci. 2023;13(08):001–11. CrossRef
6. Nurlela N, Nurfalah R, Ananda F, Ridwan T, Ilmiawati A, Nurcholis W, et al. Variation of morphological characteristics, total phenolic, and total flavonoid in A. lavenia, A. madurense, and A. platyphyllum. Biodiversitas. 2022a;23(8):3999–4005. CrossRef
7. Fauzan A, Praseptiangga D, Hartanto R, Pujiasmanto B. Characterization of the chemical composition of Adenostemma lavenia (L.) Kuntze and Adenostemma platyphyllum Cass. IOP Conf Ser Earth Environ Sci. 2017;102(1):1–8. CrossRef
8. Maeda M, Suzuki M, Fuchino H, Tanaka N, Kobayashi T, Isogai R, et al. Diversity of Adenostemma lavenia, multi?potential herbs, and its kaurenoic acid composition between Japan and Taiwan. J Nat Med. 2021;76(1):132–43. CrossRef
9. Nurlela N, Awaluddin F, Batubara I, Wahyudi ST. Computational study of kaurene diterpenoids for antivirals against SARS-CoV-2. J Appl Pharm Sci. 2022b;12(08):112–29. CrossRef
10. Singh V, Jain N, Shri R. Effect of abiotic factors on bacoside A content, acetylcholinesterase inhibitory and antioxidant activities of Bacopa monnieri (L.) Wettst. J Appl Pharm Sci. 2021;11(Supp 1):063–70.
11. Liang Y, Cossani CM, Sadras VO, Yang Q, Wang Z. The interaction between nitrogen supply and light quality modulates plant growth and resource allocation. Front Plant Sci. 2022;13:864090. CrossRef
12. Lima MC, Da-Silva CJ, Mariot MP, Freitag RA, Serpa R, Ribeiro GA, et al. Effect of shading and nitrogen fertilization on nitrogen metabolism, essential oil content, and antimicrobial activity of Achillea millefolium. Acta Sci Biol Sci. 2020;42:e46412. CrossRef
13. Mawardy WD, Karyawati AS. Effect of shade and urea fertilizer on the growth and yield of Iler plants (Plectranthus scutellarioides (L.) R. Br.). J Agric Sci. 2021;6(1):58–67. CrossRef
14. Muttaleb QA, Abdullah TL, Hassan SA, Rashid AA, Taheri S, Ahmed OA, et al. The role of shade and nitrogen on physiological traits and secondary metabolites of Piper betle L. J Hortic. 2018;5:2. CrossRef
15. Taiz, Zeiger. Plant physiology. 5th ed. Sunderland, MA: Sinauer Ass Inc Publisher; 2010.
16. Theowidavitya B, Muttaqin M, Mifthudin, Thagjoleksono A. Metabolomics analysis on the interaction of rice and bacteria. J Sumberd Hayati. 2019;5(1):18–24. CrossRef
17. BMKG [Internet]. Indonesia : Indonesian Agency for Meteorological, climatological, and Geophysics; c2022- [cited 2022 May 12]. Available from https:/dataonline.bmkg.go.id
18. AOAC. Guidelines for standard method performance requirements. 19th ed. Arlington TX: Association of Official Analytical Chemist Inc, AOAC; 2016.
19. Lukowski A, Jagiello R, Robakowski P, Adamczyk D, Karolewski P. Adaptation of a simple method to determine the total terpenoid content in needles of coniferous trees. Plant Sci. 2022;314:111090. CrossRef
20. Batubara I, Maily, Wahyuni WT, Tilaar K, Nurcholis W, Junardy FD, et al. Tyrosinase inhibition, antiglycation, and antioxidant activity of Xylocarpus granatum. Biosaintifika. 2020b;12(1):70–5. CrossRef
21. Ekawati R, Aziz SA, Andarwulan N. Shoot, total phenolic, and anthocyanin 6 productions of plectranthus amboinicus with organic fertilizing. Bul Littro. 2013;24(2):93–100.
22. Zhang H, Zhao Q, Wang Z, Wang L, Li X, Fan Z, et al. Effects of nitrogen fertilizer on photosynthetic characteristics, biomass, and yield of wheat under different shading conditions. Agronomy. 2021;11:1989. CrossRef
23. Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci. 2015;6:167. CrossRef
24. Sitorus UKP, Siagian B, Rahmawati N. Response in the growth of cocoa seedlings (Theobroma Cacao L.) on giving boiler ash and urea fertilizer at Nursery. J Agroekoteknol Univ Sumatera Utara. 2014;2(3):1021–9.
25. Budiarti E, Batubara I, Ilmiawati A. The potency of Asteraceae plant extracts as an antioxidant and antiglycation agent. J Jamu Indones. 2019;4(3):103–11. CrossRef
26. Hossain M, Sarker M, Robel H. Antioxidant and anthelminthic activities of Coccinia indica leaf extracts. J Noakhali Sci Technol Univ. 2022;5(2):73–80.
27. Kim D, Kim B, Yun E, Kim J, Chae Y, Park S. Statistical quality control of total ash, acid-insoluble ash, loss on drying, and hazardous heavy metals contained in the component medicinal herbs of “Ssanghwatang”, a widely used oriental formula in Korea. J Nat Med. 2012;67(1):27–35. CrossRef
28. Khalil JK, Sawaya WN, Hamdallah G, Devi Prasad J. Effect of nitrogen fertilization on the nutritional quality of wheat variety, Yecora Rojo. Qual Plant Plant Foods Hum Nutr. 1987;36(4):279–93. CrossRef
29. Rao Y, Xiang B. Determination of total ash and acid-insoluble ash of Chinese herbal medicine prunella spica by near-infrared spectroscopy. Pharm Sci J. 2009;129(7):881–6. CrossRef
30. Abdullahi MN, Ilyas N, Hajara I, Kabir YM. Pharmacognostic evaluation of the leaf of Microtrichia perotitii DC. (Asteraceae). J Pharmacogn Phytother. 2018;10(4):76–84. CrossRef
31. Bai X, Tsiatis AA, Lu W, Song R. Optimal treatment regimes for survival endpoints using a locally-efficient doubly-robust estimator from a classification perspective. Lifetime Data Anal. 2016;23(4):585–604. CrossRef
32. Gutiérrez-del-Río I, López-Ibáñez S, Magadán-Corpas P, Fernández-Calleja L, Pérez-Valero Á, Tuñón-Granda M, et al. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 2021;10:1264. CrossRef
33. Ormeño E, Fernandez C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr Bioact Compd. 2012;8:71979. CrossRef
34. Apak R, Özyürek M, Güçlü K, Çapano?lu E. Antioxidant activity/capacity measurement: I. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem. 2016;64(5):997–1027. CrossRef
35. Sedjati S, Supriyantini E, Ridlo A, SOenardjo N, Santi VY. Kandungan pigmen, total fenolik, dan aktivitas antioksidan Sargassum sp. J Kelaut Trop. 2018;21(2):137−44. CrossRef
36. Klau ME, Rohaeti E, Rafi M, Artika IM, Ambarsari L, Nurcholis W. Metabolite profiling of Curcuma zanthorrhiza varieties grown in different regions using UHPLC-Q-OrbitrapHRMS and chemometrics analysis. Biointerface Res Appl Chem. 2023;13(1):26. CrossRef
37. Rolnik A, Soluch A, Kowalska I, Olas B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition—comparative studies. Biomed Pharmacother. 2021;142:111982. CrossRef
38. Larrazábal-Fuentes MJ, FernándezGalleguillos C, Palma-Ramírez J, Romero-Parra J, Sepúlveda K, Galetovic A, et al. Chemical profiling, antioxidant, anticholinesterase, and antiprotozoal potentials of Artemisia copa Phil. (Asteraceae). Front Pharmacol. 2020;11:594174. CrossRef
39. Ayaz FA, Ozcan M, Kurt A, Karayigit B, Ozogul Y, Glew R, et al. Fatty acid composition and antioxidant capacity of cypselas in Centaurea s.l. taxa (Asteraceae, Cardueae) from NE Anatolia. South Afr J Bot. 2017;112:474–82. CrossRef
40. Duan L, Ding W, Liu X, Cheng X, Cai J, Hua E, et al. Biosynthesis and engineering of kaempferol in Saccharomyces cerevisiae. Microb Cell Factories. 2017;16:165. CrossRef
41. Ahmad H, Ahmad S, Shah SAA, Latif A, Ali M, Khan FA, et al. Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum. Bioorg Med Chem. 2017;25(13):3368–76. CrossRef
42. Hassan SSU, Abdel-Daim MM, Behl T, Bungau S. Natural products for chronic diseases: a ray of hope. Molecules. 2022;27(17):5573. CrossRef
43. Elmi A, Spina R, Risler A, Philippot S, Mérito A, Duval RE, et al. Evaluation of antioxidant and ntibacterial activities, cytotoxicity of Acacia seyal Del bark extracts and isolated compounds. Molecules. 2020;25(10):2392. CrossRef
44. Hanh TTH, Hang DTT, Minh CV, Dat NT. Anti-inflammatory effects of fatty acids isolated from Chromolaena odorata. Asian Pac J Trop Med. 2011;4(10):760–3. CrossRef
SUPPLEMENTARY MATERIALS
 | Figure S1. Terpenoid total in different shading and nitrogen dosages. [Click here to view] |
 | Figure S2. 11αOH-KA content in different shading (A); and nitrogen dosages (B). [Click here to view] |