INTRODUCTION
Since 1000 BC., Ayurveda has been used as a traditional medical system in India. In addition, it is used all around the world as a supplemental and alternative medical system. The Ayurvedic industry has grown considerably in recent periods. According to recent WHO research, it is estimated that almost 80% of the world’s population utilizes medications derived from natural and herbal sources. Ayurveda is presently steadily gaining relevance as a result of the problems with modern healthcare technologies and procedures seen globally. Even though Ayurveda has been refined over centuries, there are still issues with the lack of clarity surrounding high-quality medications. The international acceptance of genuine, high-quality Ayurvedic medicines will increase if these problems are resolved [1].
The concept of standardization of Ayurvedic and herbal preparations has recently gained a lot of relevance to deliver high-quality medications as per various national and international regulatory criteria. The choice of raw materials, extraction techniques, process variables, packaging mechanism, and most important storage conditions all play a part in the quality of medications [2]. Evaluating the chemical stability of these categories of Ayurvedic formulations becomes essential to quality control because they are prone to instability under specific storage circumstances. The evaluation of stability becomes challenging owing to the complex nature of a wide variety of chemistry in a polyherbal formulation. According to the reports, the stability investigations involving a specific secondary metabolite with a well-documented history of medicinal efficacy and its degradation products have received the majority of attention thus far. The conclusions drawn from these stability statistics do not always correspond to the stability of the other ingredients in the formulation [3]. Since additional constituents can influence the product’s stability, determining the constituents with recognized therapeutic activity is typically insufficient. Chemical, analytical, and active markers are three different types of components (markers) that can be found in herbal products for various determinations. Chemical indicators, which are of relevance for quality control purposes whether or not they have therapeutic efficacy, are typically characterized chemically as constituents or groupings of constituents of a herbal medical product. A chemical marker’s concentration could be a sign of a herbal medicine’s potency. To assess product quality and stability over time and establish the appropriate shelf life, chemical markers may be used. The components or collection of components used exclusively for analysis are known as analytical markers. The components or collection of components recognized to have pharmacological activity that contributes to efficacy are known as active markers. If properly stored, a product’s shelf life is the amount of time during which its chemical, physical, and microbiological stability stays within acceptable limits. The product typically has an expiration date to indicate when its shelf life has ended [4].
The topic of stability and shelf life assessment is such an important one that the Ayurvedic formulations lack a specific and organized method. The 1940 Drugs and Cosmetics Act’s Rule No. 161-B was updated by AYUSH on August 12, 2016, and it states that the shelf life must be specified and substantiated by scientific data. According to the amendment, the shelf life of Ayurvedic medications must be founded on scientific evidence and evaluated by real-time stability studies of drugs prescribed in Part I, Volume III of the Ayurvedic Pharmacopeia of India. The concurrent substances included in the polyherbal formulation may have an impact on the formulation’s overall stability. A variety of contemporary analytical techniques and methods must be used to measure the stability of Ayurvedic formulations that are solid, liquid, and semisolid to gain an understanding of the factors that affect their stability. This will provide a concise overview of the stability of the Ayurvedic formulations [4,5].
Although the AYUSH notification, which the Government of India issued on August 12, 2016, advises specifying the shelf life of Ayurvedic formulations based on scientific evidence, it is now necessary to provide a detailed justification of the scientific evidence due to improvements in process technology, packaging, and storage techniques. The reason behind the expiry of the mentioned class of Ayurvedic preparations is lacking. There is no scientific justification to support the revised shelf life of the classical Ayurvedic preparations. A validated analytical method and a stability testing module will open up avenues regarding monitoring the chemical stability of the Ayurvedic formulations. A validated protocol for performing the stability studies of Ayurvedic preparations must be designed. The protocol must include information such as the selection a batch and defined sampling plan, testing parameters, modern analytical methods, acceptance criteria and limits, storage period and conditions, testing frequency, container closure system, and appropriate stability testing methods must be considered. Pharmaceuticals are often tested using a stability-indicating analytical technique that has been validated, and an expiration date is established based on stability data from the date of production when the medicine exhibits no more than 10% degradation. These recommendations may be used in Ayurvedic medicines where percentage deterioration may be measured after being exposed to various humidity and temperature conditions. Ayurvedic or modern medicine’s notion of stability is unchanged, but the criteria used to evaluate it differ between pharmaceuticals and Ayurvedic formulations [6,7].
Ayurvedic Kushmanda Rasayanam is a multiherbal remedy that can be used to cure a variety of illnesses. The primary component of this mixture is Benincasa hispida, also referred to as Kushmanda in Sanskrit [8,9]. Amino acids, mucins, mineral salts, starch, calcium, and vitamins B and C are all present in Khushmanda. Cough, dyspnea, chest wound, phthisis, persistent fever, bleeding disease, emesis, thirst, semen shortage, weakness, emaciation, and hoarseness are among the conditions it is used to treat. Astilbin (6%), catechin (5.15%), and naringenin (3.95%) are the major flavonol components present in the extract of the fruit [10–14].
MATERIALS AND METHODS
Materials
Ultrapure water (Merck Milli Q), ACN and MeOH high performance liquid chromatography (HPLC) grade (FINAR Chemicals), Catechin, and Piperine 99% purity (Sigma–Aldrich). Khushmanda Rasayana was obtained from a local good manufacturing practices (GMPs).
Preparation of stock and working standard solution
Catechin and Piperine 10 mg were weighed, and methanol was used to dilute the mixture to 10 ml. Therefore, 1,000 μg/ml of stock standard solution was produced. This solution was further diluted with methanol, and the result was a working standard with a concentration of 10 μg/ml that contained a combination of Catechin and Piperine.
HPLC method development and validation
Shimadzu HPLC (Make: Kyoto JAPAN) was used for separation, equipped with an LC-10 ADVP quaternary pump, SIL-10 ADVP auto-injector, and an SPD M-10A VP photodiode array detector (PDA) detection system. Installed with liquid chromatography solution software. The column used for separation was C18 (250 cm × 4.6 mm, 5 μ) from Phenomenex HyperClone Base Deactivated Silica (BDS). A gradient program developed for elution.
Validation of analytical method
Specificity
The analytical method’s capacity to evaluate the marker in the presence of matrix ingredients that interfere. The composition of the herbal formulation having a complex matrix combined with matrices, degradants, and contaminants affects the determination of markers. Hence, a working standard solution mixture of 10 µg/ml concentration of Catechin and Piperine was used for determination. A system suitability solution of Catechin and Piperine was evaluated for its peak purity index.
System suitability
A mixture of Catechin and Piperine in a standard solution of a 10 µg/ml concentration was injected. Peak parameters including peak area, tailing factor, resolution, retention time, and theoretical plates were observed.
Linearity
A range of standard solutions for linearity containing a combination of Catechin and Piperine were prepared in the range of 0.05–3.1 µg/ml.
Limit of detection (LOD)
The lowest concentration of a marker that can be found in a sample using analytical methods that are only capable of detection and not quantification.
Limit of quantitation (LOQ)
The lowest concentration of a marker that can be found in a sample that can be accurately and precisely determined. LOQ is determined in assays, for the identification and quantification of impurities or degradants.
Accuracy
The similarity between the value found and a standard value serves as a measure of accuracy. A specified quantity of the marker was added to the standard samples of known concentration and the recovery of the marker was predicted. This method is known as the traditional spiking approach. The concentration levels at which accuracy was assessed for Catechin and Piperine, were 0.8, 1, and 1.2 μg/ml.
Precision
Repeatability, intermediate precision, and reproducibility are the three criteria that are typically used to evaluate the precision. To determine the intraday and interday precision, a series of six injections of standard solution were injected into the system. The retention time and peak area were examined. A set of measurement values were expressed as mean, SD, and relative SD.
Recovery of marketed formulation
Using a mortar and pestle, 5 g of Kushmanda Rasayana was crushed into a fine powder. A 50 ml volumetric flask containing 5 g of powder was filled with methanol and subjected to 45 minutes of sonication with regular shaking every 5 minutes. A sample of 2 ml of the solution was centrifuged at 1,000 rpm for 10 minutes. For analysis, 1 ml of the supernatant solution was taken, and the percentage of recovery was calculated.
Stress studies
To ascertain the existence of degradation products, stress tests were carried out using the standard solution mixture with a concentration of 10 µg/ml for Catechin and Piperine. The samples were examined using PDA detectors and optimized chromatographic conditions to determine the peak purity. The peak purity of the marker is calculated in the presence of degradant peaks to check for any potential degradation products.
Acid hydrolysis
At both heated and room temperature, hydrolysis was performed for Catechin and Piperine in an acidic medium. At room temperature, 0.1 M HCl was used to conduct the study’s initial phase. Studies were carried out at a high temperature since the deterioration that was discovered was quite nominal—around 10%. 5 mg of each of Catechin and Piperine, both accurately weighed, were added to a 50 ml round bottom flask, and 50 ml of 0.1 M HCl was used to accomplish the reaction. Furthermore, serial dilution using MeOH was done from stock standard to yield a working standard solution. The solution was neutralized using 0.1 M sodium hydroxide, to attain a pH 7. A working standard solution mixture of 10 µg/ml of Catechin and Piperine was used for analysis.
Alkaline hydrolysis
Markers were subjected at both heated and room temperature, hydrolysis was performed for Catechin and Piperine using 0.1 M sodium hydroxide for alkaline hydrolysis. Furthermore, serial dilution using MeOH was done from stock standard to yield a working standard solution and the resultant solution was neutralized to pH 7 with 0.1 M HCl. A working standard solution mixture of 10 µg/ml of Catechin and Piperine was used for analysis.
Oxidative degradation
Catechin and Piperine mixture solution and varying concentrations of peroxide solution (H2O2) were used to hydrolyze the mixture. 24-hour period of the investigation was spent using 3% and 5% v/v solutions of H2O2 in ambient conditions. Using a 30% v/v hydrogen peroxide solution, the trials were continued for up to 24 hours because there had been no indication of probable degradation for 24 hours. Catechin and Piperine final solution (10 μg/ml) was employed for the measurement.
Photolytic degradation
Accurately weighed 10 mg each of Catechin and Piperine on Petri plates and were exposed to the sun for 24 hours. A standard stock solution of Catechin and Piperine was prepared by weighing and dissolving 5 mg of each marker in MeOH. Furthermore, a working standard solution mixture of Catechin and Piperine (10 µg/ml) was injected into the HPLC system for analysis.
Thermal degradation
Accurately weighed 15 mg each of Catechin and Piperine on Petri plates. It was further subjected to storage at 80ºC for 24 hours. This working standard solution mixture of Catechin and Piperine (10 µg/ml) was employed for the measurement.
Sampling of products for stability studies
Khushmanda Rasayana was procured from a local manufacturer. Three batches were selected for the determination of the stability profile. The mean and standard deviation were used as a measure of the dispersion of data.
Evaluation of stability data
Determination of total ash
In a tared silica dish, 2 g of precisely weighed formulation were incinerated at a temperature of 200ºC. Furthermore, it was cooled and weighed. The cycle was repeated until the weight remained consistent. The percentage of ash was calculated regarding the initial weight.
Determination of acid-insoluble ash
To the complete ash-containing crucible, 25 ml of diluted hydrochloric acid was added. Collected the insoluble material on Whatman 41 filter paper and washed it with hot water. The insoluble material was transferred to the crucible on filter paper, dried, and ignited. 30 minutes later, weighed the residue after cooling. Using the initial weight, the amount of acid-insoluble ash was calculated.
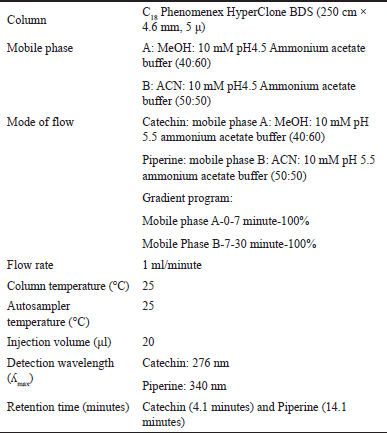 | Table 1. RP-HPLC parameters. [Click here to view] |
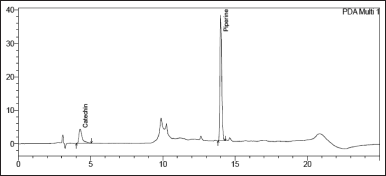 | Figure 1. Chromatogram of the optimised condition for simultaneous separation of Catechin (Rt 4.1 minutes) and Piperine (Rt 14.1 minutes). [Click here to view] |
Determination of alcohol soluble extractive
5 g of the coarsely powdered formulation was macerated with 100 ml of methanol for a total of 24 hours, with frequent shaking occurring during the first 6 hours. The mixture was then left to stand for 18 hours. 25 ml of the filtrate were filtered, evaporated in a tared dish to dryness, and then dried at 105ºC. Concerning the starting weight, the percentage of extractive that is soluble in alcohol was calculated.
Determination of water soluble extractive
The method was performed as directed for the determination of alcohol-soluble extractive, using chloroform water instead of ethanol.
Thin layer chromatography (TLC)
5 g of the sample was extracted for 30 minutes using reflux and a water bath with 75 ml of ethyl acetate. Thin layer chromatography was performed after the filtrate had been filtered and concentrated to 10 ml. 20 μl of the extract was applied to TLC plates, and using toluene: ethyl acetate (7: 3) as the mobile phase, the plates were developed to a distance of 10 cm. After development, plates were dried under air before checking them under ultraviolet and visible light.
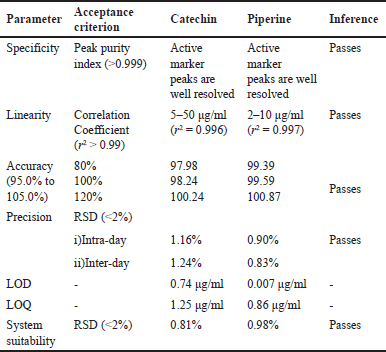 | Table 2. A summarized report of RP-HPLC validation. [Click here to view] |
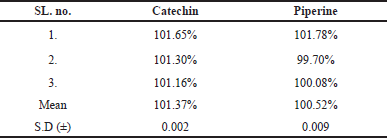 | Table 3. Mean recovery in Khushmanda Rasayana. [Click here to view] |
pH determination
The pH value of the aqueous solution of the formulation was measured.
HPLC analyses stability samples
The analyses employed the developed and validated reverse phase (RP)-HPLC method for the samples. The stability analysis involved the determination in triplicate. For the accelerated condition 40ºC 2ºC/75% relative humidity (RH) 5% RH, at periods of 0, 1, 3, and 6 months. The long-term condition involved storage for time intervals of 6, 9, and 12 months at 30ºC, 2ºC, 65% RH, and 5% RH.
Shelf-life estimation
The marker content analyses utilized the developed and validated methodologies. According to International Council for Harmonisation (ICH), stability studies were carried out. The formulation was subjected to storage at accelerated temperature conditions of 40ºC ± 2ºC and humidity of 5% RH ± 5% RH for time points of 0, 1, 3, and 6 months. Long-term conditions involved storage at a temperature of 30ºC ± 2ºC and humidity of 65% RH ± 5% RH for time points 6, 9, and 12 months. Samples were kept in a stability chamber (Thermolab Scientific, India) for the investigations. To estimate the shelf life of the formulation, Systat SigmaPlot version 15.0 (www.systatsoftware.com) was utilized, using the Arrhenius equation for extrapolation.
RESULTS AND DISCUSSION
A gradient elution program was developed using the RP-HPLC method. The final chromatographic condition for RP-HPLC is represented in below Table 1. Also, Figure 1 represents the chromatogram of the optimized RP-HPLC condition. A fingerprint profile is quite helpful in setting up standards and thus keeping a check on adulteration. In a study involving the evaluation of Catechin and Piperine in ultraviolet and visible spectroscopy using HPTLC in the analysis of various polyherbal preparations, between 287 to 550 nm were the peaks of highest responses [15].
Certain reports have evaluated the analytical responses of quercetin and rutin in B. hispida as analytical markers. The response of analytical markers can be explored futuristically. The amounts of β-sitosterol present in the B. hispida seeds extracts were obtained at 254 nm and were found to be 36% [16,17]. These reports demonstrate an array of markers that needs to be studied. Catechin and Piperine were chosen as active markers based on their evidence of being known as an active marker and their significant concentration [10–14]. Considering the availability, feasibility, and active concentration of markers present in Khushmanda Rasayana, Catechin and Piperine were chosen for the HPLC profile.
The results of method validation demonstrate the preciseness, accuracy, and reliability of the analytical method. Validation was performed as per the ICH guidelines [18]. The method demonstrates preciseness because the peak purity index for Catechin and Piperine, respectively, was 0.999 and 0.997, which is indicative of both being considerably below the threshold (>0.99). Blank determination was performed, where the active peak and diluents were monitored. There was no interference observed. In the forced degradation studies, the retention time was monitored for active peak and degradant peak. There was no evidence of interference from other components at their respective retention times. The system suitability parameters were evaluated to see whether the analytical method was suitable to the desired chromatographic parameters, and the results demonstrated acceptable standards for chromatographic separation. Since the correlation constants for Catechin and Piperine were determined to be 0.996 and 0.997, respectively, the linearity findings showed that the method was linear over a concentration range of 5–50 g/ml for catechin and 2–10 g/ml for Piperine. After determining the response standard deviation and slope, the LOD and LOQ were determined based on the signal-to-noise ratio. Catechin has concentrations of 0.74 and 1.25 μg/ml, while Piperine has concentrations of 0.007 and 0.86 μg/ml. Accuracy was determined by standard spiking method. It was assessed at three concentration levels such as 80%, 100%, and 120% of the standard marker. For Catechin and Piperine, the three concentration levels were 0.8, 1, and 1.2 µg/ml. The accuracy of the method was established at all three levels which were found within the range of 95%–105%. A typical combination solution of Piperine and Catechin was injected into the system for six injections. There were tests for repeatability as well as intermediate or between-day precision. The peak area and retention time of the markers were examined. Mean, SD, and relative SD were computed using the markers’ retention time and peak area. RSD was discovered to be within the bounds. The validation report’s executive summary is listed in Table 2 below.
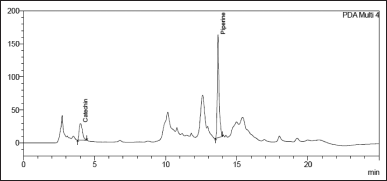 | Figure 2. Chromatogram of Khushmanda Rasayana depicting Catechin (Rt 4.2 minutes) and Piperine (Rt 13.9 minutes). [Click here to view] |
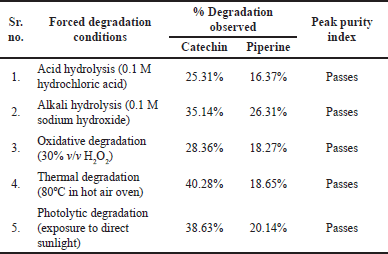 | Table 4. Stress testing. [Click here to view |
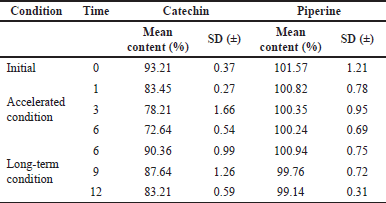 | Table 5. Mean content of markers with time. [Click here to view] |
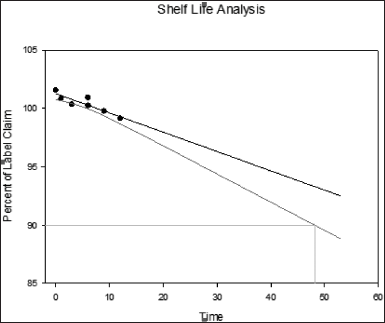 | Figure 3. Shelf life of Khushmanda Rasayana (48.1 months) considering the marker content analysis of Piperine. [Click here to view] |
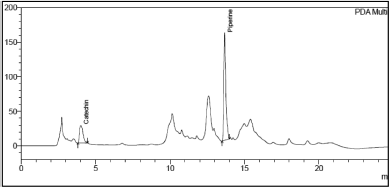 | Figure 4. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in initial (T0) time point. [Click here to view] |
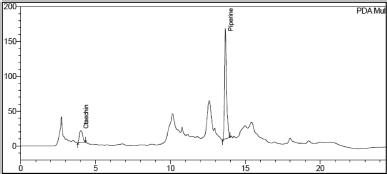 | Figure 5. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in accelerated condition at 1 month time point. [Click here to view] |
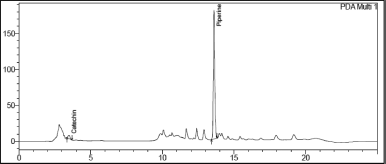 | Figure 6. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in accelerated condition at 3 months time point. [Click here to view] |
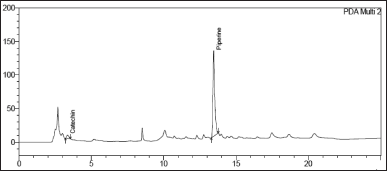 | Figure 7. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in accelerated condition at 6 months time point. [Click here to view] |
Catechin and Piperine recovered at rates of 101.37% and 100.52%, respectively. This shows that the analytical method for quantifying Catechin and Piperine in Khushmanda Rasayana is suitable and reliable. Table 3 shows the average recovery in Khushmanda Rasayana. Figure 2 depicts a chromatogram of Catechin and Piperine in Khushmanda Rasayana.
There is a potential that degradant peaks and marker peaks will co-elute during forced degradation investigations. This indicates that degradant peaks elute at the same marker retention time. As a result, the purity of the peak is demonstrated using a PDA detector. Spectral homogeneity of the marker is indicated at the λmax of Catechin (276 nm) and Piperine (340 nm). The outcomes obtained at various stress levels supported the absence of other co-eluting peaks, degradants, and contaminants. The method indicates to be specific for the simultaneous measurement of Catechin and Piperine in the presence of any degradant since no such interference with the marker peak was found. The percentage degradation is calculated using the area normalization approach. The percentage degradation observed for the markers is demonstrated in Table 4.
The changes in marker content over time are shown in Table 5. The SD stands for the triple-taken mean’s SD. Figures 4–10 depict the chromatogram of Catechin and Piperine under accelerated and long-term conditions at different time points. With variations in the marker contents examined against time and storage conditions, shelf life was projected. The software Systat SigmaPlot and the marker content analyses were used to estimate shelf life. According to Figure 3, the shelf life for Khushmanda Rasayana was calculated using Piperine to be 48.1 months. The percentage content of Catechin does not suit the curve fitting for shelf life estimation since it does not meet the internal criteria of 90%–110%. The choice of raw materials, extraction techniques, process parameters, container sealing mechanism, and most crucially, storage conditions all play a part in the quality aspect of Ayurveda [2].
Tables 6 and 7 represent the stability data of Khushmanda Rasayana at accelerated and long-term conditions at different time points. There is an increase in the pH values of the formulation over a period of time. This is suspected due to the absorption of moisture attributed to the container closure system. There is a gradual decrease in the assay value of Catechin. Figures 4–7 represent the separation of Catechin and Piperine at different time points. The TLC plates were observed with major spots at Rf 0.24 (Piperine) when observed at 366 nm. This indicates no presence of degradation products and impurities.
Investigation and root cause analysis were performed at our end to rule out the possibility of analytical errors. The pH of the formulation was checked at long-term conditions at 18 M which was found to be 5.9 and 5.3, respectively, which is beyond the pharmacopeia limit: 4.0 to 4.5. Catechin is temperature sensitive. This can be attributed to the decrease in peak area during the thermal degradation studies performed as a part of stress studies. Owing to this reason Catechin was unsuitable for curve fitting for shelf life evaluation. The content of Piperine is stable in all the temperature and humidity conditions. The estimated shelf life was 48.1 months based on the marker content of Piperine. The expiry of Rasayana preparation is 36 months as mentioned by AYUSH. The shelf-life can be extended based on real-time studies. Corrective and preventive action suggested to the local manufacturer to obtain a certificate of analysis for the containers and closures from the vendor (wall thickness and diameter). Determination of moisture vapor transmission rate for the container closure system. It was observed that there is a lack of storage instructions on the label. Hence, a suggestion based on the scientific data was given to mention storage instructions on the label to store the formulation in a cool and dry place. The concomitant substances included in the polyherbal formulation may have an impact on the formulation’s overall stability. A set of modern analytical techniques and methods must be used to measure the stability of Ayurvedic formulations that are solid, liquid, and semisolid to gain an understanding of the factors that affect their stability. This will provide a concise overview of the stability of the Ayurvedic formulations [3].
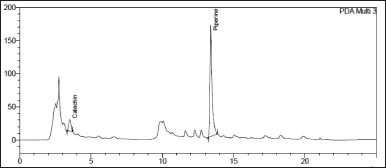 | Figure 8. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in long term condition at 6 months time point. [Click here to view] |
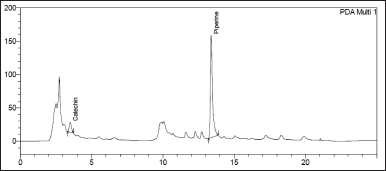 | Figure 9. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in long term condition at 9 months time point. [Click here to view] |
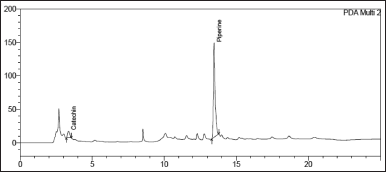 | Figure 10. Chromatogram of Catechin and Piperine in Khushmanda Rasayana in in long term condition at 12 months time point. [Click here to view] |
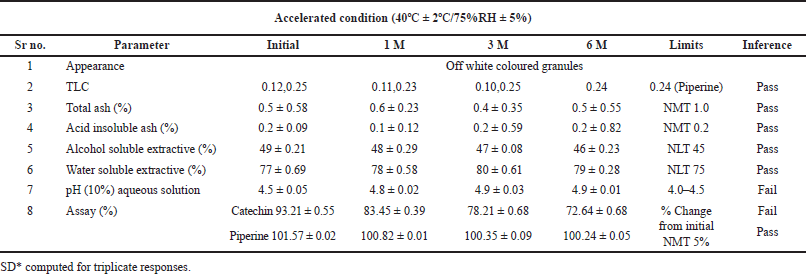 | Table 6. Stability data of Khushmanda Rasayana at accelerated condition. [Click here to view] |
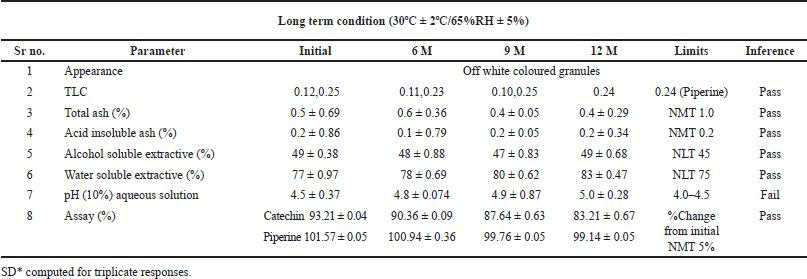 | Table 7. Stability data of Khushmanda Rasayana at long term condition. [Click here to view] |
CONCLUSION
Ayurvedic formulations must be stable under certain conditions to be of high quality. Estimating shelf life becomes crucial to quality as a result. It is extremely difficult to set quality benchmarks and limit characteristic ranges due to the complexity of these phytoconstituents. The AYUSH ministry issued a regulation on August 12, 2016, that requires AYUSH items to include a shelf-life statement based on scientific evidence. Real-time stability assessments of drugs should be used to identify a drug’s expiration date, according to the Ayurvedic Pharmacopoeia of India. Small-scale industries prescribe the expiry date in the formulations as per the Ayurvedic Pharmacopoeia of India. The shelf life for Khushmanda Rasayana based on Catechin and Piperine was found to be 48.1 months. The mentioned shelf life of Khushmanda Rasayana is 3 years (36 months) as per Ayurvedic Pharmacopoeia of India. Small-scale manufacturers state their compliance to GMPs as per schedule T requirements of the Drugs and Cosmetics Act, for Ayurvedic, Siddha Unani drugs. But specify the shelf life in formulations by the guidelines set forth by the Indian Ayurvedic Pharmacopoeia. Without any supporting scientific evidence, the formulation’s stated shelf life is presented. Only the historical data serves the purpose of the evidence of the safety and efficacy of these formulations. The shelf life can be extended from 36 to 48 months with the relevant scientific evidence on chemical stability. Based on scientific data, the shelf life of certain Ayurvedic preparations requires immediate attention to be mentioned. Establishing acceptable standards for shelf life assessment becomes crucial when taking into account the various classes of phytoconstituents included in Ayurvedic formulations. Although Catechin and Piperine were chemical markers in our study, other active markers may be used in more research. Thus, a fingerprinting profile to assess the quality and stability inclusive of active markers, chemical markers, and general markers will prove substantial in estimating shelf life. To assess the quality and stability of Khushmanda Rasayana, various manufacturing procedures, container closure systems, and other stability-indicating characteristics must be investigated. The formulation’s established expiration date was restricted to the randomly chosen batches for research. The shelf life of Khushmanda Rasayana was estimated in the study and was computed based on a 12-month study that can be extrapolated further for 3 years. A real-time commercial study taking into account the complete shelf life should be conducted to assess the shelf life of Khushmanda Rasayana considering polyherbal constituents. The study would be a crucial quality control tool for monitoring the quality of the formulation throughout the shelf life.
ACKNOWLEDGMENT
The authors are grateful to the Department of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, for providing the necessary facility to conduct the research work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research received funding from the TMA Pai Fellowship, MAHE, in the Fiscal Year 2019–2022.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Day 2019. The emergence of ayurveda health Hindustan times [Internet]. [cited 2019 Apr 25]. Available from: https://www.hindustantimes.com/health/world-health-day-2019-the-emergence-of-ayurveda/story-LyI564Lb97puXEfiaUZPTJ.html
2. Rastogi S. Translational ayurveda. Singapore: Springer; 2018. CrossRef
3. Gafner S, Bergeron C. The challenges of chemical stability testing of herbal extracts in finished products using state-of-the-art analytical methodologies. Curr Pharm Anal. 2005 Jun 1;1(2):203–15. CrossRef
4. The Ayurvedic Pharmacopoeia of India. Part I. New Delhi, India: Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha, and Homoeopathy; 2007.
5. Gupta V, Jain A, Shankar MB, Sharma RK. Shelf life of ayurvedic dosage forms in regulatory perspectives. Int J Adv Ayurveda Yoga Unani Siddha Homeopath. 2017;6(1):360–9. CrossRef
6. Comment DFOR. Stability testing of active. 2017;2017(January):1–53. Available from: http://www.who.int/medicines/areas/quality_safety/quality_assurance/regulatory_standards/en/
7. ICH. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use ICH harmonised tripartite guideline stability testing of new drug substances and products Q1A (R2). ICH [Internet]; 2003. Available from: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf
8. The Ayurvedic Pharmacopoeia of India. Part II. Formulations. New Delhi, India: Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha, and Homoeopathy; 2008.
9. The Ayurvedic formulary of India. Part I. Delhi, India: Government of India, Ministry of Health and Family Planning, Department of Health; 2008
10. Shadad M, Alhakamy NA, Aldawsari HM, Husain M, Kotta S, Abdullah ST. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in a 549 lung cancer cells. Pharmaceuticals. 2020 Jul 15;13(7):152. CrossRef
11. Doshi G, Nalawade V, Mukadam A, Chaskar P, Zine S, Somani R. Elucidation of flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida plant extracts by hyphenated technique of liquid chromatography-mass spectroscopy. Pharmacogn Res. 2016;8(4):281. CrossRef
12. Doshi G, Nalawade V, Mukadam A, Chaskar P, Zine S, Somani R. Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: mass spectroscopy. Pharmacogn Res. 2015;7(3):282. CrossRef
13. Doshi GM, Chaskar PK, Une HD. Revelation of β-sitosterol from Benincasa hispida seeds, Carissa congesta roots and Polyalthia longifolia leaves by high performance liquid chromatography. Pharmacogn J. 2016 Oct 1;8(6):610–3. CrossRef
14. Doshi G, Une H. Quantification of quercetin and rutin from Benincasa hispida seeds and Carissa congesta roots by high-performance thin layer chromatography and high-performance liquid chromatography. Pharmacogn Res. 2016;8(1):37. CrossRef
15. Garg S, Mishra A, Gupta R. Fingerprint profile of selected ayurvedic churnas/preparations: an overview. Altern Integr Med. 2013;02(06):1. CrossRef
16. Gaurav MD, Vivek VN, Aaditi SM, Pratip KC, Sandeep PZ, Rakesh RS, et al. Elucidation of flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida plant extracts by hyphenated technique of liquid chromatography?mass spectroscopy. Phcog Res. 2016;8:281–6. CrossRef
17. Gaurav MD, Pratip KC, Une HD. Revelation of β-sitosterol from Benincasa hispida seeds, Carissa congesta roots and Polyalthia longifolia leaves by high performance liquid chromatography. Pharmacogn J. 2016;8(6):610–3. CrossRef
18. ICH. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use ICH harmonised tripartite guideline validation of analytical procedures: text and methodology Q2 (R1). ICH [Internet]. Available from: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf