INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common and disabling nerve compression syndrome [1,2]. CTS treatment options include both traditional and surgical intervention. While surgical intervention significantly alleviates symptoms in the majority of cases, approximately 20% of patients may experience persistent postoperative symptoms because nerve damage before surgery may not recover completely [3,4]. Thus, the treatment of CTS prompts a multimodal approach, and conventional treatment is just as important as surgical treatment [5]. However, as the prevalence of CTS rises, so does the demand for additional conventional treatments [6,7]. Over the past few decades, it has been shown that the accumulation of free radicals caused by mechanical compression can perpetuate nerve damage and prevent remission [8,9]. Antioxidant agents have been proposed as one of the promising conventional treatments for effectively treating CTS patients. Alpha-lipoic acid (ALA) has been shown to reduce lipid peroxidation and prevent nerve ischemia by improving the nerve blood flow as an antioxidant agent [10]. Currently, it is widely used for the treatment of diabetic neuropathy and has proven to be effective [11]. Several studies have also found that ALA plays an important role in reducing inflammation and promoting neuroprotection and neuroregeneration in the case of compressive neuropathy [12]. Based on these findings, it is possible that ALA could be used as an alternative conservative treatment in CTS patients. However, there is still limited evidence, and no clear consensus on the role of ALA in CTS treatment has been reached. As a result, we conducted a systematic review of existing studies and a meta-analysis to investigate and quantify the efficacy of ALA in CTS patients.
MATERIALS AND METHODS
Study registration and methodology
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria were used to report this study [13]. The protocol for this study has been registered in the International prospective register of systematic reviews (CRD42021289390).
Search strategy and study selection
A literature search was carried out with multiple electronic databases, such as PubMed, ScienceDirect, EBSCO, ProQuest, and Google Scholar from inception until March 28, 2023. The search was performed by three independent reviewers using keywords [(“alpha-lipoic acid” or “ALA”) AND (“Carpal tunnel syndrome” or “CTS”)].
The keywords described above were used to find articles. After using the EndNote program to remove duplicates, retrieved articles were screened based on their titles and abstracts. Following that, potential eligible full-text articles were thoroughly screened using the inclusion and exclusion criteria outlined below. Any emerging discrepancies would be resolved by consensus among all authors. The planned procedure is illustrated in Table 1.
Inclusion and exclusion criteria
The inclusion criteria were the following: (a) randomized controlled trial studies (RCTs); (b) patients diagnosed with CTS both clinically and electrodiagnostically are included in this study. There are no age, race, occupation, economic or social status restrictions, religion, country, or underlying conditions restrictions. (c) studies evaluating the effect of ALA or ALA-containing nutraceutical products for the treatment of CTS, (d) patients treated with placebo and/or any supplemental or noninvasive therapy as a comparison, and (e) studies evaluating primary outcomes of either/both the clinical and electrophysiological impact of ALA in CTS patients. Clinical outcome was evaluated by patient-reported symptoms and function on the Boston Carpal Tunnel Questionnaire (BCTQ), and the electrophysiological outcome was evaluated by median motor nerve distal latency (MDL) and sensory nerve conduction velocity (SNCV). The secondary outcomes were pain reduction measured with the visual analog score (VAS). The exclusion criteria were the following: (a) other nonRCT studies including nonrandomized clinical trials, cross-sectional, cohort, case control, case reports or series, review studies (narrative or literature reviews), book sections, conference papers, letters to the editor, and correspondences; (b) papers with unavailable full text; and (c) study not available in English.
Data extraction
The following data were extracted from the studies selected for inclusion: (1) first author; (2) publication year; (3) study origin; (4) study design; (5) sample size; (6) age; (7) gender; (8) intervention; (9) other treatment; (10) follow up; and (11) trial registry. All information was extracted by two independent authors, and conflicting data were resolved with consensus among all the authors. For the meta-analysis, we extracted several clinical and electrophysiological outcomes, including (a) BCTQ score, (b) Visual Analog Scale (VAS) score, (c) median MDL, and (d) SNCV.
One of the most common missing outcome data in many studies has been the SD of the mean change pre/postintervention or delta (^). To obtain this data, several steps can be taken, including calculating an estimated number with review manager (RevMan), contacting the corresponding authors of the included studies to request their datasets or the mean and SDchanges, or performing a manual calculation. Due to a lack of data to calculate the estimation number and an inability to obtain the data from the corresponding authors, the current study used the calculation method used in many other meta-analyses. The formula was written as follows [14,15]:
SDchange means the SD of the mean changes from baseline. SDbaseline represents the SD from the pretest or preintervention, SDfinal corresponds to the SD of the post-test or postintervention, and r symbolizes the correlations between the baseline and final measurements which are usually not presented in the studies. A previous meta-analysis reported assigning the value of 0.7 to the r in the formula to provide a conservative estimate [16]. Further missing outcome data resulted in the study’s exclusion.
Quality assessment
For this systematic review and meta-analysis, we used the recommended Cochrane Risk of Bias (RoB2) tool to assess the quality of the included RCTs [17]. All three researchers independently evaluate the quality of each study with any discrepancies resolved through consensus.
Meta-analyses
Standardized mean differences (SMD) or mean differences (MD) of the delta (^) value of dependent variables (BCTQ, VAS, MDL, and SNCV) with a confidence interval (CI) of 95% were used to determine the efficacy of ALA for the treatment of CTS. SMD was used when the outcomes were measured in different methods or units across trials, whereas MD was used when all studies reported the outcomes using the same method and scale. Either a fixed-or random-effects model would be used depending on the study heterogeneity. We used Cochrane’s Q test of homogeneity and Higgins I² statistics to assess the heterogeneity of included studies. If the data are sufficient, subgroup analysis will be conducted to find the possible cause of heterogeneity. A funnel plot was used to assess publication bias visually when there were at least 10 studies as recommended by the Cochrane Handbook. An asymmetric funnel plot suggested that publication bias was possible. In cases where the number of studies collected was less than 10, the Begg and Mazumdar’s rank correlation test and Egger’s regression test, which used the SE of the observed outcomes as a predictor, were used to statistically check for funnel plot asymmetry and the presence of publication bias. To further assess the possibility of publication bias, a fail-safe N analysis would be performed. Detected publication bias would be corrected using Duval and Tweedie’s Trim and Fill Method. Furthermore, sensitivity analysis was performed to confirm the robustness of this meta-analysis. All statistical tests were done using RevMan 5.3 and comprehensive meta-analysis 3.0.
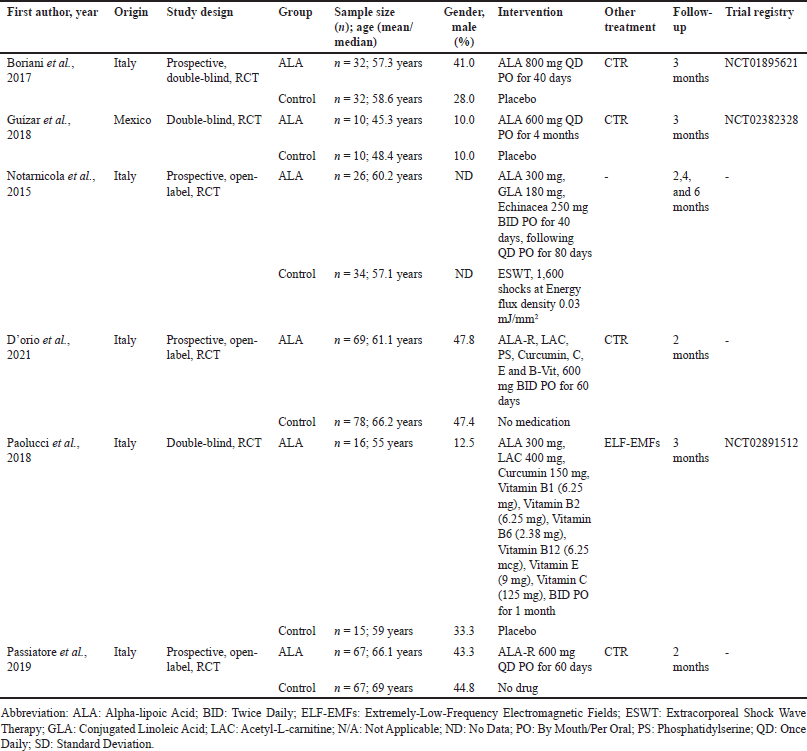 | Table 1. Characteristics of studies. [Click here to view] |
Level of evidence of the meta-analyses
Each meta-analysis result was graded by applying the grading of recommendations, assessment, development, and evaluations (GRADE) method as described in the GRADE handbook and recommended by the Cochrane Handbook for Systematic Reviews of Interventions and Cochrane Collaboration’s tool for assessing the RoB2in randomized trials. GRADE was used to determine the confidence in cumulative evidence. Judgment was made using several indicators including the presence of study limitations, consistency, directness, imprecision, and/or reporting bias. Overall certainty of the evidence was shown as high, moderate, low, or very low quality.
RESULTS
Search result
An initial search from the electronic database yielded 10,186 studies, of which 1,144 were duplicates and therefore excluded. After screening the remaining 9,031 studies by title and abstract, and 11 studies were further assessed for eligibility. Finally, six studies were included in our systematic review and five studies were included in the final meta-analysis. The search term strategy and selection methods of this study following PRISMA guidelines are illustrated in Figure 1.
Study characteristics
All the included studies were conducted in Italy, except for one study [18] which was conducted in Mexico. Most of the study subjects were female adults, aged 48–69 years. ALA 600 to 1,200 mg daily, and treatment duration was between 40 days and 4 months after decompression surgery. The control group, however, was given either a placebo or nothing, with one study [19] comparing the efficacy of ALA and shock wave therapy. Follow-up time across studies varied from2 to 6 months. In addition, only three out of six RCTs had their trial protocol registered before the study was initiated. The characteristics of individual studies are summarized in Table 1.
Only two [20,21] out of six RCTs had an overall low RoB2, with two studies concerning risk and the other two with a high RoB2. Two studies [10,18] showed some concerns of bias due to the randomization process and/or selection of the reported result as a result of not describing how the subjects were randomized or not having their trial protocol registered beforehand to compare the planned procedure with the published data. A high RoB2 [19,22] mainly arose during data collection, as the scoring system was subject to subjectivity even though the study used the same measurement tool. The quality assessment of individual studies is presented in Figure 2.
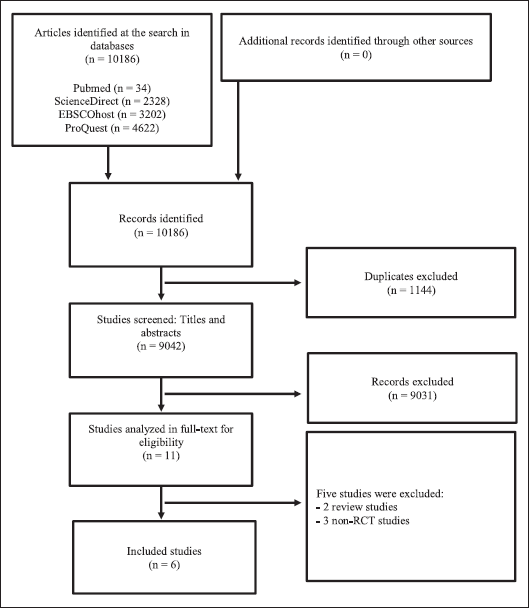 | Figure 1. PRISMA flow diagram of study selection. [Click here to view] |
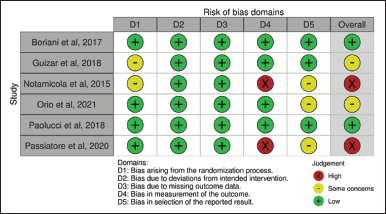 | Figure 2. Methodological quality of the RCTs included in the present study. [Click here to view] |
Meta-analyses
Figure 3a shows a forest plot of three studies and the pooled result in the meta-analysis. We found that there was a significant improvement in BCTQ score in the ALA group compared to the control/placebo group (SMD = –0.44; 95% CI: –0.80 to –0.09; p = 0.01). To address the substantial group heterogeneity found in this finding, subgroup analysis was done and demonstrated low group heterogeneity found in the RCT studies (I² = 31%) [23].
Figure 3b shows a forest plot of a study with two separate data. This study also evaluated post-treatment pain reduction in participants using the VAS score. The pooled analysis demonstrated that the reduction of VAS score was significantly higher in the ALA group compared to the control group (MD = –3.40; 95% CI: –3.64 to –3.16; p < 0.00001). The group heterogeneity in the RCT studies was found to be low (I² = 0%) [23].
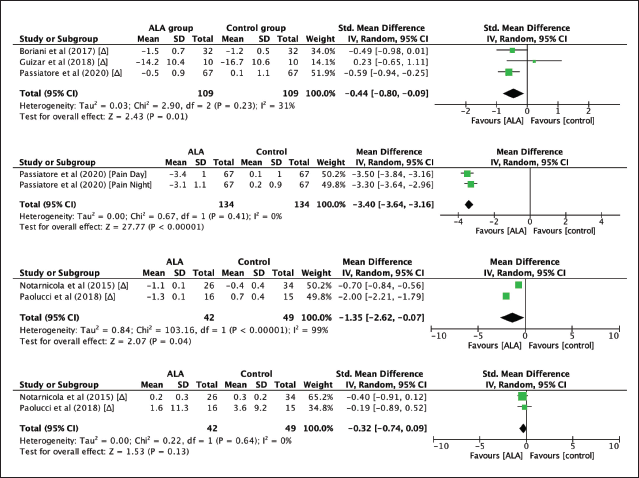 | Figure 3. (a) Meta-analysis of the reduction in BCTQ scores in post-CTR patients who received ALA 600–800 mg once daily for 2 to 3 months compared to placebo. The vertical line indicates no significant difference between the groups compared. Diamond shapes and horizontal lines represent odds ratios and 95% CIs. Squares indicate point estimates, and the size of each square indicates the weight of each study included in this meta-analysis. (b) Meta-analysis of the reduction in VAS scores in post-CTR patients who received ALA-R 600 mg once daily for 2 months compared to no drug. The vertical line indicates no significant difference between the groups compared. Diamond shapes and horizontal lines represent odds ratios and 95% CIs. Squares indicate point estimates, and the size of each square indicates the weight of each study included in this meta-analysis. (c) Meta-analysis of MDL in nerve conduction studies among patients with CTS who did not have surgery but were given ALA 300 mg once daily for 1 to 6 months compared to ESWT or placebo. The vertical line indicates no significant difference between the groups compared. Diamond shapes and horizontal lines represent odds ratios and 95% CIs. Squares indicate point estimates, and the size of each square indicates the weight of each study included in this meta-analysis. (d) Meta-analysis of SNCV in nerve conduction studies among patients with CTS who did not have surgery but were given ALA 300 mg once daily for 1 to 6 months compared to ESWT or placebo. The vertical line indicates no significant difference between the groups compared. Diamond shapes and horizontal lines represent odds ratios and 95% CIs. Squares indicate point estimates, and the size of each square indicates the weight of each study included in this meta-analysis. ALA: alpha-lipoic acid; χ2: chi-square statistic; CI: confidence interval; df: degrees of freedom; I2: I-square heterogeneity statistic; IV: weighted mean difference; p: p-value; RCT: randomized controlled trials; SD: standard deviation; Z: Z statistic. [Click here to view] |
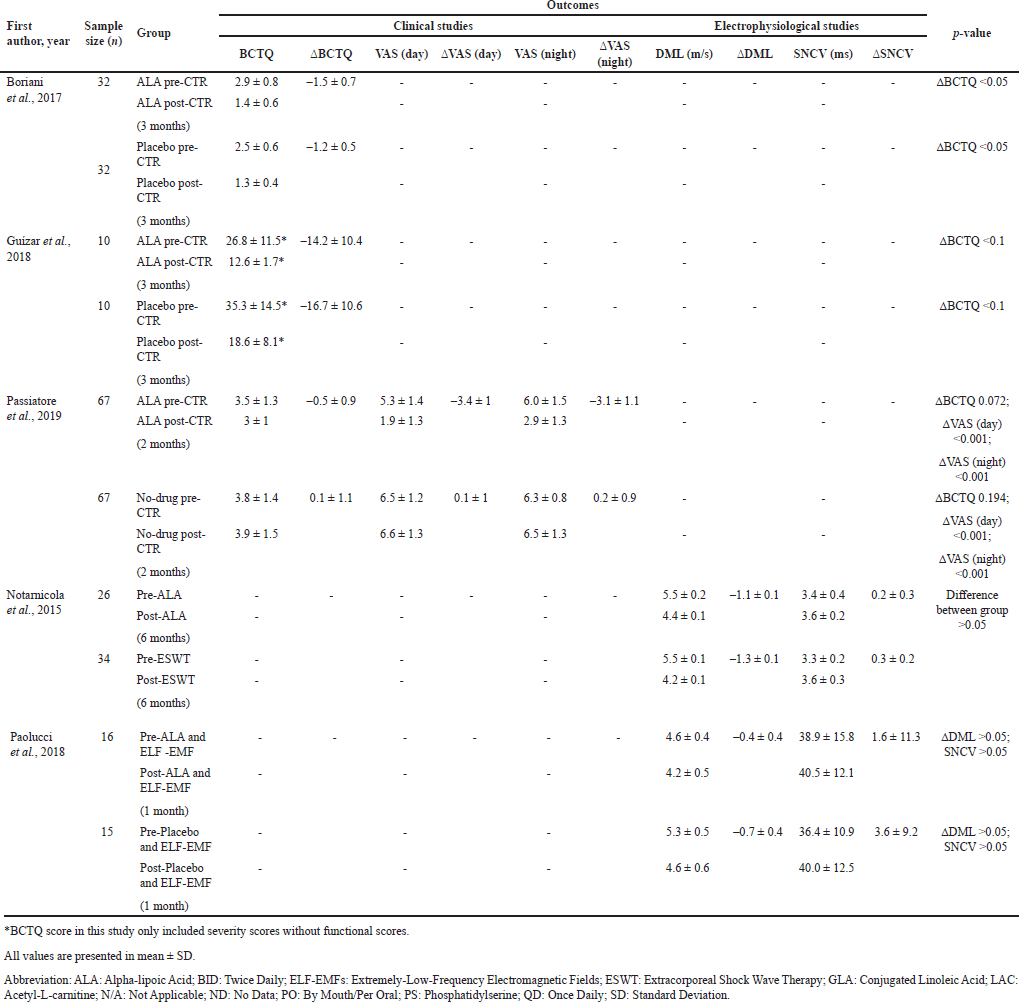 | Table 2. Outcomes of studies included in the meta- analysis. [Click here to view] |
This study also evaluated the effect of ALA on the improvement of electrophysiological parameters such as median MDL and SNCV. Sensory nerve distal latency was not included in the meta-analysis due to a lack of data. Figures 3c and d show the forest plot of electrophysiological outcomes. There was a significant improvement in median MDL (MD = –1.35; 95% CI: –2.62 to –0.07; p = 0.04), but not in median SNCV (p = 0.13) in the ALA group compared to the control group, with substantially high heterogeneity among the MDL group (I = 97%) [23]. Table 2 summarizes the outcomes of each study included in the meta-analysis.
Publication bias
Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 1 and p = 0.62, respectively). The Rosenthal approach to fail-safe N analysis yielded 6.000 studies (p = 0.002), with a null effect required to reduce the result to nonsignificant. This showed a strong significant result in the present study. Due to no publication bias observed, Duval and Tweedie’s Trim and fill method was not performed.
Level of evidence of the meta-analyses
GRADE assessment results are shown in Table 3. Two outcomes were rated as having moderate-quality evidence, and two outcomes were rated as low-quality evidence. Due to a lack of power, the publication bias for each outcome was undetected, even though rank correlation, regression test, and fail-safe N analysis all revealed no publication bias.
DISCUSSION
In this meta-analysis, we investigated the efficacy of ALA in CTS patients. We evaluated the clinical and electrophysiological function of the nerve in CTS patients after receiving ALA compared to placebo or other noninvasive interventions such as extracorporeal shock-wave therapy (ESWT). Clinical assessment including the BCTQ and VAS was assessed in ALA given after carpal tunnel release (CTR), whereas the electrophysiological outcomes of median MDL and SNCV were assessed in ALA given without any surgery or CTR.
BCTQ showed a significant reduction in the ALA group (SMD = –0.44; 95% CI: –0.80 to –0.09; p = 0.01), with a homogenous population in general (I² = 31%). The BCTQ is a 19-item self-reported questionnaire that examines symptom severity and overall functional status of patients with CTS, and each item ranges from 1 to 5 with 1 as no difficulty and 5 as difficult [24]. It should be noted that all three RCTs included in this analysis used ALA as supportive treatment postsurgery, and surgical release of CTS alone was an effective intervention to relieve patients’ symptoms and function as shown in significant improvements in all items of both elements of BCTQ (SSS and FSS) in 3 months [25]. To support our hypothesis, we explored the studies used in this analysis. A study by Boriani et al. [20] showed that the pre-post treatment changes in the ALA group and placebo group were statistically significant [ALA mean –1.5 ± 0.7 (–2.5 to 0.1) vs. Placebo mean –1.3 ± 0.5 (–2.3 to 0.4); p <0.05]. Another study by Guízar et al. [18] also showed that ALA supplementation for 1 month before surgical decompression may significantly lower the BCTQ score taken before surgery (ALA –10.3 vs. Placebo –2.9; p <0.01) with both groups having similar baseline BCTQ score (ALA 37.1 ± 9.7 vs. Placebo 38.1 ± 10.9). These data supported the fact that changes in BCTQ score were affected by the ALA supplementation, not merely due to the surgical release of CTS alone. The reduction in this score after ALA supplementation is due to its positive effects such as antioxidant properties, vasorelaxation, positive metabolic profile, as well as anti-inflammatory potential. Senoglu et al. [26] demonstrated the actions of ALA in an animal model using compressive sciatic nerve injury in rats, they found that ALA reduced oxidative stress by increasing catalase and superoxide dismutase activity and reduced the concentration of malondialdehyde. A similar mechanism may explain the therapeutic effect of ALA in CTS as the ischemia/reperfusion process triggers the disease [26].
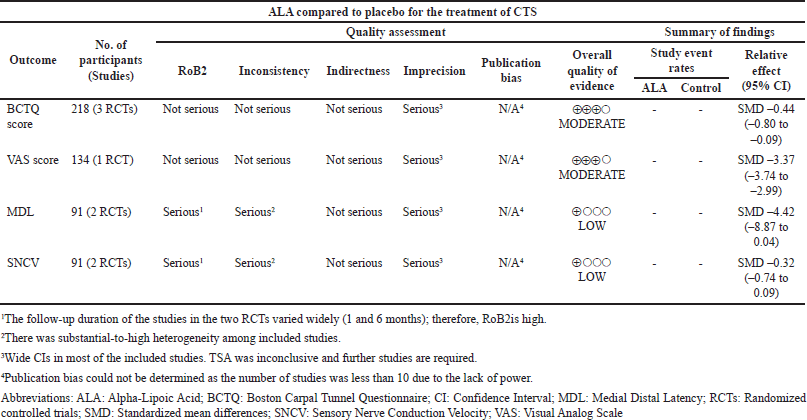 | Table 3. GRADE evidence profile. [Click here to view] |
VAS score that was pooled from a single study with two sets of data also showed a significant reduction in the ALA group (MD = –3.40; 95% CI: –3.64 to –3.16; p = <0.00001) with a homogenous population in general (I² = 0%). The VAS score is a validated, subjective, and widely used scoring system to assess acute and chronic pain. Scores are obtained using a handwritten mark on a 10 cm line representing the continuum between “no pain” and “severe pain”[27]. The study by Passiatore et al. [22] showed a reduction of night pain/night VAS score in the ALA group after 2 months of supplementation but an increase in pain score in the placebo group (ALA –3.1 vs. Placebo +0.2; p < 0.0001), with the similar result observed in day pain/day VAS score (ALA –3.4 vs. Placebo +0.1; p < 0.0001). ALA has been shown in several studies to have a powerful neuroprotective effect, significantly reducing thermal hyperalgesia, cold allodynia, lipid peroxidation, nitric oxide levels, glutathione levels, and axonal degeneration. It also had prolonged effects that were sustained even during the treatment-free period. Several chronic pain studies also showed that ALA was beneficial in diabetic neuropathy, post-trauma peripheral nerve pain, and sciatic pain [28–30].
There was a significant improvement in median MDL (MD = –1.35; 95% CI: –2.62 to –0.07; p = 0.04) but no significant improvement in median SNCV (p = 0.13). Although MDL improved in this meta-analysis, careful interpretation is required because the follow-up period of the two included studies ranged from 1 to 6 months, with high heterogeneity (I2 = 99%). Guízar et al. [18] conducted one study that assessed electrophysiological assessment but was excluded from this analysis because it was conducted after surgery, whereas the two studies included in this analysis were not. Guízar et al. [18] also showed ALA supplementations after surgery improved motor (Motor mean –1.3; p < 0.01) and sensory (Sensory mean –1.12; p < 0.01) parameters but not in the placebo group. This could be explained by ALA’s multimodal mechanism as a potent antioxidant, acting as a free radical scavenger as well as a regenerator of vitamins C and E, both beneficial in reducing oxidative stress in peripheral nerves [18].
This study has several limitations: (1) The majority of the data came from a single country and (2) the ALA dosages varied between trials. Only one of the five RCTs included in the meta-analysis used an 800 mg dose, while the other four used a 600 mg dose. No study examined or compared the effects of various doses. A subgroup analysis of the 600 mg dosage study showed a decrease in BCTQ score; however, the difference was no longer significant (MD = –2.90; 95% CI: –1.07 to 0.49; p = 0.47). Because the difference in dose is small and yet within the recommended daily dose range of 600 to 1,800 mg, [31] the authors concluded that it had no serious effect on the outcome of our analyses. (3) The active forms of ALA differ between trials. Only one of the five RCTs included in the meta-analysis employed ALA-R, an R-form enantiomer of ALA known to be more effective than the synthetic form of ALA. A subgroup analysis of ALA studies without ALA-R still resulted in a slight decrease in BCTQ score, although the result became nonsignificant (MD = –0.23; 95% CI: –0.90 to 0.45; p = 0.51). (3) The studies’ follow-up periods ranged from 1 to 6 months, but the analyses were done by limiting the data to a narrow range. Future research can be carried out in a large and homogenous population, comparing low-dose versus high-dose but still within the recommended daily dose, or even comparing the ALA with the ALA-R form, which is known to be more effective.
CONCLUSION
Postoperative ALA supplementation with a dose of 600–800 mg once daily for 2–3 months is potentially beneficial in improving the clinical function of CTS as shown by a reduction in BCTQ and VAS scores. Although ALA significantly improved MDL, this outcome was graded as low evidence. SNCV remained unchanged. Further studies are required to further elucidate this conclusion.
LIST OF ABBREVIATIONS
ALA, Alpha-lipoic acid; BCTQ, Boston carpal tunnel questionnaire; CI, Confidence interval; df, degree of freedom; I2, I-square heterogeneity statistic; IV, weighted mean difference; MD, Mean differences or unstandardized mean differences; MDL, Medial distal latency; RCTs, Randomized controlled trials; SMD, Standardized mean differences; SNCV, Sensory nerve conduction velocity; VAS, Visual analog scale; Z, Z statistic.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gelfman R, Melton LJ 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33–41. CrossRef
2. Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg Am. 2001;26:460–6. CrossRef
3. Tahririan MA, Moghtaderi A, Aran F. Changes in electrophysiological parameters after open carpal tunnel release. Adv Biomed Res. 2012;1:46. CrossRef
4. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;2008:CD001552. CrossRef
5. Huisstede BM, Randsdorp MS, Coert JH, Glerum S, vanMiddelkoop M, Koes BW. Carpal tunnel syndrome. part II: effectiveness of surgical treatments-a systematic review. Arch Phys M. 2010;91:1005–24. CrossRef
6. Atroshi I, Englund M, Turkiewicz A, Tagil M, Petersson IF. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch Intern Med. 2011;171:943–4. CrossRef
7. Pourmemari MH, Heliovaara M, Viikari-Juntura E, Shiri R. Carpal tunnel release: lifetime prevalence, annual incidence, and risk factors. Muscle Nerve. 2018;58:497–502. CrossRef
8. Bellomo G, Mirabelli F, Richelmi P. Glutathione-mediated mechanism of defense against oxygen free radical-induced hepatotoxicity. Hum Toxicol. 1989;8:152. CrossRef
9. Bellomo G, Mirabelli F, Salis A, Vairetti M, Richelmi P, Finardi G, et al. Oxidative stress induced plasma membrane blebbing and cytoskeletal alterations in normal and cancer cells. Ann NY Acad Sci. 1988;551:128–30. CrossRef
10. D’Orio M, Vitis RD, Taccardo G, Rocchi L, Ferrari F, Perna A, et al. Clinical usefulness of nutraceutics with acetyl-L-carnitine, a-lipoic acid, phosphatidylserine, curcumin, C, E and B-group vitamins in patients awaiting for carpal tunnel release during COVID-19 pandemic: a randomized controlled open label prospective study. Acta Biomed. 2021;92:e2021539. CrossRef
11. Ziegler D, Papanas N, Schnell O, Nguyen BDT, Nguyen KT, Kulkantrakorn K, et al. Current concepts in the management of diabetic polyneuropathy. J Diabetes Investig. 2021;12:464–75. CrossRef
12. Abushukur Y, Knackstedt R. The impact of supplements on recovery after peripheral nerve injury: a review of the literature. Cureus. 2022;14:e25135. CrossRef
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. CrossRef
14. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Effect sizes based on means. In: Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons; 2009. Available from: https://www.meta-analysis.com/downloads/Meta-analysis%20Effect%20sizes%20based%20on%20means.pdf
15. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (Updated March 2011). UK: The Cochrane Collaboration; 2011 [Online]. [cited 2023 Feb 01]. Available from: https://handbook-5-1.cochrane.org
16. Yagiz G, Esedullah A, Kubis HP, Owen JA. The effectsof resistace training on architecture and volume of the upper extremity muscles: a systematic review of randomised controlled trials and meta-analyses. Appl Sci. 2022;12:1593. CrossRef
17. Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). UK: Cochrane; 2022 [Online]. [cited 2023 Feb 01]. Available from: www.training.cochrane.org/handbook
18. Guízar EAM, Benavides LG, Plascencia ARA, González SP, Sutto SET, Muñoz EGC, et al. Effect of alpha-lipoic acid on clinical and neurophysiologic recovery of carpal tunnel syndrome: a double-blind, randomized clinical trial. J Med Food. 2018;21:521–6. CrossRef
19. Notarnicola A, Moretti B. The biological effects of extracorporeal shock wave therapy (ESWT) on tendon tissue. Muscles Ligaments Tendons J. 2012;2:33–7. CrossRef
20. Boriani F, Granchi D, Roatti G, Merlini L, Sabattini T, Baldini N. Alpha-lipoic acid after median nerve decompression at the carpal tunnel: a randomized controlled trial. J Hand Surg Am. 2017;42:236–42. CrossRef
21. Paolucci T, Piccinini G, Nusca SM, Marsilli G, Mannocci A, La Torre G, et al. Efficacy of dietary supplement with nutraceutical composed combined with extremely-low-frequency electromagnetic fields in carpal tunnel syndrome. J Phys Ther Sci. 2018;30:777–84. CrossRef
22. Passiatore M, Perna A, De-Vitis R, Taccardo G. The use of alfa-lipoic acid-R (ALA-R) in patients with mild-moderate carpal tunnel syndrome: a randomised controlled open label prospective study. Malays Orthop J. 2020;14:1–6. CrossRef
23. Schunemann H. GRADE handbook. Canada: GRADE Working Group; 2013 [Online]. [cited 2023 Feb 01]. Available from: https://www.rama.mahidol.ac.th/ceb/sites/default/files/public/pdf/journal_club/2017/GRADE handbook.pdf
24. Mintken P. Boston Carpal Tunnel Questionnaire. USA: American Physical Therapy Association; 2017 [Online]. [cited 2023 Feb 01]. Available from: https://www.apta.org/patient-care/evidence-based-practice-resources/test-measures/boston-carpal-tunnel-questionnaire-bctq
25. Mustafa A, Almigdad A. Post-operative outcome of surgical decompression for carpal tunnel syndrome. RMS. 2021;28:60–70.
26. Senoglu M, Nacitarhan V, Kurutas EB, Senoglu N, Altun I, Atli Y, et al. Intraperitoneal alpha-lipoic acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009;4:22. CrossRef
27. Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2:e088. CrossRef
28. Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic acid and diabetic neuropathy. Rev Diabet Stud. 2009;6:230–6. CrossRef
29. Neerati P, Prathapagiri H. Alpha lipoic acid attenuated neuropathic pain induced by chronic constriction injury of the sciatic nerve in rats. Clin Phytosci. 2021;7:21. CrossRef
30. Wang J, Lou Z, Xi H, Li Z, Li L, Li Z, et al. Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model. Open Life Sci. 2021;16:222–8. CrossRef
31. Derosa G, D’Angelo A, Preti P, Maffioli P. Safety and efficacy of alpha lipoic acid during 4 years of observation: a retrospective, clinical trial in healthy subjects in primary prevention. Drug Des Devel Ther. 2020;14:5367–74. CrossRef