INTRODUCTION
Oral diseases, such as dental caries and pharyngitis, are major health concerns caused by various factors, including bacterial overgrowth. The oral microbiome is a collection of microorganisms that reside in the mouth, with the genus Streptococcus being the most prevalent [1]. Certain Streptococcus species, such as Streptococcus mutans and Streptococcus sobrinus, are prevalent in the progression of dental caries [2,3]. Additionally, orthodontic treatment has been linked to an increasing S. mutans and Candida albicans population in the oral cavity [4]. Streptococcus pyogenes can cause pharyngitis and tonsillitis, as well as more serious, potentially fatal diseases [5–7]. Various antibacterial products such as chlorhexidine, fluorides, and phenol derivatives have been widely used in dentistry to control bacterial growth in the mouth. However, excessive use of these products can lead to microbial resistance, tooth discoloration, erosion, and abrasion [8].
Extensive utilization of Boesenbergia rotunda (L.) Mansf. (commonly known as krachai or fingerroot), classified under the family Zingiberaceae, is observed as traditional medicine in Southeast Asia for the treatment of cough, stomach ache, and postnatal care in women. It displays antimicrobial, anti-allergic, antioxidant, and anti-tumor activities [9,10]. Boesenbergia rotunda demonstrates remarkable antimicrobial activity against various pathogenic bacteria, including Helicobacter pylori, Escherichia coli, S. mutans, S. sobrinus, S. pyogenes, Staphylococcus aureus, Staphylococcus epidermidis, and Bacillus subtilis [11–13]. Essential oils in B. rotunda (BREO) contain geranyl formate, geranyl propionate, geraniol, neral, myrcene, isoborneol, and beta-pinene [14]. There have been no reports of BREO with active components identified against oral pathogenic bacteria [15]. In this study, we isolated BREO components and assessed their antibacterial activities. These findings can help in the development of an effective natural product to control oral pathogenic bacteria.
MATERIALS AND METHODS
Preparation of essential oil
Boesenbergia rotunda was sourced locally from the Muang district, Chiang Mai province. The authentication of plant rhizomes was conducted at the Faculty of Science, Maejo University, Chiang Mai, Thailand, with the assignment of a specimen voucher number MJU 2102-005. Cleaned rhizomes were chopped using a kitchen blender. The chopped rhizomes were then placed into a distillation flask along with distilled water and then heated. The steam with essential oil is cooled and condensed to produce a liquid mixture of water for 6 hours and essential oil was separated by using a Clevenger-type apparatus [16]. The obtained essential oils were dried over anhydrous sodium sulfate and subsequently stored at −20°C before analysis.
Bacterial strains and culture conditions
All tests were performed using S. pyogenes Department of Medical Science, Ministry of Public Health, Thailand (DMST) 30563, S. mutans DMST 18777, and S. sobrinus DMST 35719. The test strains were obtained from the DMST, cultured on brain heart infusion agar (BHA), and incubated at 37°C for 24 hours in an anaerobic jar, using a Gaspak system (Becton, Dickinson and Company) which limited oxygen to ≤1% and ensured a carbon dioxide level of ≥13%.
Disc diffusion assay
The disc diffusion method was performed [17] using Mueller–Hinton agar (MHA), and the bacteria were standardized to the 0.5 McFarland standard. The paper disks containing 10 μl BREO at a concentration of 0.863 g/ml were placed on the cultured plate. The diameter of the inhibition zone was determined following incubation at 37°C for a period of 24 hours. Commercially available antibiotic discs of tetracycline and erythromycin (30 μg/disc; Oxoid, UK) were designated as controls.
Broth microdilution method
The broth dilution method was performed as previously described [17]. Briefly, the minimum inhibitory concentration (MIC) of the BREO was examined using a 96-well microtiter plate assay. Broth microdilution was performed using Mueller–Hinton broth (MHB). BREO was prepared at concentrations ranging from 0.06 to 512 mg/ml in 10% dimethyl sulfoxide (DMSO). A solution containing 10% DMSO without BREO was used as the negative control. The last well of each row contained MHB without BREO as a positive control. The tested bacterial strains were adjusted to a 0.5 McFarland standard turbidity and diluted 1:100 in beta-hydroxybutyrate. A 50 μl bacterial suspension was added to each well of the microtiter plate. The plates were gently tapped at the corners to ensure thorough mixing of the solutions and then incubated at 37°C for 24 hours in an anaerobic jar. The lowest BREO concentration that showed no visible growth of the tested organisms was determined as the MIC. The MIC assay wells showing no observable proliferation were subjected to culturing on MHA, followed by a 24-hour incubation period at 37°C. The minimal bactericidal concentration (MBC) was identified as the lowest concentration of BREO that could inhibit microbial proliferation on MHA plates.
Thin layer chromatography-bioautography
The BREO active compounds were separated, and their antibacterial effect was evaluated using TLC plates. Previously described methods [18] were modified and performed in parallel on three TLC plates (I, II, and III) made of Silica Gel 60 F254 (from MERCK, Germany). One microliter of BREO was added to each plate. All chemicals used for TLC were of analytical grade. BREO was separated using a mobile phase of toluene:ethyl acetate (95:5). To eliminate the solvent, the plates were left to dry in a fume hood for 15 minutes.
Plate I was visualized under ultraviolet (UV) light to identify the separated compounds. Plate II was sprayed with a 5% sulfuric acid spray, while Plate III was coated with bacteria-infused molten BHA for bioautography purposes. After a 24-hour humid incubation at 37°C, Plate III was treated with a 1% solution of thiazolyl blue tetrazolium bromide, followed by a 10-minute room temperature incubation. The presence of an antibacterial agent is indicated by clear spots on a purple background.
Plates I and II were compared to plate III to identify spots with the same retention factor (Rf) values as the antibacterial zone on plate III. These spots were scraped off and eluted from silica gel plates with dichloromethane. The eluted compounds were analyzed using gas chromatography–mass spectrometry (GC-MS).
Gas chromatography mass spectrometry
The BREO and active compounds extracted from the TLC plate were analyzed by GC-MS using an Agilent 6890 Plus instrument with an HP-5MSI column (0.25 mm × 30 m × 0.25 μm) and a Hewlett Packard 5973 detector. The procedures were modified from those previously described [19] with a slight alteration in the temperatures. The oven temperature was set to 60°C for the first 3 minutes, increased to 200°C at 3°C/minutes, and further increased to 280°C at 15°C/minutes. The temperature was maintained at 280°C for an additional 5 minutes. Helium was used as the carrier gas at a flow rate of 1 ml/minute, and 1 μl of BREO (diluted 1:100 with dichloromethane) was injected into the system. To extract the active compounds obtained from TLC, a 200 μl volume of dichloromethane was used for elution. The eluate was then filtered using a 0.2 μm membrane and subsequently injected into the GC-MS instrument. For the GC-MS analysis, the transfer line temperature was maintained at 150°C, while the ion source temperature was set to 230°C. To calculate the Kovats retention indices, a homologous series of C9–C20 n-alkanes were injected using identical conditions. Compound identification was verified by comparing the results with the Wiley 7 and Nist 05 libraries, as well as relevant literature data [20]. Geraniol (Sigma-Aldrich, St. Louis, MO) served as the standard compound.
Time-kill kinetics assay
In the time-kill assay, the antibacterial effects of BREO and geraniol were evaluated against S. pyogenes, S. mutans, and S. sobrinus. A suspension of bacteria was prepared in a normal saline solution to match the turbidity of a 0.5 McFarland standard. To the bacterial suspension, either BREO or geraniol was added, with final concentrations ranging from 0.5 to 4 times the MIC. Subsequently, the mixture was incubated at 37°C in a reciprocal shaker operating at 150 rpm. Viable counting was performed in triplicate at 0, 1, 5, 10, 30, and 60 minutes, and the cultures were additionally observed for 24 hours on BHA. The procedure was performed in triplicate, and the log10 colony-forming units (CFU)/ml were plotted against time. The extract-free medium was used as a non-treated control [21]. The percentage kill was calculated as follows:
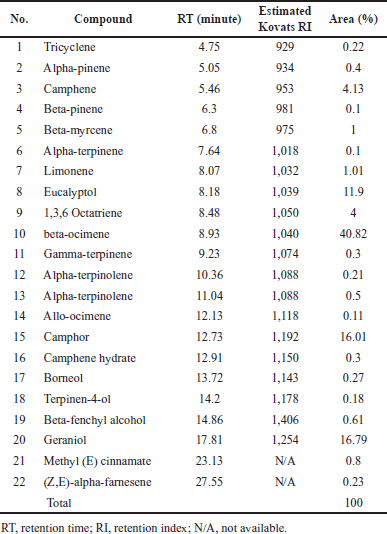 | Table 1. Chemical constituents of the BREO by GC-MS analysis. [Click here to view] |
Statistical analysis
The experiments were performed in triplicate, and the inhibition zones were analyzed using a one-way analysis of variance followed by Duncan’s multiple range test to identify significant differences. International Business Machines Statistical Package for the Social Sciences Statistics 2 software was used with a p-value < 0.05 regarded as significant.
RESULTS AND DISCUSSION
Chemical composition analysis
The yield of BREO from fresh rhizomes was 4.5 ml per 1,000 g at a density of 0.863 g/ml. GC-MS revealed that BREO comprised 22 chemical components (Table 1). The major constituents were beta-ocimene (40.82%), geraniol (16.79%), and camphor (16.01%), which together accounted for more than 70% of the essential oil. These findings provide insights into the specific chemical compounds present in BREO and their potential role in its medicinal properties. A previous study [22] on the BREO also used GC-MS to analyze its chemical composition and identified nerol (39.6%) and L-camphor (36.0%) as the main constituents. While both essential oils contain high levels of camphor, there are notable differences in their chemical compositions, with BREO in this study having higher concentrations of beta-ocimene and geraniol.
Antibacterial activity
This study investigated the antibacterial activity of BREO against three oral pathogenic bacteria, S. pyogenes, S. sobrinus, and S. mutans. The agar disc diffusion assay revealed that the BREO had significant inhibitory effects on the growth of these bacteria (Table 2, Fig. 1). The MIC and MBC values for BREO (Table 3, Fig. 2) were 6.25–12.5 mg/ml, indicating the bactericidal effects of the extract on all tested bacteria. These findings are consistent with those of a previous study [23], demonstrating the antimicrobial properties of B. rotunda extracts against S. pyogenes and other oral pathogens with suggested mechanisms of action associated with the cell wall and cell membrane damage [24]. In addition to its antibacterial effects, B. rotunda extracts have inhibitory effects on biofilm formation by oral bacteria and anti-periodontitis activity. Furthermore, B. rotunda extracts have anti-inflammatory properties, inhibiting the synthesis of pro-inflammatory cytokines and the expression of certain enzymes involved in inflammation [25].
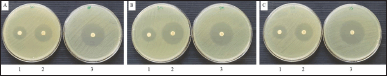 | Figure 1. Inhibition zone of BREO against tested bacteria (1), tetracycline (2) and erythromycin (3) against S. pyogenes (A) S. mutans (B) and S. sobrinus (C) [Click here to view] |
 | Table 2. Inhibition zone of the BREO against tested bacteria (statistical analysis shows differences in the activity for each species subjected to three different treatments). [Click here to view] |
 | Figure 2. MIC microtiter plate of BREO against tested bacteria [S. pyogenes (a), S. mutans (b), S. sobrinus (c)] Row A-C (BREO) Row D-F (geraniol) Well 12A - 12F (positive control) Well 12G (negative control). [Click here to view] |
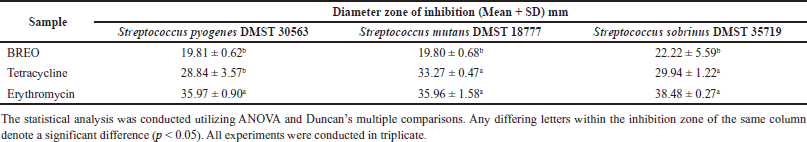
TLC-bioautography and GC-MS
The TLC-bioautography analyses revealed that the crude extract of B. rotunda comprised a single antibacterial component with an Rf value of 0.28 against the tested bacteria (Fig. 4). This component was subsequently identified as geraniol through GC-MS analysis of BREO separation on a TLC plate (Fig. 3A–C). Although geraniol was not the most concentrated component in the BREO, the TLC-bioautography results demonstrated its antibacterial activity against all tested bacterial strains. A previous study [10] showed that B. rotunda possessed promising bioactive compounds exhibiting antioxidant and antibacterial properties. However, the specific active components responsible for these properties have not yet been identified. Our study suggests that geraniol is a key active antibacterial component of BREO against S. pyogenes, S. sobrinus, and S. mutans. These findings further support the promise of BREO as a source of antimicrobial substances and highlight the importance of investigating its potential medicinal uses.
 | Table 3. MICs and MBCs of the BREO against tested bacteria. [Click here to view] |
 | Figure 3. GC-MS analysis of the BREO (A), components with antibacterial zone from TLC (B), and standard geraniol (C). [Click here to view] |
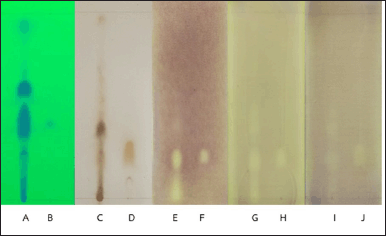 | Figure 4. TLC of BREO (A) and geraniol (B) on Silica Gel 60 F254 under UV 254. TLC of BREO (C) and geraniol (D) sprayed with 5% sulfuric acid. Bioautography of BREO (E) and geraniol (F) against S. pyogenes. Bioautography of BREO (G) and geraniol (H) against S. mutans. Bioautography of BREO (I) and geraniol (J) against S. sobrinus. [Click here to view] |
Time-kill kinetics assay
A time-kill kinetics assay of BREO and geraniol against the tested bacteria was performed to characterize their bactericidal action (Fig. 5A–F). The cell survival patterns and reduction in cell numbers using varied concentrations of BREO, and geraniol were similar across the bacteria used in this study. BREO and geraniol displayed concentration-dependent kinetics. Higher concentrations resulted in rapid bacterial death, suggesting a dose-dependent action. The 1× MIC of BREO lowered the CFU count against all examined bacteria by ≥2 log10 CFU/ml in 1 minute, whereas geraniol was not considered bactericidal at this time.
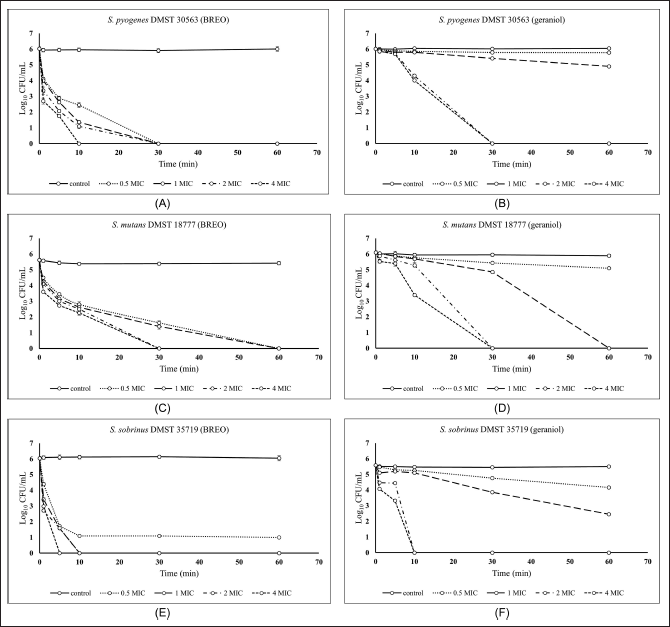 | Figure 5. Time-kill kinetics curve of BREO against S. pyogenes (A), S. mutans (C), and S. sobrinus (E) and geraniol against S. pyogenes (B), S. mutans (D), and S. sobrinus (F) at different concentrations of BREO and geraniol. [Click here to view] |
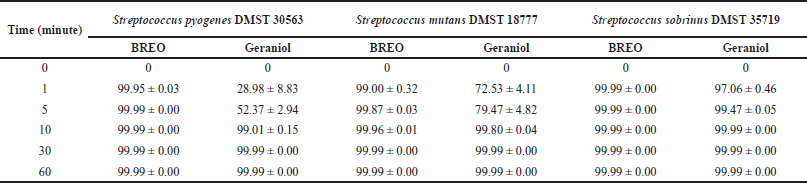 | Table 4. Average percentage kill (mean ± SD) of tested bacteria in the presence of 4 × MIC of the BREO and geraniol. [Click here to view] |
Table 4 shows the percentage kill of bacteria over 60 minutes using 4 × MIC of the tested bacteria. BREO reduced ≥99% of all tested bacteria in 1 minute. Geraniol reduced ≥99% of S. pyogenes and S. mutans in 10 minutes and ≥99% of S. sobrinus in 5 minutes. BREO exhibited bactericidal activity at 1 minute and demonstrated a higher percentage kill of all tested bacteria than geraniol at 5 and 10 minutes. In a previous study, Geraniol increased the rate of potassium leaked from intact cells and increased the membrane fluidity of C. albicans, as measured by fluorescence polarization [26]. The findings of our study demonstrate that BREO exhibits considerably greater efficacy in killing the tested bacteria than geraniol alone. The potential synergistic effects of other components in BREO must be further investigated as BREO contains several components that may contribute to its bactericidal activity.
CONCLUSION
The BREO has potent antibacterial activity against S. pyogenes, S. mutans, and S. sobrinus. A time-kill assay demonstrated the superior efficacy of BREO against these bacteria compared to geraniol at an equivalent MIC. As these bacteria are common pathogens associated with oral diseases, BREO has the potential to serve as a valuable source of antimicrobial agents for oral hygiene. Furthermore, the outcomes of this investigation suggest that BREO is more effective than geraniol alone, suggesting that further investigation of the synergistic effects of other components in BREO could be valuable.
AUTHORS’ CONTRIBUTION
N.W. and D.S. conceived and designed the project. K.B. and R.W. performed the experiments. N.W, D.S., and P.S. analyzed the results. N.W. drafted the manuscript. N.W., D.S., and P.S. revised and finalized the manuscript which was reviewed and approved by all authors.
FINANCIAL SUPPORT
This study was supported by Naresuan University and Maejo University, Thailand.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
This research article encompasses all the data that has been generated and analyzed, which is readily available within this document.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40(1):50–76. doi: https://doi.org/10.1111/j.1600-0757.2005.00148.x
2. Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7(10):e47722. doi: https://doi.org/10.1371/journal.pone.0047722
3. Saraithong P, Pattanaporn K, Chen Z, Khongkhunthian S, Laohapensang P, Chhun N, et al. Streptococcus mutans and Streptococcus sobrinus colonization and caries experience in 3- and 5-year-old Thai children. Clin Oral Investig. 2015;19(8):1955–64. doi: https://doi.org/10.1007/s00784-015-1437-0
4. Yang F, Dinis M, Haghighi F, He X, Shi W, Tran NC. Oral colonization of Candida albicans and Streptococcus mutans in children with or without fixed orthodontic appliances: a pilot study. J Dent Sci 2022;17(1):451–8. doi: https://doi.org/10.1016/j.jds.2021.07.026
5. Ferretti JJ, Stevens DL, Fischetti VA. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016.
6. Han X, An J, Zhang Y, Gong X, He Y. Risk factors for life-threatening complications of maxillofacial space infection. J Craniofac Surg. 2016;27(2):385–90. doi: https://doi.org/10.1097/SCS.0000000000002416
7. Bali RK, Sharma P, Gaba S, Kaur A, Ghanghas P. A review of complications of odontogenic infections. Natl J Maxillofac Surg. 2015;6(2):136–43. doi: https://doi.org/10.4103/0975-5950.183867
8. Paolantonio M, D’Ercole S, Pilloni A, D’Archivio D, Lisanti L, Graziani F, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a xanthan-based chlorhexidine gel in the treatment of periodontitis: a randomized multicenter trial. J Periodontol. 2009;80(9):1479–92. doi: https://doi.org/10.1902/jop.2009.090050
9. Chahyadi A, Hartati R, Wirasutisna KR, Elfahmi. Boesenbergia pandurata Roxb., an Indonesian medicinal plant: phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014;13:13–37. doi: https://doi.org/10.1016/j.proche.2014.12.003
10. Atun S, Handayani S, Rakhmawati A. Potential bioactive compounds isolated from Boesenbergia rotunda as antioxidant and antimicrobial agents. Pharmacogn J. 2018;10(3);513–8. doi: https://doi.org/10.5530/pj.2018.3.84
11. Bhamarapravati S, Juthapruth S, Mahachai W, Mahady G. Antibacterial activity of Boesenbergia rotunda (L.) Mansf. and Myristica fragrans Houtt. against Helicobacter pylori. Songklanakarin J Sci Technol. 2006;28(S1):157–63.
12. Zainin NS, Lau KY, Zakaria M, Son R, Abdull Razis AF, Rukayadi Y. Antibacterial activity of Boesenbergia rotunda (L.) Mansf. A. extract against Escherichia coli. Int Food Res J. 2013;20(6):3319–23
13. Raja Mazlan RNA, Zakaria MPM, Rukayadi Y. Antimicrobial activity of fingerroot Boesenbergia rotunda (L.) Mansf. A.] extract against Streptococcus mutans and Streptococcus sobrinus. J Pure Appl Microbiol. 2016;10(3):1755–61.
14. Jantan IB, Yassin MSM, Chin CB, Chen LL, Sim NL. Antifungal activity of the essential oils of nine Zingiberaceae species. Pharm Biol. 2003;41(5):392–7. doi: https://doi.org/10.1076/phbi.41.5.392.15941
15. Rosdianto AM, Puspitasari IM, Lesmana R, Levita J. Bioactive compounds of Bosenbergia sp. and their anti-inflammatory mechanism: a review. J App Pharm Sci. 2020;10(7):116–26. doi: https://doi.org/10.7324/JAPS.2020.10715
16. Wongkattiya N, Akekawatchai C, Sanguansermsri P, Fraser IH, Pratoomsoot C, Sanguansermsri D. Chemical composition and biological properties of essential oils from Zanthoxylum rhetsa (Roxb.) DC and Zanthoxylum limonella Alston. Afr J Tradit Complement Altern Med. 2018;15(2):12–8. doi: https://doi.org/10.21010/ajtcamv15i2.2
17. CLSI. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. CLSI standard M07. 11th ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2018
18. Lomarat P, Phanthong P, Wongsariya K, Chomnawang MT, Bunyapraphatsara N. Bioautography-guided isolation of antibacterial compounds of essential oils from Thai spices against histamine-producing bacteria. Pak J Pharm Sci. 2013;26(3):473–7.
19. Wongkattiya N, Sanguansermsri P, Fraser IH, Sanguansermsri D. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J Complement Integr Med. 2019;16(4). doi: https://doi.org/10.1515/jcim-2018-0195
20. NIST. NIST chemistry WebBook, NIST standard reference database number 69. Gaithersburg (MD): National Institute of Standards and Technology; 2022. doi: https://doi.org/10.18434/T4D303
21. Aiyegoro OA, Afolayan AJ, Okoh AI. In vitro antibacterial activities of crude extracts the leaves of Helichrysum longifolium in combination with selected antibiotics. Afr J Pharm Pharmacol. 2009;3(6):293–300.
22. Baharudin MKA, Hamid SA, Susanti D. Chemical composition and antibacterial activity of essential oils from three aromatic plants of the Zingiberaceae family in Malaysia. J Phys Sci. 2015;26(1):71–81.
23. Thomas BT, Adeleke AJ, Raheem-Ademola RR, Kolawole R, Musa OS. Efficiency of some disinfectants on bacterial wound pathogens. Life Sci J. 2012;9(2):752–55.
24. Limsuwan S, Voravuthikunchai SP. Bactericidal, bacteriolytic, and antibacterial virulence activities of Boesenbergia pandurata (Roxb.) Schltr. extract against Streptococcus pyogenes. Trop J Pharm Res. 2013;12(6)1023–8. doi: https://doi.org/10.4314/tjpr.v12i6.23
25. En-Chong T, Yean-Kee L, Chin-Fei C, Choon-Han H, Sher-Ming W, Li-Ping CT, et al. Boesenbergia rotunda: from ethnomedicine to drug discovery. Evid Based Complement Alternat Med. 2012;12:473637. doi: https://doi.org/10.1155/2012/473637
26. Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids. 1988;23(6)534–8. doi: https://doi.org/10.1007/BF02535593