INTRODUCTION
The use of antibiotics as growth promoters in poultry feed has been restricted in several countries [1]. Using an antibiotic growth promoter inconsistent with the recommended dosage and continuous administration can cause antibacterial resistance, livestock product residues [2], weakening disease resistance, and environmental pollution [3]. Feed antibiotics have been prohibited in the European Union since January 2006 (Regulation EC/1831/2003). Antibiotic bans can result in economic losses. Prohibition of the use of antibiotics has encouraged efforts to develop alternatives to antibiotics [4], namely traditional antibiotics as feed additives. Various feed additives have been utilized as antibacterial agents, including phytobiotics, probiotics, prebiotics, synbiotics, enzymes, and organic acids. Plant organic acids have been widely investigated, whereas those of animal origin have rarely been studied. Several additional organic acids also exhibit antibacterial effects. Insect-based biorefineries are a potential alternative for manufacturing feeds and foods because of the increasing demand for renewable and sustainable products [5]. One potential source of animal organic acid is lauric acid obtained from black soldier fly oil (BSFO). BSFO contains lauric acid (C:12) as the main fatty acid, which is a medium-chain fatty acid, which has the potential as an antibacterial agent, and boosts immunity [6–9]. BSFO contains 44.55% of lauric acid. Lauric acid can damage bacterial cell membranes and walls through physicochemical processes.
Broiler performance can be partially explained by the improvement in intestinal morphology and microbial balance associated with the modulation of intestinal microflora and pathogen inhibition [10]. Toxic emissions from pathogenic microorganisms compromise the physical integrity of the small intestine cell wall, which is crucial for vitamin absorption. Traditional antibiotics can be easily administered through drinking water; however, BSFO, in the form of an oil phase, does not dissolve in water. One way to overcome this solubility problem is to use nanotechnology as nanoemulsions [in the self-nanoemulsifying drug delivery system (SNEDDS)]. Self-emulsifying drug delivery systems are utilized to overcome poorly mixed and insoluble substances by improving their solubility composed of a mixture of oils, surfactants, and cosurfactans [11]. SNEDDS are homogenous anhydrous liquid solutions of cosurfactant, surfactant, and oil. With its potential to speed up and expand the absorption of drugs with low water solubility properties, SNEDDS has been an exciting object of study [12]. When these naturally occurring oil-in-water nanoemulsions were diluted with water while being gently stirred, they had a size of approximately 5–100 nm [11]. The nanoparticles can dissolve in water [13]. Nanoparticles are gaining attraction for functional applications in interdisciplinary fields. In general, the nanoparticles are 10−9 cm in size. The size of nanoemulsions ranges from 1 to 100 nm [14]. In sick animals, the beneficial effect of nanoparticles is that active substances are more quickly and effectively carried to the site of infection. SNEDDS has been proposed to improve the formulations’ solubility [15]. The BSFO has been used as a novel feedstock for preparing nanoemulsions [5]. However, BSFO has limited oral applications owing to its low oral bioavailability. In this study, a new SNEDDS was created to improve the solubility and absorption of BSFO when administered to poultry via drinking water.
MATERIALS AND METHODS
The primary material, BSFO, was acquired from a black soldier fly farmer in Bekasi Regency, West Java, Indonesia, utilizing a mechanical pressing machine. Then, 14-day-old fresh black soldier fly larvae were dried at 60ºC for 24 hours. Black soldier fly larvae that had been dried were pulverized and pressed to produce oil. After letting the oil remain for 2 days, the sediment was separated. In order to create SNEDDS, the solution was then filtered in the laboratory using Whatman filter paper. The chemicals and other components used were: Tween 80 and polyethylene glycol 400 (PEG 400) are both available from PT. Brataco in Cikarang, Bekasi, Indonesia, and Sigma Aldrich in Germany, respectively. Distilled water (Jaya Santosa, Indonesia), hydrochloric acid (37%), and sodium chloride (MERCK, Germany) were used to make artificial gastric fluid (AGF).
SNEDDS formulation of BSFO
BSFO SNEDDS preparation
BSFO, Tween 80, and PEG 400 were mixed in various proportions to generate SNEDDS with a ratio of 1:1:1 to 1:8:1. BSFO is first combined with PEG 400 by means of a magnetic stirrer for 10 minutes at 37ºC, 250 rpm. The Tween 80 was then added and thoroughly mixed for another 20 minutes at 37ºC, 250 rpm. The formulations were sonicated using an ultrasonic cleaner (Aczet model number CUB 10-liter Frequency 40 KHz power 120 W Germany) at 40ºC for 10 minutes and visual observation was carried out (Table 1).
Measurement of transmittance
Five milliliters of distilled water and one hundred microliters of BSFO SNEDDS were vigorously mixed using a vortex for 2 minutes. The self-nanoemulsification effectiveness of SNEDDS was shown by transmittance values of the formulations exceeding 90% [16]. The transmittance percentage was measured using a UV spectrophotometer at 650 nm wavelength [17].
Measurement of emulsification time
To determine the emulsification time, separate glass beakers set at 37ºC were set, and 50 ml of AGF with a pH of 1.42 was added along with 100 μl of BSFO SNEDDS. The mixture was then gently stirred using a magnetic stirrer at 100 rpm until the solution became clear. The amount of time it took for the solution to become clear was considered self-emulsification [18]. The SNEDDS with good emulsification and solubility characteristics along with BSFO, Tween 80, and PEG 400 in various ratios were selectively chosen.
Optimization of BSFO SNEDDS
In a further experimental study, a three-part system, comprising X1, the BSFO phase; X2, the surfactant (Tween 80); and X3, the cosurfactant (PEG 400), was used. The responses (Y1 = emulsification time; Y2 = transmittance) were interpreted using Design Expert software version 13.0.1.0. For emulsification time and transmittance, the results of the BSFO SNEDDS were replicated in triplicate. A t-test using a single sample was used to evaluate the data.
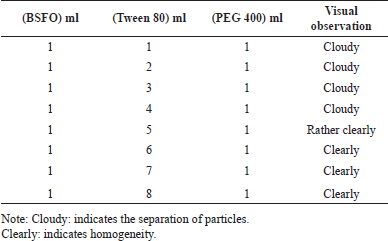 | Table 1. The effect of surfactant ratio on visual observation. [Click here to view] |
 | Table 2. The components that were examined using an I-optimal mixture design ranged in value. [Click here to view] |
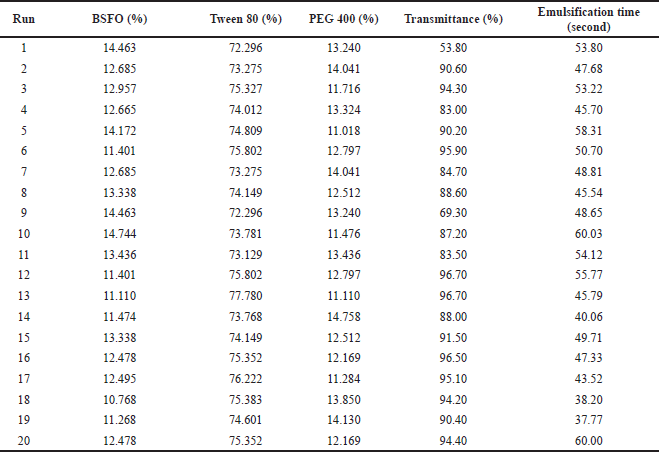 | Table 3. Designing a formulation (Design Expert Ver. 13.0.1.0). [Click here to view] |
Characterization of BSFO SNEDDS
Measurement of droplet size
Using a Zetasizer Nano (Malvern, Germany), the polydispersity index (PDI) and nanoemulsion droplet size were calculated [19]. The experiment was carried out in triplicate, with the samples diluted with distilled water at a ratio of 1: 1,000 (v/v) [20].
Measurement of zeta potential (ZP)
Using a particle size analyzer from Malvern Instrument Germany, dynamic light scattering (DLS) was utilized to estimate the ZPs of the most optimal formulations. Results were captured once the samples were put into clear disposable cuvettes [21]. The samples were thoroughly mixed with distilled water at a ratio of 1:1,000 (v/v), and the experiment was conducted thrice [20].
Measurement of viscosity
Employing a small-sample adapter (Brookfield digital viscometer DV-I Prime, Brookfield Engineering Laboratories, INC, Middleboro, MA 02346), BSFO SNEDDS and nanoemulsion viscosities were evaluated. The viscosity of BSFO SNEDDS was evaluated using Spindle 03 at 100 rpm for 15 seconds at room temperature (25ºC) in triplicate. The Spindle 02 was used to measure the nanoemulsion’s viscosity at 100 rpm at room temperature (25ºC) for 15 seconds in triplicate.
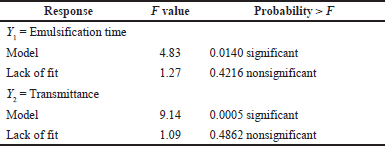 | Table 4. Quadratic model analysis. [Click here to view] |
Measurement of morphology
Applying a Joel JEM-100 CX (Joel, Tokyo, Japan) transmission electron microscopy, The morphology of BSFO SNEDDS was carefully investigated. A sample drop taken from SNEDDS samples was diluted with water (1:1,000) and stained for 30 seconds with a 2% phosphotungstic acid solution, followed by being deposited on a copper grid [22].
Studies on the thermodynamic stability of BSFO SNEDDS
Additional research on the thermodynamic stability of the ideal formula was conducted. The first step was heating and cooling cycles. Six cycles were examined, each lasting between 4ºC and 45ºC for at least 48 hours. Centrifugation was applied on formulations that passed it through this temperature without exhibiting any indications of instability (cracking or creaming). Second was centrifugation. The mixtures were centrifuged at 3,500 rpm for 30 minutes. The freeze–thaw cycle was implemented for formulations that demonstrated no evidence of instability (cracking or creaming). The last step was the cycle of freezing and thawing. The formulations were stored between −21ºC and 25ºC for no less than 48 hours at each temperature (using a deep freezer). As described by Parmar et al. [23], the formulations were centrifuged for 5 minutes at 3,000 rpm.
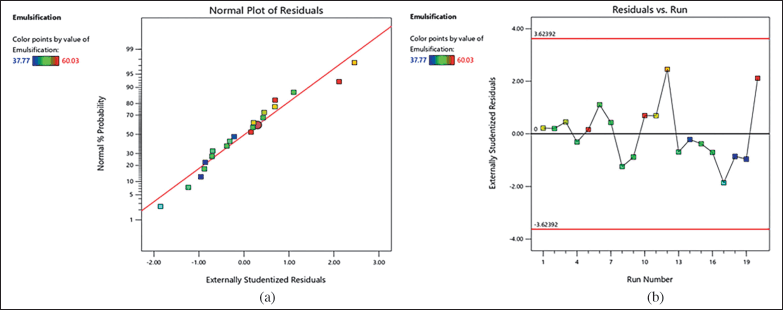 | Figure 1. Graph of a residual parameter of emulsification time. [Click here to view] |
RESULTS AND DISCUSSION
Formulation optimization of BSFO SNEDDS
Table 2 shows the effects of the BSFO, Tween 80, and PEG 400 ratios on visual observation. Because of the clarity of the observations, the ideal ratio was calculated using the formula 1:6:1. The BSFO: Tween 80: PEG 400 were 1: 5: 1 (lower limit) and 1:7:1 (upper limit) (v/v), in a respective manner. Lower and upper limits, which are shown in Table 2, were established using this data. With the aid of response surface methodology and considering three independent parameters (BSFO, PEG 400, and Tween 80) effect on two dependent variables (transmittance and emulsification time), this study seeks to identify the variables’ levels at which the product is of the highest quality. The selectively chosen formulation relied on the “trading off” among multiple variable responses, i.e., increasing transmittance percentage while reducing emulsification time. In order to identify the ideal formula for an array of formulas, this study optimized using an I-optimal mixture design (Table 3). Transmittance and emulsification values were determined using the 20 formulas in Table 3. The responses were transmittance and emulsification time. Table 4 displays the result from the analysis of variance and lack-of-fit tests.
Emulsification time
The hues that correspond to the light spectrum serve as an illustration of the emulsification time [24]. The closer distance to light green indicates a high emulsification time. The residuals’ normal probability plot for emulsification time appears in Figure 1(a). The obtained data revealed no evidence of whether the straight line contains any outliers; the residuals are normally distributed. Meanwhile, the data in Figure 1(b) are centered on the zero line, and no particular value sticks out. The model (Fig. 2) clearly illustrates the relationship between quantity (BSFO, Tween 80) and response (emulsification time). The projected value and the actual values were very closely matched, as shown by the lack of fit analysis (Table 4), which revealed no significance. For creating emulsions in gastric fluid with SNEDDS properties, emulsification time is a crucial component to consider [25]. When in contact with biological fluids, the nonionic surfactant Tween 80 quickly disperses due to its low toxicity, strong solubility, and good emulsification ability [25]. Figure 5 shows the amount of time needed for quicker emulsification with a higher Tween 80 content. In nanoemulsion formulations, reduced BSFO is frequently employed to enhance solubility and bioavailability. A single-sample t-test with Open-Stat was used to confirm the outcomes of the optimum formula. The emulsification time’s p-value was 1,000 (Table 5), which was practically perfect and showed no difference between the expected and actual data.
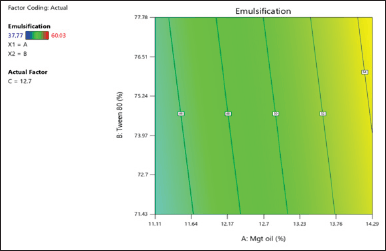 | Figure 2. Special model cubic of emulsification time graphic values. [Click here to view] |
 | Table 5. Single sample t-test verification of the optimal formula. [Click here to view] |
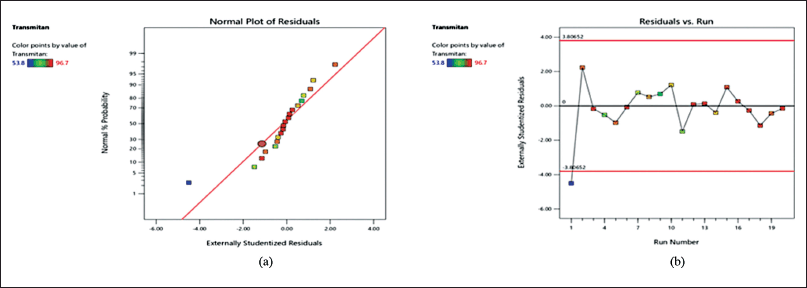 | Figure 3. Graph of a residual parameter of transmittance. [Click here to view] |
Percentage of transmittance
The solution’s transmittance percentage was utilized to well document the process of self-emulsification while the solution was dissolving and the process of self-emulsification was taking place [26]. The colors that follow the light spectrum serve as an example of the transmittance value [24]. A normal probability plot of the transmittance residuals is shown in Figure 3(a). There is no evidence of the potential of outliers, and the straight line displays residuals with a normally distributed distribution. The data are close to the zero line, as shown in Figure 3(b), and nobody sticks out. In the unique cubic model (Fig. 4), the redder the shift, the greater the transmittance value. The transmittance increases as the BSFO% decreases. The fit analysis result in Table 4 did not provide any significant results, demonstrating that the predicted values and the observed data were closely matched. With desirability of one, the suggested ideal formulas for BSFO, Tween 80, and PEG 400 were 11.426%, 73.364%, and 11.177%, respectively (Fig. 5). Through the use of Open-Stat software and a single-sample t-test, the outcomes of the optimum formula were confirmed. Since the transmittance’s p-value was 0.999 (Table 5), higher than 0.05, no statistically significant discrepancy existed between the projected value and the observed data.
Characterization of BSFO SNEDDS
Particle size and PDI
Assuming the presence of spherical particles, the mean particle size and PDI were derived using the volume, intensity, and bimodal distribution. According to the sample’s size, the PDI calculates the sample’s heterogeneity [27]. This instrument allows the measurement of particle homogeneity with a size range from 0.0 to 1.0 [11]. It was discovered that the preferred formulation’s particle size distribution was 10.02 ± 0.12 nm (Fig. 6), indicating a small size distribution of the system. The PDI was (0.36% ± 0.0%), indicating the homogeneity distribution of the system. As it controls the pace and volume of drug release and absorption and increases bioavailability, droplet size is a crucial component in SNEDDS formulations [23]. Fewer surfactants are needed since the shock wave that results from ultrasonication can efficiently mix the layers. Consequently, smaller droplet emulsions within the size distribution were produced [28]. The interfacial coating was condensed and stabilized by the addition of Tween 80 to the BSFO nanoemulsion.
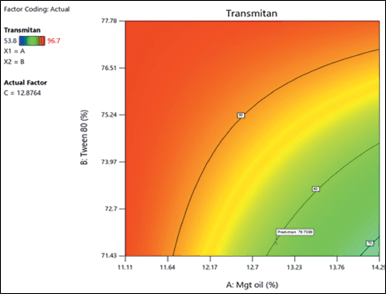 | Figure 4. Special model cubic of transmittance graphic values. [Click here to view] |
 | Figure 5. Superimposed image of particular model cubic emulsification time and transmittance. [Click here to view] |
Zeta potential
The BSFO nanoemulsion droplet had a ZP of −19.4 mV (Fig. 7). The ZP measurements showed that the SNEDDS formulation was stable. According to Balakumar et al. [20], the formulation’s inclusion of fatty acids was likely the reason for the significant negative charge. The use of ZP measurements with DLS measurements to determine particle size and surface charge has grown in popularity [29].
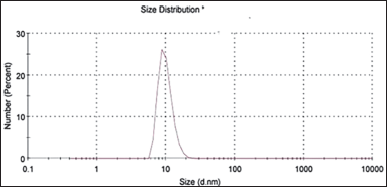 | Figure 6. Optimum formula particle size distribution graph. [Click here to view] |
Viscosity measurement
BSFO SNEDDS and nanoemulsion had viscosities of 387.33 ± 22.81 mPa·s and 10.47 ± 0.42 mPa·s, respectively. The smaller droplet size is primarily responsible for the nanoemulsion’s reduced viscosity. Tween 80 has a high viscosity (425 mPa·s); therefore, adding PEG 400 could enhance self-emulsification [30].
Morphology of BSFO SNEDDS
With a spherical shape and uniform droplet size, the BSFO nanoemulsion was visible as a bright spot against a dark background (Fig. 8). Numerous parameters, including emulsion composition, homogenization, and environment, must be tuned to produce stable nanoemulsions [31]. The cosurfactant’s surfactant ratio impacted the nanoemulsions morphology [5]. Lauric acid-containing samples were more spherical and included remnants of the membrane [32].
Test for thermodynamic stability
The environmental conditions, including temperature, markedly affect the stability of nanoemulsion [33]. This study used centrifugation and freeze–thaw cycle techniques in thermodynamic stability tests to find metastable formulations [34]. The thermodynamic stability tests were successful for the ideal formulation (Table 6). The system’s stability was demonstrated by the best formulation, which displayed no signs of instability. In order to create a nanoemulsion, BSFO SNEDDS should spontaneously emulsify in the digestive system. The BSFO SNEDDS system needs to be of high enough caliber to resist instability and prevent creaming, cracking, or precipitation. The prepared BSFO SNEDDS was verified to be stable for the shortest time using thermodynamic stability. The chosen formulation was centrifuged, exposed to a freeze-thaw cycle, and subjected to a heating-cooling cycle [35].
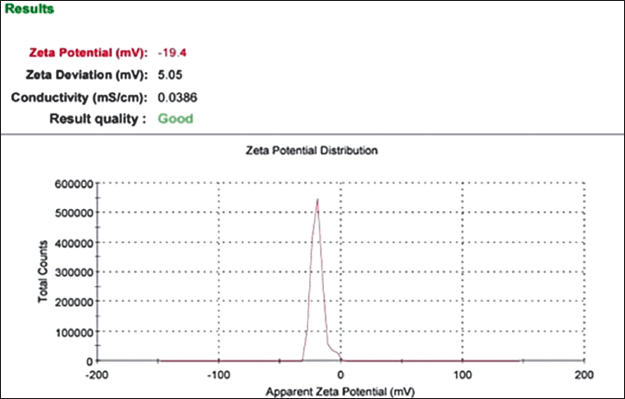 | Figure 7. Zeta potential distribution. [Click here to view] |
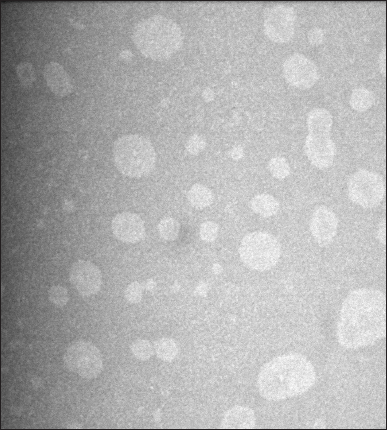 | Figure 8. Transmission electron micrograph of BSFO self-nanoemulsifying drug delivery system with spherical shape. [Click here to view] |
 | Table 6. Test for thermodynamic stability. [Click here to view] |
CONCLUSION
The ratios of 11.426% BSFO, 73.364% of Tween 80, and 11.177% PEG 400 were used to create the SNEDDS of BSFO. The formulation’s properties included a high percent transmittance of 95.2 ± 0.72, a brief emulsification time of 47.89 ± 0.78 seconds, a small droplet size of 10.02 ± 0.12 nm with a PDI of 0.36% ± 0.0%, a ZP of −19.4 mV, and BSFO SNEDDS; and nanoemulsion had viscosities of 387.33 ± 22.81 mPa·s and 10.47 ± 0.42 mPa·s. BSFO SNEDDS passed the thermodynamic stability test and showed signs of having a sphere-like form. This investigation showed that this formulation might increase BSFO water solubility and stability. If this formula is improved, it would be possible to employ it as an antibacterial agent and a possible antibiotic substitute in the production of chicken.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Institute for Research and Community Service, Universitas Sebelas Maret for research funding, also Biopharmaceutics Laboratory, Department of Pharmaceutics, Faculty of Pharmacy, and Department of Animal Science Learning Center (ASLC) Faculty of Animal Science Universitas Gadjah Mada Yogyakarta Indonesia for providing facilities in this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors also would like to address gratitude to RKAT PTNBH Universitas Sebelas Maret for the 2023 fiscal year through a research scheme PENELITIAN DISERTASI DOKTOR (PDD-UNS) with the number of the Research Assignment Agreement letter: 228/UN27.22/PT.01.03/2023 for the funding to conduct a significant part of the study.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Khan RU, Naz S. The applications of probiotics in poultry production. Worlds Poult Sci J. 2013;69(3):621–32.
2. Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Barkat RA, Gabr AA, et al. Potential role of important nutraceuticals in poultry performance and health – a comprehensive review. Res Vet Sci. 2021;137:9–29.
3. Salim HM, Huque KS, Kamaruddin KM, Beg MAH. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci Prog. 2018;101(1):52–75.
4. Cheng G, Hao H, Xie S, Wang X, Dai M, Huang L, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol. 2014 May 13;5:217.
5. Chou TH, Nugroho DS, Cheng YS, Chang JY. Development and characterization of nanoemulsions based on oil extracted from black soldier fly larvae. Appl Biochem Biotechnol. 2020;191(1):331–45.
6. Dierick NA, Decuypere JA, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols containing medium-chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition in vitro screening of the release of MCFAs from selected fat sources by. Livest Prod Sci. 2002;75(2):129–42.
7. Park SI, Chang BS, Yoe SM. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol Res. 2014;44(2):58–64.
8. Spranghers TJ, Michiels J, Vrancx A, Ovyn M, Eeckhout P, De Clercq S, et al. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim Feed Sci Technol. 2018;235:33–42.
9. Irawan AC, Astuti DA, Wibawan IWT, Hermana W. Impact of the feeding with the black soldier fly (Hermetia illucens) on egg physical quality, egg chemical quality and lipid metabolism of laying hens. J Phys Conf Ser. 2019;1351:1–8.
10. Abudabos AM, Al-Batshan HA, Murshed MA. Effects of prebiotics and probiotics on the performance and bacterial colonization of broiler chickens. S Afr J Anim Sci. 2015;45(4):419–28.
11. Prajapati BG, Prajapati B, Khunt D. Formulation and evaluation of self-nanoemulsifying drug delivery system for improved oral delivery of exemestane hydrochloride. IP Int J Compr Adv Pharmacol. 2023;8(1):42–8.
12. Prajapati BG, Paliwal H, Shah PA. In vitro characterization of self-emulsifying drug delivery system-based lipsticks loaded with ketoconazole. Future J Pharm Sci. 2023;9(1):1–2.
13. Abd El-Hack ME, El-Saadony MT, Saad AM, Salem HM, Ashry NM, Abo Ghanima MM, et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult Sci. 2022;101(2):1–21.
14. Neethirajan S, Jayas DS. Nanotechnology for the food and bioprocessing industries. Food Bioproc Tech. 2011;4(1):39–47.
15. São Pedro A, Santo I, Silva C, Detoni C, Albuquerque E. The use of nanotechnology as an approach for essential oil-based formulations with antimicrobial activity. Microb Pathog Strategies Combating. 2013;2:1364–74.
16. Narkhede R, Gujar K, Gambhire V. Design and evaluation of self-nanoemulsifying drug delivery systems for nebivolol hydrochloride. Asian J Pharm. 2014;8(3):200–9.
17. Sharma B, Sharma A, Arora S, Gupta S, Bishnoi M. Formulation, optimization and evaluation of atorvastatin calcium loaded microemulsion. J Pharm Drug Deliv Res. 2012;01(03):1–7.
18. Ghosh G, Khan D. Chemotherapeutic impact of natural antioxidant flavonoids gallic acid rutin quercetin and mannitol on pathogenic microbes and their synergistic effect. Int J Sci Technol Res. 2015;4(8):243–56.
19. Prajapati BG, Patel AG, Paliwal H. Fabrication of nanoemulsion-based in situ gel using moxifloxacin hydrochloride as model drug for the treatment of conjunctivitis. Food Hydrocoll Health. 2021;1:100045.
20. Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B. 2013;112:337–43.
21. Nasr A, Gardouh A, Ghorab M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8(3):1–29.
22. Zhao Y, Wang C, Chow AHL, Ren K, Gong T, Zhang Z, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2010;383(1–2):170–7.
23. Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine lnoaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf B. 2011;86(2):327–38.
24. Wiwiek IA, Martodihardjo SSJ, Ngurah Budiana IGMM. Preparation and in-vitro characterization of self-nano emulsifying system of C-phenylcalix-[4]-resorcinaryl octacinnamate and C-methylcalix-[4]-resorcinaryl octabenzoate as ultraviolet absorbers. Bali Med J. 2017;6(3):569–77.
25. Ujilestari T, Martien R, Ariyadi B, Dono ND, Zuprizal Z. Self-nanoemulsifying drug delivery system (SNEDDS) of Amomum compactum essential oil: design, formulation, and characterization. J Appl Pharm Sci. 2018;8(6):14–21.
26. Villar AMS, Naveros BC, Campmany ACC, Trenchs MA, Rocabert CB, Bellowa LH. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int J Pharm. 2012;431(1–2):161–75.
27. Mudalige T, Qu H, Van Haute D, Ansar SM, Paredes A, Ingle T. Characterization of nanomaterials: tools and challenges. Nanomater Food Appl. 2019 Jan 1;11:313–53.
28. Cabrera-Trujillo MA, Filomena-Ambrosio A, Quintanilla-Carvajal MX, Sotelo-Díaz LI. Stability of low-fat oil in water emulsions obtained by ultra turrax, rotor-stator and ultrasound homogenization methods. Int J Gastron Food Sci. 2018;13:58–64.
29. Bhattacharjee S. DLS and zeta potential – what they are and what they are not? J Control Release. 2016;235:337–51.
30. Rowe RC, Sheskey PJ, Quinn ME. Handbook pharmaceutical excipient. 6th ed. London, UK: The Pharmaceutical Press and The American Pharmacist Association; 2009.
31. Montes de Oca-Ávalos JM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. Curr Opin Food Sci. 2017;16:1–6.
32. Farkuh L, Hennies PT, Nunes C, Reis S, Barreiros L, Segundo MA, et al. Characterization of phospholipid vesicles containing lauric acid: physicochemical basis for process and product development. Heliyon. 2019;5(10):1–10.
33. Mohite P, Rajput T, Pandhare R, Sangale A, Singh S, Prajapati BG. Nanoemulsion in management of colorectal cancer: challenges and future prospects. Nanomanufacturing. 2023;3(2):139–66.
34. Winarti L, Suwaldi, Martien R, Hakim L. Formulation of self-nanoemulsifying drug delivery system of Bovine serum albumin using HLB (Hydrophilic-Lypophilic Balance) approach. Indonesian J Pharm. 2016;27(3):117–27.
35. Bandyopadhyay S, Katare OP, Singh B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf B. 2012;100:50–61.