INTRODUCTION
Dietary supplements, including mushrooms, have become increasingly popular due to their potential anti-disease properties. Numerous studies have emphasized the health, nutritional, and medicinal benefits of mushrooms, as they are abundant in essential nutrients and minerals. Research has demonstrated their ability to stimulate the immune system and exhibit anticancer properties [1]. Moreover, mushrooms have been linked to a decreased risk of coronary heart disease, the regulation of blood sugar levels, as well as possessing anti-inflammatory and antibacterial properties [2,3].
Boletus griseipurpureus Corner (family Boletaceae), also known as Hed-Sa-Med, is a popular mushroom consumed locally in Thailand’s southern and northeastern regions, including Narathiwat. Boletus griseipurpureus is a seasonal ectomycorrhizal fungus. It is naturally found in melaleuca, sea pine, and eucalyptus forests. Its distinctive features include an umbrella-shaped cap with a curved and prominent form, resembling a wok. When young, the buds have fine hairs reminiscent of a Camellia flower and are grayish-purple or pale purple. The gills underneath the cap are small and whitish, turning pale pinkish-brown when fully open. The stem is grayish-purple, the flesh is white, and the base is bulbous. Basidia, club-like structures, measure 24–40 × 8–10 μm, while basidiospores are pear-shaped, smooth, and sized 3–4 × 7–10 micrometers [4,5]. Boletus griseipurpureus has been subject to several studies, revealing the presence of alkaloids, flavonoids, saponins, and tannins through crude ethanolic screening of active compounds [6–8]. Moreover, the crude extract from B. griseipurpureus has antioxidant and antibacterial properties against Staphylococcus aureus and Escherichia coli [9–11]. In terms of nutritional content, the mushroom is rich in protein (31.4%), carbohydrates (33.3%), fiber (15%), and Ash (8.6%), while being low in fat (0.9%) [12]. These characteristics make it a potential dietary health food, with its bioactive compounds effectively resisting oxidative processes and acting as natural antioxidants [1,13]. Furthermore, B. griseipurpureus extract has the potential to reduce constipation, excretion, hypertension, Alzheimer’s disease, cholesterol levels, and blood glucose levels [6,14]. Traditional medicine practices among the indigenous people of Kelantan, Malaysia, also utilize this mushroom to treat diabetes, cervical cancer, and breast cancer [6]. While the development of B. griseipurpureus as a medicinal drug holds significant benefits, there is limited understanding of its safety and bioactivity. It is crucial to conduct toxicity studies on medicinal mushroom extracts in animals to ensure safety and address public health concerns, thus enhancing the value of local mushrooms. The assessment of acute and subacute toxicity is employed to evaluate the safety, mode of action, and potential risks of these compounds [15]. Acute toxicity testing involves administering a single dose of a substance to animals to observe symptoms and assess toxicity levels. Subacute toxicity studies follow acute tests, providing additional insights into the effects of repeated doses on specific tissues or organs [16].
Therefore, the objective of this study was to evaluate the safety of B. griseipurpureus Corner extracts by examining their potential acute and subacute toxic effects on mice. This assessment was conducted through histopathological and biochemical examinations.
MATERIALS AND METHODS
Mushroom material and preparation of crude extract
Basidiomes of B. griseipurpureus Corner were harvested from melaleuca forests in Yi-ngo, Narathiwat, Thailand, in July 2022. A voucher specimen (Collection No. BGC-2566-01) was identified as B. griseipurpureus Corner by Natthapat Srihanant. It was collected at the Faculty of Natural Resources, Prince of Songkla University, Hat Yai, Songkhla, Thailand. The Mushroom extraction was performed with some modifications to the protocol [6,7]. Fresh basidiomes were cleaned, oven-dried at 60°C for 24 hours, finely ground, and then immersed in 95% ethanol (ratio 1:4) for 3 days at room temperature. The extraction process was repeated twice, and the resulting extract was filtered using Whatman filter paper No.1. The ethanol was subsequently removed using a rotary evaporator (Heidolph Hei-VAP Precision) until dry, resulting in a dark brown viscous crude extract. Yields were calculated as a percentage based on the dry weight of the basidiomes. The extracts were stored at room temperature and diluted with distilled water before use.
Experimental animals
A total of 30 male Institute of Cancer Research mice, aged 6 weeks and weighing 25–35 g, were obtained from Nomura Siam International Co. Ltd., Bangkok, Thailand. After a 7-day acclimation period, the mice were housed and fed at the Laboratory Animals Unit of the Research Institute for Health Sciences, Walailak University. Random assignment was done to allocate the mice into control and treatment groups. The mice were kept in cages maintained at 22°C ± 2°C with a 12-hour light-dark cycle, 50%–70% relative humidity, and were provided ad libitum access to standard food and water throughout the experiment. All procedures were conducted by the code of ethics for the care and use of animals for scientific purpose, as outlined by the Institute of Animals for Scientific Purposes Development and the National Research Council of Thailand. Surgeries were performed under sodium pentobarbital anesthesia, and measures were taken to minimize pain and discomfort.
Acute toxicity study
The administration of the divided dose of B. griseipurpureus Corner extract followed the guidelines outlined in the Organization of Economic Co-operation and Development (OECD) Guideline 420 for acute toxicity testing [16]. In the study, five mice were subjected to a single oral gavage of the extract at a dose of 2,000 mg/kg, while the control group of five mice was given distilled water. The number of samples was calculated using the Federer formula [17]. Close monitoring for signs of toxicity and mortality was conducted at intervals of 30 minutes, 4 hours, 24 hours, and once daily for 14 days. Throughout the 14-day study, daily measurements of body weight, water intake, and food consumption were recorded. At the end of the observation period, the mice were sacrificed, and blood samples were obtained via cardiac puncture. Furthermore, the liver and kidney were collected for histopathological examination.
Subacute toxicity study
The toxicity testing in this study was conducted according to the OECD guideline 420 [16]. Male mice were assigned randomly to four groups, with each group containing five mice. The number of samples was calculated using the Federer formula [17]. The treated group received oral doses of the extract at 50, 300, and 2,000 mg/kg/day, while the control group was administered distilled water. Daily observations were made over 14 days to monitor symptoms of toxicity and death. Throughout the test, measurements of body weight, water intake, and food consumption were recorded. At the end of the study, surviving mice were sacrificed for microscopic examination of kidney and liver cells. Hematological and biochemical analyses were performed by collecting blood samples through cardiac puncture.
Blood analysis
Hematological parameters consist of red blood cell count (RBC), white blood cell count (WBC), hemoglobin concentration (Hb), hematocrit (Hct), platelet count, and mean corpuscular volume (MCV), which were measured using an automatic hematological analyzer (Automated MEK-6410 Hematology analyzer). Serum biochemical parameters were assessed using commercially available enzyme marker kits to evaluate liver and kidney function. Liver function tests measured enzyme activity such as alkaline phosphatase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Kidney function tests measured creatinine (CRE), blood urea nitrogen (BUN), and uric acid. Lipid and blood sugar tests measured triglycerides, cholesterol, high-density lipoprotein (HDL), and fasting blood sugar (FBS) were analyzed using a clinical chemistry Olympus AU400 analyzer (Olympus, Tokyo, Japan).
Histopathological analysis
Liver and kidney tissues were fixed in 4% paraformaldehyde, dehydrated with graded alcohols, cleared with xylene, and embedded in paraffin. Paraffin blocks were cut into 3–5 μm sections using a rotary microtome 4060E (Germany) and stained with hematoxylin and eosin (H&E). The sections were examined under an optical microscope (Olympus’ BX53 upright microscope, Tokyo, Japan) and analyzed using cellSens Imaging software.
Statistical analysis
Results were displayed as mean ± SD. The data were analyzed statistically using Graphpad Prism software version 5.0 (Graphpad Software, Inc., San Diego, CA) by performing one-way ANOVA followed by Dunnett’s multiple comparison tests. A significance level of p < 0.05 was used to determine the statistical significance of the analysis.
RESULTS
Acute toxicity analysis
No clinical symptoms such as abnormal respiration, salivation, sleep disturbances, tremors, convulsions, aggression, lack of hair erection, or signs of death were observed after a single oral dose of B. griseipurpureus Corner extracts at 2,000 mg/kg body weight. Additionally, there were no abnormal morphological changes in the color, texture, and weight of internal organs like the liver and kidneys. The intake of 2,000 mg/kg body weight of B. griseipurpureus extract did not significantly affect body weight gain or the organ-to-body weight index compared to the control group. However, the treatment group showed a significant increase (p < 0.05) in food intake compared to the control group, while water intake did not differ between the two groups (Table 1).
Subacute toxicity analysis
After a 14-day testing period, male mice treated with B. griseipurpureus extract doses of 50, 300, and 2,000 mg/kg did not experience any death. Food intake was significantly increased (p < 0.05) in mice administered 300 and 2,000 mg/kg doses of the extract. However, there were no significant changes in body weight and organ-to-body-weight index across all extract doses administered (Table 2).
Hematological parameters
Acute studies in mice treated with B. griseipurpureus extract at a dose of 2,000 mg/kg showed a significant increase (p < 0.05) in erythrocyte volume, while Hb and Hct levels were significantly decreased (p < 0.05) compared to the control group (Table 3). Subacute studies administering B. griseipurpureus extract at 50 and 2,000 mg/kg resulted in significant reductions (p < 0.05) in Hb and Hct levels. Although not statistically significant, there was a decrease in Hb and Hct at the 300 mg/kg extract dose compared to the control group. However, after 14 days of extract ingestion, RBC, WBC, and mean blood cell mass did not show significant differences compared to controls (Table 4).
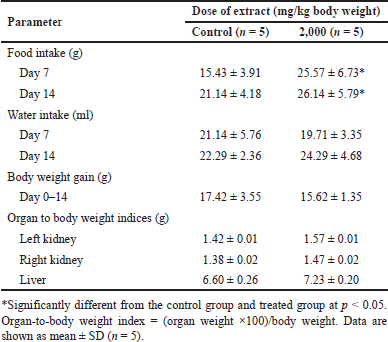 | Table 1. Food consumption, water consumption, growth parameters, and organ-to-body weight indices of mice exposed to an acute dose of extracts. [Click here to view] |
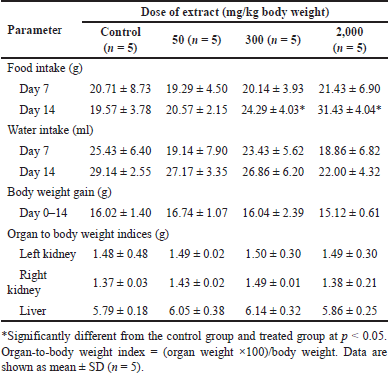 | Table 2. Food consumption, water consumption, growth parameters, and organ-to-body weight indices of mice exposed to a subacute dose of extracts at various doses. [Click here to view] |
Serum biochemical studies
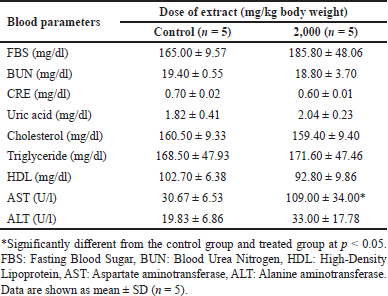
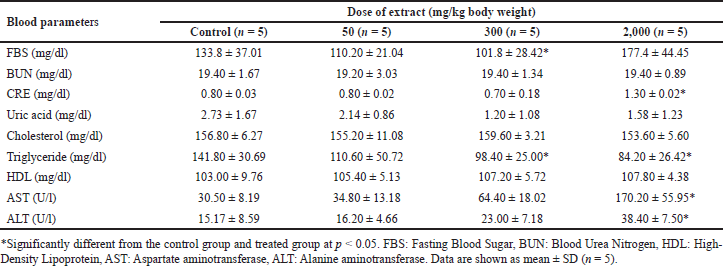
In acute toxicity studies, mice treated with 2,000 mg/kg ethanolic B. griseipulpureus extract showed a significant increase (p < 0.05) in AST levels compared to the control group (Table 5). In the subacute group, FBS was significantly reduced (p < 0.05) at a dose of 300 mg/kg extract. Levels of AST, ALT, and CRE were significantly increased (p < 0.05) in mice treated with 2,000 mg/kg extract compared to the control group. BUN levels showed a nonsignificant increase in all groups receiving B. griseipurpureus extract. Triglyceride levels were significantly reduced (p < 0.05) in mice treated with 300 and 2,000 mg/kg. However, other parameters did not show significance compared to the control group (Table 6).
Histopathological observations
The control group showed normal histology of mice liver (Fig. 1A), whereas mice treated with ethanol B. griseipulpureus extracts exhibited hepatocyte shrinkage or degeneration, necrotic and pyknotic nuclei, cytoplasmic hepatocyte vacuoles, accumulation of inflammatory cells around the portal triad, and damage to the endothelial lining of the central vein (Figs. 1B–E).
Control mouse kidney sections showed intact Bowman’s capsule and typical proximal and distal convoluted glomeruli with no capillary congestion or bleeding (Figs. 2A and B). In contrast, the kidney sections of the ethanol B. griseipulpureus extract-treated group displayed histological changes, including glomerular distortion, capillary congestion, hemorrhage, and tubular damage (Figs. 2C–J).
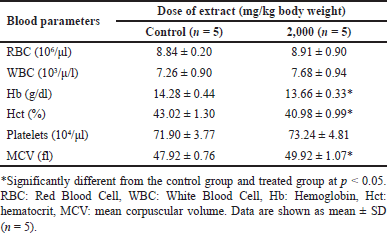 | Table 3. Blood hematological parameters of mice exposed to an acute dose of extracts at various doses. [Click here to view] |
DISCUSSION
The primary objective of evaluating the safety of medicinal plants is to identify any negative effects and determine the level of exposure at which these effects occur. Alterations in the consumption of food, water, and fluctuations in body weight indicate the general well-being of animals under study [18]. Complex biological processes govern food intake to maintain a relatively consistent body weight over an extended period [19]. The appetite is crucial in monitoring food consumption and regulating body weight. In the acute toxicity investigation of B. griseipurpureus extract on male mice, the administration of a single oral dose of 2,000 mg/kg did not result in any abnormal signs or mortality. There were no significant differences in body weight gain compared to the control group (Table 1). However, water intake was reduced, while food intake showed a significant increase (p < 0.05) (Table 1). These findings suggest that the LD50 of the extract is likely higher than 2,000 mg/kg. These results align with previous studies conducted on other plants [20,21] and indicate that the extract can be considered relatively safe according to the globally harmonized system (GHS) system of classification and labeling of chemicals for acute toxicity tests [22]. Therefore, the B. griseipurpureus extract can be classified as category five according to GHS standards.
Subacute toxicity studies are critical in providing further insights beyond the limited scope of acute toxicity data in clinical applications. When dealing with chronic illnesses, it becomes essential to administer repeated doses of substances to assess their toxicity. This is because long-term usage can gradually impact tissues and organs due to accumulation [23]. Subacute toxicity analyses are vital in evaluating the effects on specific organs and examining hematological or biochemical changes caused by extracts that may not be detected in acute toxicity tests. They play a crucial role in ensuring human safety, particularly in the development of pharmaceuticals [15]. In the subacute toxicity analysis of B. griseipurpureus extract in male mice, the extract was continuously administered at doses of 50, 300, and 2,000 mg/kg/day for 14 days. The study employed four dose levels (high, mid, low, and vehicle), which were modified based on the GHS criteria for oral toxicity categories: <5, 50, 300, and 2,000 mg/kg [16,22]. No mortality or abnormal symptoms were observed in the treated mice during the study period. The treated group exhibited a significant increase (p < 0.05) in food intake, while water intake showed no significant decrease compared to the control group (Table 2). There were no significant changes in body weight or relative organ weights between the treated and control groups (Table 2). However, the results of organ-to-body weight indices in the acute and subacute tests showed an increase compared to control and lower dose levels (Tables 1 and 2). Changes in organ weight serve as valuable indicators of toxicity, effects on enzymes, disruptions in physiological processes, and organ-specific injuries [24]. An increase in organ weight suggests hypertrophy, while a decrease indicates necrosis in the target organ. However, it is crucial to interpret organ weight data alongside gross structure pathology and histopathology examinations to obtain a comprehensive understanding [25]. These findings suggest that prolonged use of the extract may harm the liver and kidneys. It is recommended to compare these results (Tables 1 and 2) with histopathological findings (Figs. 1A–E and 2A–J).
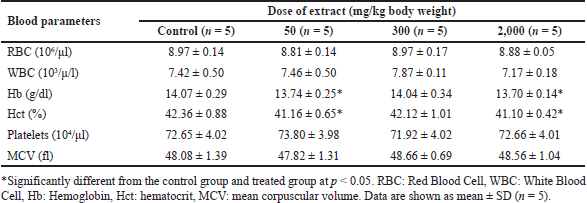 | Table 4. Blood hematological parameters of mice exposed to a subacute dose of extracts at various doses. [Click here to view] |
 | Table 5. Blood biochemical parameters of mice exposed to an acute dose of extracts at various doses. [Click here to view] |
Toxicity studies commonly include hematological tests to evaluate the impact of the test substance on the hematopoietic system, encompassing platelets, WBC, and RBC. These tests provide insights into the effects of the substance on hematopoiesis and the overall functioning of the hematopoietic system [26]. In the present study, the administration of B. griseipurpureus extract led to a significant decrease (p < 0.05) in Hb and Hct values (Tables 3 and 4). These measurements are commonly used to assess anemia, characterized by a reduction in the number of RBCs or the amount of Hb within each RBC [27]. However, all groups of mice showed normal values for other hematological parameters (Tables 3 and 4). Therefore, it is likely that the extract may impact the hematopoietic system. In the acute toxicity studies, the average Hb concentration decreased, while the mean erythrocyte density and measured erythrocyte volume significantly increased (p < 0.05) (Table 3). However, the measured erythrocyte volume in acute toxicity studies showed increased levels without statistical significance (Table 3). These changes in results may be attributed to the production of RBC in response to dehydration and blood loss, indicating an increase in hematopoiesis [28]. Biochemical measurements in the blood serve as valuable indicators of organ function, particularly in vital organs like the liver, pancreas, and kidneys, which are crucial for animal survival [29]. Enzymes such as ALT and AST reflect the cellular integrity of the liver, while total protein and albumin levels indicate its functionality [25]. Elevated serum levels of ALT and AST can indicate liver damage, as hepatocytes primarily produce these enzymes. Increased liver enzyme levels may suggest the potential for hepatocellular toxicity, while decreased levels may indicate enzyme inhibition [30]. However, ALT is a more sensitive marker for liver damage or toxicity compared to AST, as AST is also present in other organs like the kidneys, heart, testes, and skeletal muscles [23]. The standard blood test for kidney function involves measuring levels of CRE, dissolved salts, and urea. Kidney function is assessed through CRE clearance, which reflects the glomerular filtration rate [31]. In the acute toxicity analysis, there was a decrease in CRE levels and an increase in ALT levels, although these results did not reach statistical significance, making their interpretation unclear (Table 5). However, significant differences were observed in the subacute toxicity analysis compared to the control group, with significantly increased (p < 0.05) levels of ALT, AST, and CRE (Table 6). These findings indicate liver and kidney damage and toxicity [32]. Furthermore, significant alterations (p < 0.05) were observed in the levels of ALT, AST, and CRE when comparing our extract to the control group, suggesting that the extract may have harmful effects on the liver and kidneys. Moreover, administration of the extract at doses of 300 and 2,000 mg/kg in mice significantly reduced (p < 0.05) triglyceride levels compared to the control group (Table 6). However, at a dose of 50 mg/kg, the decrease in triglycerides was not statistically significant (Table 6). FBS levels also showed a significant decrease (p < 0.05) at the dose of 300 mg/kg (Table 6). These findings suggest that the extract may improve insulin sensitivity, leading to better glucose uptake and lower FBS levels. The observed reduction in triglyceride levels could be attributed to physical activity and other ethanolic extracts, possibly due to the presence of antioxidant compounds [33]. These results hold importance for future studies.
 | Table 6. Blood biochemical parameters of mice exposed to a subacute dose of extracts at various doses. [Click here to view] |
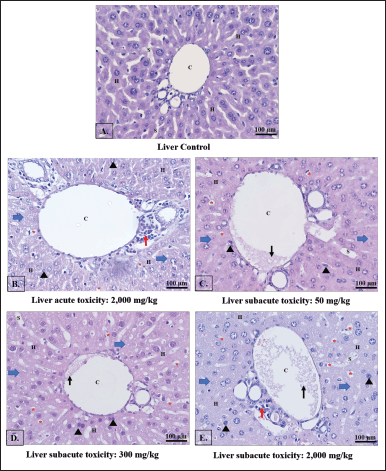 | Figure 1. Light photomicrographs of liver sections were examined to assess the effects of different doses of B. griseipurpureus extract. The control group showed a normal liver appearance (A), while the acute group was treated with 2,000 mg/kg b.w. exhibited changes (B). In the subacute groups, the liver sections of mice treated with 50 mg/kg b.w. (C), 300 mg/kg b.w. (D), and 2,000 mg/kg b.w. (E) displayed distinct characteristics. The key features observed in the images included hepatocytes (H), central veins (C), sinusoids (S), pyknotic nuclei (black arrowheads), increased accumulation of lipid droplets (red asterisks), degenerated hepatocytes (blue arrows), increased accumulation of lymphocyte (red arrows) and congestion of central vein (black arrows). H&E staining ×20 magnification. The scale bar represents 100 μm. [Click here to view] |
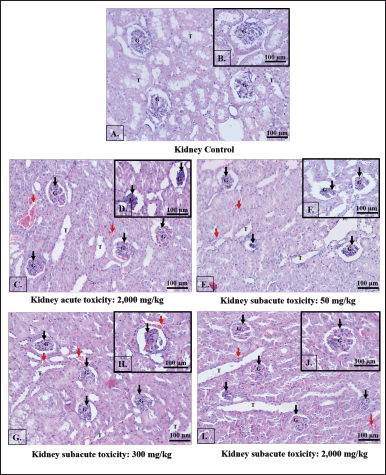 | Figure 2. Light photomicrographs of kidney sections were examined to assess the effects of different doses of B. griseipurpureus extract. The control group showed a normal kidney appearance (A and B), while the acute group was treated with 2,000 mg/kg b.w. exhibited changes (C and D). In the subacute groups, the kidney sections of mice treated with 50 mg/kg b.w. (E and F), 300 mg/kg b.w. (G and H), and 2,000 mg/kg b.w. (I and J) displayed distinct characteristics. The key features observed in the images included renal tubules (T), glomeruli (G), glomerular damage (Black arrows), and capillary hemorrhage (Red arrows). H&E staining ×20 magnification. The scale bar represents 100 μm. [Click here to view] |
Histological investigations provide valuable evidence to complement hematological and biochemical analyses. The examination of all toxicity tests through histopathology (Figs. 1B–E and 2C–J) revealed the presence of lesions and abnormalities in the internal organs of mice treated with the B. griseipurpureus extract. These findings strongly suggest that the extract may possess toxic properties and could be detrimental to the liver and kidneys of mice. These results align with previous studies that have demonstrated the radical activity of plant extracts containing flavonoids, alkaloid saponins, and tannins. Free radicals have the potential to initiate chain reactions, induce protein glycation, deactivate enzymes, and disrupt the structure and function of membranes, including collagen basement membranes, leading to biological damage. Notably, the alcoholic leaf extract exhibited higher efficacy as a stable free radical scavenger compared to the aqueous leaf extract [34,35]. Furthermore, histopathological analysis of the internal organs revealed abnormal characteristics (Figs. 1B–E and 2C–J), consistent with previous research findings [21,34]. These results of subacute toxicity analysis are supported by a statistically significant increase (p < 0.05) in AST, ALT, and CRE levels (Table 6), which are reliable indicators of liver and kidney function [36,37]. Consequently, these findings indicate that the administration of the extract over a period of 14 days leads to significant harm to the kidneys and livers of the treated animals.
CONCLUSION
Based on the available information, the administration of B. griseipurpureus Corner extracts to mice resulted in toxicity, although it did not cause death or noticeable behavioral changes. However, histopathological assessments revealed toxicological alterations in the liver and kidney as a result of the treatment. These alterations included apoptotic nuclei, inflammation in sinusoidal capillaries, dilated sinusoids, expanded interstitial spaces, disruption of glomeruli and tubules, and other changes. Blood analysis of the liver and kidney showed abnormalities, with elevated serum ALT, AST, and CRE levels. The severity of these abnormalities varied depending on the duration and dosage of the administered extract. Consequently, these findings strongly suggest that the B. griseipurpureus Corner extracts may possess toxic properties specifically targeting the liver and kidney. To gain a comprehensive understanding of the nature and mechanism of this toxicity, further research involving higher animal models, chronic studies, and the isolation of secondary metabolites within the extract is necessary.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Research Institute for Health Sciences at Walailak University and the Faculty of Medicine at Princess of Naradhiwas University, Thailand for providing the research laboratory. The authors extend special thanks to MS. Rungruedi Kimseng for her valuable technical support and Dr. Natthapat Srihanant for her assistance in identifying the mushroom.
AUTHOR’S CONTRIBUTIONS
AK was involved in the experimental design, performed the experiments, analyzed, interpreted the data, drafted, critically revised the manuscript, the material supported, and funded. OS contributed to the execution of the experiments, the analysis, the interpretation of the data, and the material supported. PS, GP, and WP contributed to the execution of the experiments, the analysis, and the interpretation of the data. All authors have reviewed and approved the manuscript, figures, and references before submission.
FINANCIAL SUPPORT
This study was granted by the Faculty of Medicine, Princess of Naradhiwas University, Thailand.
CONFLICTS OF INTEREST
The authors declared they have no conflicts of interest.
ETHICAL APPROVALS
All procedures in the experiment were approved by the Animal Ethics Committee, Walailak University (Reference No. WU-ACUC-64039). Date of approval is November 4, 2021.
DATA AVAILABILITY
All the data generated and analyzed are included in this report.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ferreira ICFR, Barros L, Abreu RMV. Antioxidants in wild mushrooms. Curr Med Chem. 2009;16(12):1543–60. doi: https://doi.org/10.2174/092986709787909587
2. Hamza A, Zouari N, Zouari S, Jdir H, Zaidi S, Gtari M, et al. Nutraceutical potential, antioxidant and antibacterial activities of Terfezia boudieri Chatin, a wild edible dessert truffle from Tunisia arid zone. Arab J Chem. 2016;9(3):383–9. doi: https://doi.org/10.1016/ j.arabjc.2013.06.015
3. Zhang JJ, Li Y, Zhou T, Xu DP, Zhang P, Li S, et al. Bioactivities and health of mushrooms mainly from China. Molecules. 2016 Jul;21(7):938. doi: https://doi.org/10.3390/molecules 21070938
4. Seehanan S, Petcharat V. Some species of wild Boletes in Thailand. J Agric Technol. 2008;4(1):109–18.
5. Lau MF, Tajuddin R, Mohd MH, Zakaria L. Identification and factors that affect the growth of the indigenous mushroom, Boletus sp. in Bachok Kelantan, Malaysia. Pertanika J Trop Agric Sci. 2017 Feb;40(1):53–72.
6. Muniandy S, Daud F, Senafi S, Noor MM, Kunaran M, Alwi ANAM, et al. Active compound, antioxidant, antiproliferative, and effect on stz-induced zebrafish of various crude extracts from Boletus griseipurpureus. Malays Appl Biol. 2016;45(1):69–80.
7. Seekhaw P, Rachinta S, Thurnkul N, Surayot P. Determination of total phenolic and flavonoid contents, antioxidant and antibacterial activities of four wild edible mushrooms from the community market in Loei province. PSRU J Sci Technol. 2020;5(3):60–73.
8. Naksuwankul K, Chamnnantap N, Saisong A, Champasit K. Antioxidant activity, total phenolic, flavonoid, and tannin contents from phlebopus colossus (R.Heim) singer and Boletus griseipurpureus Corner crude extract with various solvents. KKU Res J (Graduate Studies). 2023;23(1):132–43.
9. Aung-aud-chariya A, Bangrak P, Lumyong S, Phupong W, Aggangan NS, Kamlangdee N. RNA polymerase II secondary largest molecular identification of Boletus griseipurpureus Corner from Thailand and antibacterial activity of basidiocarp extracts. Jundishapur J Microbiol. 2015;8(3):e15552. doi: https://doi.org/10.5812/jjm.15552
10. Angajchariya A, Naranong S, Phichairat D, Kaewsongsang L, Phupong W, Mahae N. Mycelial growth, antioxidant and antibacterial properties of Boletus griseipurpureus from South Thailand. Int J Agric Technol. 2017;13(4):521–9.
11. Sudjaroen Y, Thongkao K. Screening of nutritive values, in vitro antioxidant, anticancer and antimicrobial activities from Boletus griseipurpureus. Int J Green Pharm. 2017;11(1):174–81.
12. Aung-aud-chariya A, Bangrak P, Dell B, Lumyong S, Kamlangdee N. Preliminary molecular identification of Boletus griseipurpureus Corner from Thailand and its nutritional value. J Agric Technol. 2012;8(6):1991–8.
13. Rai M, Tidke G, Wasser SP. Therapeutic potential of mushrooms. Nat Prod Rad. 2005;4:246–57.
14. Suttisansanee U, On-nom N, Charoenkiatkul S, Thiyajai P, Srichamnong W. The database development of nutritive values, bioactive compounds and functional properties of edible mushroom in Amnat Charoen province [Final Report]. Nakhon Pathom, Thailand: Institute of Nutrition, Mahidol University; 2016.
15. Li Y, Zhuang Y, Tian W, Sun L. In vivo acute and subacute toxicities of phenolic extract from rambutan (Nephelium lappaceum) peels by oral administration. Food Chem. 2020 Aug 1;320:126618. doi: https://doi.org/10.1016/j.foodchem.2020.126618
16. OECD. Guidance document on acute oral toxicity testing. Paris, France: Environment, Health and Safety Publications, OECD. Series on Testing and Assessment No. 24. [Internet]. 2001 [cited 2022 Jan 5]. Available from: https://www.oecd.org/officialdocuments/publicdisplay documentpdf/?cote=env/jm/mono(2001)4&doclanguage=en.
17. Kosala K, Widodo MA, Santoso S, Karyono S. In vitro and in vivo anti-inflammatory activities of Coptosapelta flavescens Korth root’s methanol extract. J App Pharm Sci. 2018;8(9):042–8. doi: https://doi.org/10.7324/JAPS.2018.8907
18. El Hilaly J, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91(1):43–50. doi: https://doi.org/10.1016/ j.jep.2003.11.009
19. Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007 May;48(3):338–44. doi: https://doi.org/10.1016/j.appet.2006.09.013
20. Konan NA, Bacchi EM, Lincopan N, Varela SD, Varanda EA. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.). J Ethnopharmacol. 2007;110(1):30–8. doi: https://doi.org/10.1016/j.jep.2006.08.033
21. Alelign T, Chalchisa D, Fekadu N, Solomon D, Sisay T, Debella A, et al. Evaluation of acute and sub-acute toxicity of selected traditional antiurolithiatic medicinal plant extracts in Wistar albino rats. Toxicol Rep. 2020 Oct 6;7:1356–65. doi: https://doi.org/10.1016/ j.toxrep.2020.10.001
22. Anonymous. Globally harmonized system of classification and labelling of chemicals (GHS). The purple book. New York, Geneva: United Nations Economic Commission for Europe; 2005.
23. Bariweni MW, Yibala OI, Ozolua RI. Toxicological studies on the aqueous leaf extract of Pavetta crassipes (K. Schum) in rodents. J Pharm Phcog Res. 2018;6(1):1–16.
24. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007 Aug;35(5):742–50. doi: https://doi.org/10.1080 /01926230701595292
25. Ugwah-Oguejiofor CJ, Okoli CO, Ugwah MO, Umaru ML, Ogbulie CS, Mshelia HE, et al. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon. 2019 Jan;5:e01179. doi: https://doi.org/10.1016/ j.heliyon.2019.e01179
26. Feldman BF, Zinkl JG, Jain NC. Schalm’s veterinary hematology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 1120–4.
27. Hume R, Dagg JH, Goldberg A. Refractory anemia with dysproteinemia: long-term therapy with low-dose corticosteroids. Blood. 1973;41(1):27–35. doi: https://doi.org/10.1182/blood. V41.1.27.27
28. d’Yvoire MB, Bremer S, Casati S, Ceridono M, Coecke S, Corvi R. ECVAM and new technologies for toxicity testing. In: Balls M, Combes RD, Bhogal N, editors. New technologies for toxicity testing. Advances in experimental medicine and biology. New York, NY: Landes Bio Science and Springer Science+Business Media Inc; 2012. p. 171.
29. Angkhasirisap W, Inala P, Sirimontaporn A, Inpunkaew R, Rungrojejinda K, Kengkoom K, et al. Blood chemistry profiles of outbred Sprague-Dawley rat in the facility of National Laboratory Animal Centre. In: Svasti J, editor. Proceedings of the 28th Congress on Science and Technology of Thailand; 2002 Oct 26-30; Queen Sirikit National Convention Center, Bangkok, Thailand. Bangkok, Thailand: The Science Society of Thailand Under the Patronage of His Majesty the King; 2002.
30. Akanji MA, Yakubu MT, Kazeem MI. Hypolipidemic and toxicological potential of aqueous extract of Rauwolfia vomitoria Afzel root in Wistar rats. J Med Sci. 2013;13(4):253–60. doi: https://doi.org/10.3923/jms.2013.253.260
31. Lum G, Leal-Khouri S. Significance of low serum urea nitrogen concentrations. Clin Chem. 1989 Apr;35(4):639–40.
32. De Oliveira P. Mushroom poisoning. Med Intern. 2009;16:232–8.
33. Amarowicz R, Pegg RB. Protection of natural antioxidants against low-density lipoprotein oxidation. Adv Food Nutr. 2020;93:251–91. doi: https://doi.org/10.1016/bs.afnr.2020.04.002
34. Alebachew M, Kinfu Y, Makonnen E, Bekuretsion Y, Urga K, Afework M. Toxicological evaluation of methanol leaves extract of Vernonia bipontini Vatke in blood, liver, and kidney tissues of mice. Afr Health Sci. 2014 Dec;14(4):1012–24. doi: https://doi.org/10.4314/ ahs.v14i4.33
35. Kaewnarin K, Suwannarach N, Kumla J, Saisamorn L. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase, and hyperglycemic inhibitory activities. J Funct Foods. 2016;27:352–64. doi: https://doi.org/ 10.1016/j.jff.2016.09.008
36. Hall RL. Principles of clinical pathology for toxicity studies. In: Hayes WA, editor. Principles and methods of toxicology. 4th ed. Philadelphia, PA: Taylor and Francis; 2001. pp. 1001–38.
37. Satyanarayana PS, Singh D, Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol. 2001 May;23(4):175–81. doi: https://doi.org/10.1358/mf.2001.23.4.634641