INTRODUCTION
Malaria is a highly prevalent infectious disease caused by Plasmodium. In 2021, the World Health Organization (WHO) reported that there were 241 million malaria cases and 672 1,000 deaths due to malaria, with 90% of deaths occurring due to patients experiencing various complications such as severe anemia, multiorgan dysfunction, acidosis, and Cerebral malaria (CM) [1].
CM is a complication of malaria characterized by decreased consciousness and other neurological symptoms caused by Plasmodium falciparum infection [2]. The P. falciparum parasite has unique characteristics and the capacity to infect red blood cells, causing the infected red blood cells to stick to the inner lining of blood vessels in the brain, leading to sequestration. Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1), located on the outer erythrocyte membrane, facilitates the attachment of infected erythrocytes to brain vascular endothelial cells (ECs) [3]. PfEMP1 binds to various receptors on ECs such as intracellular adhesion molecule-1, vascular cell adhesion molecule-1, CD36, CD31, thrombospondin, chondroitin sulfate A, and cytokine-activated endothelial protein C receptor [4–6]. Infected red blood cell buildup in the brain microvascular blocks blood flow, resulting in hypoxia, coma, and death [7]. Furthermore, PfEMP-1 can trigger signaling pathways in ECs that cause conformational changes in the blood-brain barrier (BBB) resulting in blood leakage [8]. Proinflammatory cytokines (TNF-α, IL-1, IL-6, and IL18) cause brain inflammation in CM, eventually resulting in multiorgan failure and death if not treated immediately [9].
WHO has recommended CM therapy, including specific antimalarial treatment, adjunctive therapy, and supportive care. Emergency treatment for CM aims to swiftly repair disturbed physiological functions and provide active, efficient, and rapid parasiticidal drugs. Currently, artemisinin derivatives (artemether and artesunate) and the cinchona alkaloids (quinidine, quinine, and cinchonine) are administered to treat human CM [2,10]. However, parasite resistance to antimalarial drugs is a major obstacle, so antimalarial drug development is ongoing using natural and synthetic ingredients, such as alkaloids, polyphenols, terpenes, and quinones, secondary metabolites with antimalarial activity [11].
Several studies have shown that apigenin and ursolic acid demonstrate antimalarial activity through various mechanisms in both in vitro and in vivo experiments [12–15]. Apigenin causes glutathione efflux through ATP-binding cassette (ABC) transporters (ABCC1), calcium transporter inhibitors, and inhibits proinflammatory cytokines [16–18] in erythrocytes, whereas ursolic acid, a class of terpenoid acid, inhibits isoprenoid biosynthesis in apicoplasts [19,20]. Due to their anti-inflammatory properties, apigenin and ursolic acid may be used for treating CM by inhibiting the release of proinflammatory cytokines such as IL-6, INF-γ, IL-4, IL-5, and TNF-α [21,22].
The latest malaria treatment is artemisinin-based combination therapy which has been shown to lower cases of chloroquine and sulfadoxine-pyrimethamine resistance [23]. Combination treatments have several benefits, including preventing toxicity and reducing the dose while raising or maintaining the same level of effectiveness [24]. Some successful compound combinations in the field of malaria research are flavonoids (luteolin, apigenin, and quercetin) and combinations of chloroquine and kaemferol, which have been reported to have greater antimalarial activity compared to the single compounds [12,25]. Hence, this study evaluated the combination of apigenin and ursolic acid in inhibiting experimental CM in mice infected with Plasmodium berghei.
MATERIALS AND METHODS
Mice
The mice were male Swiss Webster mice aged 7–8 weeks obtained from the Center for Biological Sciences, Institut Teknologi Bandung. The mice were kept in standard light and temperature conditions and given water and food. The experiments were conducted in compliance with the animal handling guidelines and received approval from the preclinical ethics committee of the Faculty of Pharmacy, Universitas Jenderal Achmad Yani, Cimahi, West Java, Indonesia, Number 9006/KEP-UNJANI/II/2022.
Parasites
The Antwerpen-Kasapa (ANKA) strain of Plasmodium berghei was obtained from the Malaria Laboratory, Faculty of Pharmacy, Universitas Jenderal Achmad Yani, and intraperitoneally injected into Swiss Webster mice. Parasitemia was assessed using thin blood smears stained with Giemsa stain (Merck). The mice in the experimental group were infected with blood containing 1 × 107 P. berghei in 200 μl.
Drug treatment and animal grouping
Apigenin was purchased from Hefei Dielegance Biotechnology Co., China, while ursolic acid was purchased from Chengdu Biopurify Phytochemicals Ltd, China. All compounds were suspended in HPMC (Merck) at 0.5%. The mice were assigned to eleven groups (n = 5 in each group) as follows: the uninfected mice as the normal group; the infected mice that received 0.5% HPMC as the control group; the infected mice that received 20 mg/kg chloroquine; 75, 100, and 125 mg/kg apigenin; 50, 75, and 100 mg/kg ursolic acid; a combination of 50 mg/kg apigenin + 50 mg/kg ursolic acid; and 75 mg/kg apigenin + 50 mg/kg ursolic acid (Fig. 1). The treatment was given orally for 4 days.
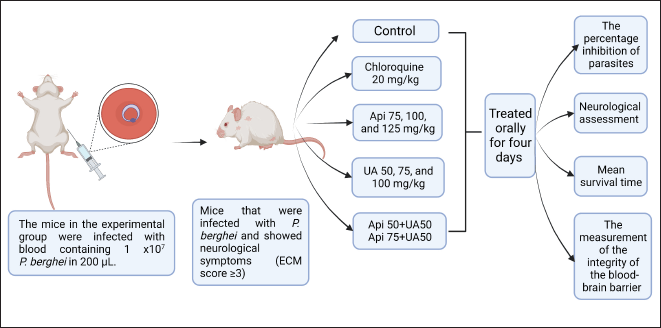 | Figure 1. Design of CM in a murine model. [Click here to view] |
Assessment of parasitemia and neurological symptoms
The neurological symptoms were assessed and scored as follows: 0 = without symptoms; 1 = ruffled fur; 2 = hunching; 3 = wobbly gait; 4 = limb paralysis; 5 = convulsions; 6 = coma. Then the group ECM score was developed in a timeframe [26].
Tail-tip blood was drawn to make thin blood smears, which were then used to check parasitemia daily over 4 days. The blood smear was fixed with methanol, stained with 10% Giemsa for 20 minutes and observed using a microscope (Olympus BX-53) at 1,000× magnification with immersion oil. The percentage parasitemia and percentage parasite inhibition were calculated using the following formula:
Mean survival time (MST)
The group MST was measured using the Kaplan–Meier estimate by calculating the average survival days from the infection until the mice died in one observation for 30 days [27].
Evaluation of BBB integrity
The BBB integrity was evaluated according to the method described by Schmidt et al. [26] with some modifications. On day 4, the mice were injected with 2% Evans blue (EB) dye solution (4 ml/kg) intravenously and after 1 hour, the mice were euthanized using CO2 in a closed container. The mice were dissected to harvest the brains, which were weighed to calculate the brain index using the following equation:
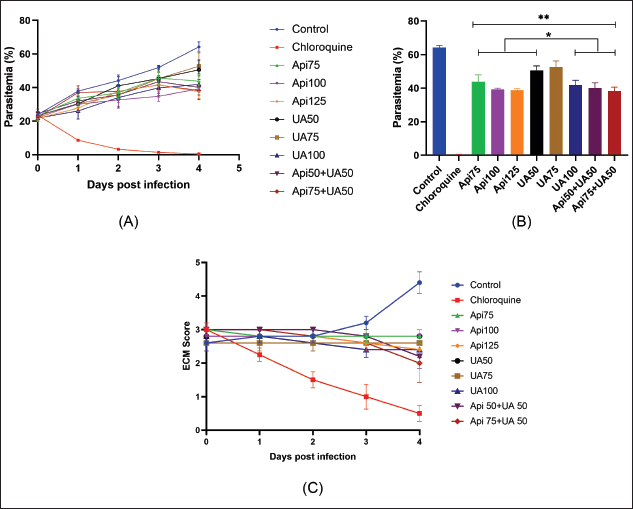 | Figure 2. The level of parasitemia and neurological symptoms in experimental CM after administration of a combination of apigenin and ursolic acid. (A) Parasitemia levels of different experimental groups during 4 days of observation. (B) Statistical analysis of the level of parasitemia on day 4. (C) ECM scores after administration of apigenin and ursolic acid were given separately or in combination. Data are shown as mean ± SEM, n = 5, *p < 0.05 compared to the control group and **p < 0.05 compared to chloroquine. Api = apigenin; UA = ursolic acid; ECM = experimental CM. [Click here to view] |
The brains were placed in a 4 ml container with dimethylformamide and extravasated for 48 hours before the absorbance was measured using a UV-Vis spectrophotometer at 630 nm. An EB calibration curve (0.625, 1.25, 2.5, 5, and 10 μg/ml) was plotted.
Statistical analysis
The data are presented as mean ± standard error and differences were assessed by one-way ANOVA using GraphPad Prism 8.3 software. The Kaplan–Meier method was used to calculate the cumulative long-term survival. A p-value of 0.05 or less was regarded as significant.
RESULTS
Estimating parasitemia and the neurologic symptoms
The parasitemia was assessed daily for 4 days, as shown in Figure 2A and B, and increased in the control group but decreased in the mice administered chloroquine to 0% by day 4. The level of parasitemia in the mice treated with apigenin, ursolic acid, or both apigenin and ursolic acid was significantly different from the control group (p < 0.05). The highest parasite inhibition was observed with the combination of 75 mg/kg apigenin and 50 mg/kg ursolic acid (40.42%). In addition, apigenin at 125 mg/kg and ursolic acid at 100 mg/kg both inhibited parasite growth by 34.78% and 39.64%, respectively. The assessment of neurological symptoms in mice with CM in Figure 2C indicates that the control group had the highest ECM score, with the lowest ECM score in the group given chloroquine. Apigenin, ursolic acid, or the combination of apigenin and ursolic acid treatment resulted in lower ECM scores than the control group, with the combination of 75 mg/kg apigenin and 50 mg/kg ursolic acid scoring lower than apigenin or ursolic acid alone.
Mean survival time
The MST analyzed using the Kaplan–Meier estimate showed that all mice in the control group, those treated with apigenin, ursolic acid, or the combination of apigenin and ursolic acid, had a 100% mortality rate (Fig. 3). However, mice treated with chloroquine had a 100% survival rate up until the end of the observation period. The mice given either apigenin, ursolic acid, or a combination of apigenin and ursolic acid had a longer MST (over 5 days) than the control group. The combination of 75 mg/kg apigenin and 50 mg/kg ursolic acid demonstrated the longest MST, which lasted 7.4 days and was significantly different from the control (Table 1).
Evaluation of BBB integrity
The BBB integrity in Figure 4A shows that all experimental groups displayed considerably different and greater amounts of EB in the brain than the uninfected mice (p < 0.05). The intensity of the color variations in the brain showed differences in EB levels (Table 2). The intact brain in the control group had a higher color intensity of EB deposited in the brain compared to the group given chloroquine, apigenin, ursolic acid, or a combination of both. Chloroquine at a dose of 20 mg/kg and a combination of 75 mg/kg apigenin and 50 mg/kg ursolic acid showed lower levels of EB and were significantly different compared to the control group (p < 0.05). Figure 4B displays the average change in each brain index, showing that the mice treated with chloroquine, apigenin, ursolic acid, as well as apigenin and ursolic acid combination, displayed a lower organ index and were statistically different from the control group (p < 0.05).
DISCUSSION
CM is a neurological complication caused by P. falciparum infection. Since the pathophysiology of CM in humans is still poorly understood, research on in vitro testing and animal models of CM is required. In murine CM, the parasite commonly used is P. berghei ANKA, which can easily infect various strains of mice, such as CBA or C57BL/6, ICR, and Swiss Webster [28]. The development of CM does not solely arise from the sequestration of infected red blood cells within the microvasculature of the brain. The pathogenesis of CM arises from a complex interplay of both parasite-related and host-related factors. However, the underlying mechanism of brain damage could be different. Previous studies on CM in murine models demonstrated the critical involvement of CD8 + T cells in the pathogenesis of brain damage in experimental CM in murine models, which is still unclear in humans [29].
This study’s observed parameters included percent parasite inhibition, neurologic assessment, meantime survival, and BBB integrity test. In the observation parameter of percentage inhibition of parasites by apigenin and ursolic acid, it was seen that there was a correlation between dose and response. A compound with a percentage of parasite inhibition above 30% is considered active [30]. Our findings showed that apigenin, ursolic acid, and the combination had a percentage of parasite inhibition above 30%. So, it can be concluded that apigenin, ursolic acid, and their combination have antimalarial activity. This is in accordance with previous studies which reported that apigenin and ursolic acid have antimalarial activity in a mouse model [13,14].
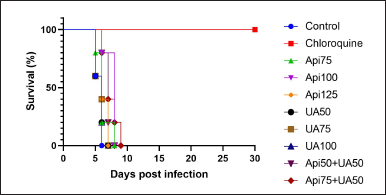 | Figure 3. MST of mice with CM. Survival analysis using the Kaplan -Meier estimate. Api = apigenin; UA = ursolic acid. [Click here to view] |
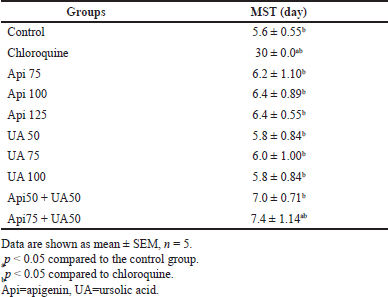 | Table 1. Statistical results of the MST of the different experimental groups. [Click here to view] |
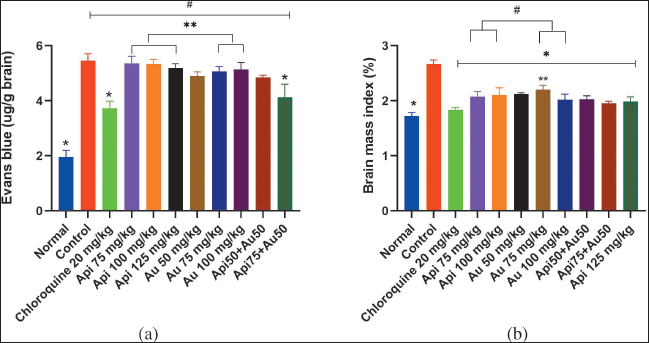 | Figure 4. The BBB integrity was measured using EB dye. (A) The level of EB in mice brains was quantified using a spectrophotometer. (B) Brain mass index of the mice with experimental CM.*p < 0.05 compared to the control group, **= p < 0.05 compared to the group given chloroquine, #p < 0.05 when compared to uninfected mice. Api=apigenin, UA = ursolic acid. [Click here to view] |
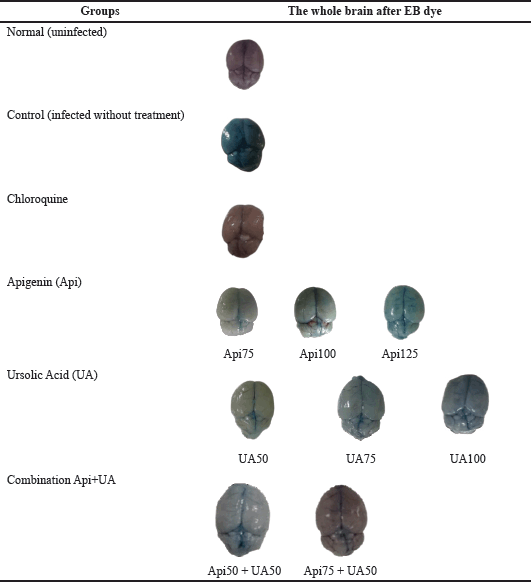 | Table 2. Image of the intact brain in mice with CM to test the integrity of the BBB. [Click here to view] |
In this study, parasitemia in the control group increased daily along with the ECM score. In contrast, in the groups treated with apigenin, ursolic acid, or both, the ECM score decreased daily, in line with the parasitemia suppression induced by apigenin, ursolic acid, or both. However, they did not completely clear parasites like chloroquine.
MST is one of the critical parameters in assessing antimalarial activity, and a substance is considered antimalarial if its MST is longer than the control group [31]. From the results of the MST study in the control group, the average was 5.6 days. Short MST is related to a higher level of parasitemia and is directly associated with infected RBC sequestration, cytoadhesion, inflammation, and endothelial dysfunction, resulting in microvascular obstruction, brain hypoxia, and death [32]. Our findings showed that after administration of apigenin, ursolic acid, or their combination, there was an increase in MST time compared to the control group. This is linked to apigenin and ursolic acid’s capacity to prevent parasite growth and avoid the severity of CM that affects MST. The EB dye injected into the tail vein will bind to albumin and circulate, thus a marker of BBB integrity [33]. If the BBB is damaged, it allows EB to penetrate the brain. The intensity of EB stain deposited in the brain (dark blue) correlates with the severity of CM [34]. In the present study, the control group brain exhibited higher levels of EB and darker color intensity, consistent with the previous studies on BBB changes [35]. Additionally, cerebral edema may occur due to the BBB’s damage. This is shown in the brain index organ of the control group, which has the highest value. The results of this study are in accordance with previous studies, which reported that increased BBB permeability caused by BBB damage was associated with increased brain edema [36]. The group given chloroquine, apigenin, and ursolic acid, as well as the combination of apigenin and ursolic acid, showed lower levels of EB and organ index than the control group.
Apigenin, ursolic acid, and combinations can reduce BBB damage by inhibiting parasite growth. In addition, apigenin and ursolic acid have anti-inflammatory activity, so they are thought to protect against BBB damage. This aligns with previous studies that revealed apigenin could protect the BBB and prevent early brain injury by inhibiting TLR4-mediated inflammatory injury [37]. In contrast, ursolic acid was reported to reduce early brain injury and protect the BBB by reducing oxidative stress [38]. In this study, there was a limitation where no evaluation of proinflammatory cytokines was carried out, which is one factor that plays a role in the pathogenesis of CM. In addition, evaluating the effect of the combination of apigenin and ursolic acid on proinflammatory cytokines in the CM mouse model can be one of the studies of the mechanism of action. Future research on the combination of apigenin and ursolic acid still needs to be developed, considering that this combination has the potential as an adjuvant therapy for CM patients.
CONCLUSION
The combination of apigenin and ursolic acid could reduce the severity of CM in P. berghei ANKA-infected mice. The combination of apigenin and ursolic acid, apart from being able to inhibit parasite growth, can also protect against BBB damage in the CMl mouse model.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for funding this research. We extend our heartfelt gratitude to the Doctoral Program in Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, and the Faculty of Pharmacy, Universitas Jenderal Achmad Yani, for their invaluable support and outstanding facilities that have greatly contributed to the success of this research publication.
FINANCIAL SUPPORT
The funding for this study were provided by the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia and Universitas Padjadjaran.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
The experiments were conducted in compliance with the animal handling guidelines and received approval from the preclinical ethics committee of the Faculty of Pharmacy, Universitas Jenderal Achmad Yani, Cimahi, West Java, Indonesia, Number 9006/KEP-UNJANI/II/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Brian De Souza J, Riley EM. Cerebral malaria: The contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 2002;4(3):291–300.
2. Idro R, Jenkins NE, Newton CRJ. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4(12):827–40.
3. Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF. The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood [Internet]. 2006;108(1):370–8. Available from: http://dx.doi.org/10.1182/blood-2005-11-4624
4. Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11(May):1–29.
5. Avril M, Bernabeu M, Benjamin M, Brazier AJ, Smith JD. Interaction between endothelial protein C receptor and intercellular adhesion molecule 1 to mediate binding of Plasmodium falciparum-infected erythrocytes to endothelial cells. MBio. 2016;7(4):1–10.
6. Lennartz F, Adams Y, Bengtsson A, Olsen RW, Turner L, Ndam NT, et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe [Internet]. 2017;21(3):403–14. Available from: http://dx.doi.org/10.1016/j.chom.2017.02.009
7. MacPherson GG, Warrell MJ, White NJ, Looareesuwan S. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119(3):385–401.
8. Nishanth G, Schlüter D. Blood–brain barrier in cerebral malaria: pathogenesis and therapeutic intervention. Trends Parasitol [Internet]. 2019;35(7):516–28. Available from: https://doi.org/10.1016/j.pt.2019.04.010
9. Dunst J, Kamena F, Matuschewski K. Cytokines and chemokines in cerebral malaria pathogenesis. Front Cell Infect Microbiol. 2017;7:324.
10. Brejt JA, Golightly LM. Severe malaria: update on pathophysiology and treatment. Curr Opin Infect Dis. 2019;32(5):413–8.
11. Tajuddeen N, Van Heerden FR. Antiplasmodial natural products: an update. Malar J [Internet]. 2019;18(1):1–62. Available from: https://doi.org/10.1186/s12936-019-3026-1
12. Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes. 2008;5:1–5.
13. Bero J, Hérent MF, Schmeda-Hirschmann G, Frédérich M, Quetin-Leclercq J. In vivo antimalarial activity of keetia leucantha twigs extracts and in vitro antiplasmodial effect of their constituents. J Ethnopharmacol [Internet]. 2013;149(1):176–83. Available from: http://dx.doi.org/10.1016/j.jep.2013.06.018
14. Amiri M, Nourian A, Khoshkam M, Ramazani A. Apigenin inhibits growth of the Plasmodium berghei and disrupts some metabolic pathways in mice. Phytother Res. 2018 Sep;32(9):1795–802.
15. Beaufay C, Ledoux A, Jansen O, Bordignon A, Zhao S, Teijaro CN, et al. In vivo Antimalarial and Antitrypanosomal Activity of Strychnogucine B, a Bisindole Alkaloid from Strychnos icaja. Planta Med. 2018 Aug 1;84(12–13):881–5.
16. Fallatah O, Georges E. Apigenin-induced ABCC1-mediated efflux of glutathione from mature erythrocytes inhibits the proliferation of Plasmodium falciparum. Int J Antimicrob Agents [Internet]. 2017;50(5):673–7. Available from: http://dx.doi.org/10.1016/j.ijantimicag.2017.08.014
17. Palacz-Wrobel M, Borkowska P, Paul-Samojedny M, Kowalczyk M, Fila-Danilow A, Suchanek-Raif R, et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed Pharmacother [Internet]. 2017;93:1205–12. Available from: http://dx.doi.org/10.1016/j.biopha.2017.07.054
18. Adeoye AO, Olanlokun JO, Tijani H, Lawal SO, Babarinde CO, Akinwole MT, et al. Molecular docking analysis of apigenin and quercetin from ethylacetate fraction of adansonia digitata with malaria-associated calcium transport protein: an in silico approach. Heliyon [Internet]. 2019;5(9):e02248. Available from: https://doi.org/10.1016/j.heliyon.2019.e02248
19. Goulart HR, Kimura EA, Peres VJ, Couto AS, Duarte FAA, Katzin AM. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48(7):2502–9.
20. Heloisa Berti Gabriel RAS, Gabriel HB, Kimura EA, Rodrigo AC Sussmann EAK, Rodriguez AAM, Rodriguez AAM, et al. Terpenes as potential antimalarial drugs. Intech [Internet]. 2016;i(tourism):13. Available from: https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics
21. Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci [Internet]. 2004 [cited 2022 Sep 10];96(3):229–45. Available from: https://pubmed.ncbi.nlm.nih.gov/15539763/
22. Gudoityte E, Arandarcikaite O, Mazeikiene I, Bendokas V, Liobikas J. Ursolic and oleanolic acids: plant metabolites with neuroprotective potential. Int J Mol Sci. 2021 May 1;22(9).
23. Eastman R, Fidock D. ACTs: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7(12):864–74.
24. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81.
25. Somsak V, Damkaew A, Onrak P. Antimalarial activity of kaempferol and Its combination with chloroquine in Plasmodium berghei infection in Mice. J Pathog. 2018;2018:3912090.
26. Schmidt KE, Kuepper JM, Schumak B, Alferink J, Hofmann A, Howland SW, et al. Doxycycline inhibits experimental cerebral malaria by reducing inflammatory immune reactions and tissue-degrading mediators. PLoS One. 2018;13(2):1–20.
27. Bewick V, Cheek L, Ball J. Statistics review 12: survival analysis. Crit Care. 2004;8(5):389–94.
28. Thiengsusuk A, Chaijaroenkul W, Na-Bangchang K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol Res. 2013;112(4):1475–81.
29. Albrecht-Schgoer K, Lackner P, Schmutzhard E, Baier G. Cerebral malaria: current clinical and immunological Aspects. Front Immunol. 2022;13(April):1–11.
30. Gorobets NY, Sedash Y V., Singh BK, Poonam, Rathi B. An overview of currently available antimalarials. Curr Top Med Chem [Internet]. 2017 Feb 23 [cited 2022 Apr 1];17(19). Available from: https://pubmed.ncbi.nlm.nih.gov/28137228/
31. Kumatia EK, Ayertey F, Appiah-Opong R, Bolah P, Ehun E, Dabo J. Antrocaryon micraster (A. Chev. And Guillaumin) stem bark extract demonstrated anti-malaria action and normalized hematological indices in Plasmodium berghei infested mice in the Rane’s test [Internet]. J Ethnopharmacol. 2021;266:113427. Available from: https://doi.org/10.1016/j.jep.2020.113427
32. Cunnington AJ, Walther M, Riley EM. Piecing together the puzzle of severe malaria. End Nostalg Mex Confronts Challenges Glob Compet. 2013;5(211):10–39.
33. Neill AL, Chan-Ling T, Hunt NH. Comparisons between microvascular changes in cerebral and non-cerebral malaria in mice, using the retinal whole-mount technique. Parasitology. 1993;107(5):477–87.
34. Clark IA, Hunt NH, Cowden WB. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988;96(3):579–89.
35. Basir R, Rahiman SSF, Hasballah K, Chong WC, Talib H, Yam MF, et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 2012;7(4):62–74.
36. Ng FC, Churilov L, Yassi N, Kleinig TJ, Thijs V, Wu TY, et al. Microvascular dysfunction in Blood-Brain Barrier Disruption and Hypoperfusion Within the Infarct Posttreatment Are Associated with Cerebral Edema. Stroke [Internet]. 2022 May 1 [cited 2023 Mar 5];53(5):1597–605. Available from: https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.036104.
37. Zhang T, Su J, Guo B, Wang K, Li X, Liang G. Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int Immunopharmacol. 2015 Sep;28(1):79–87.
38. Zhang T, Su J, Wang K, Zhu T, Li X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci Lett. 2014 Sep 5;579:12–7.