INTRODUCTION
Reactive oxygen species (ROS) are widely acknowledged to have a role in several physiological processes of human cells, such as cellular signal transduction, cell division, differentiation, and apoptosis (Snezhkina et al., 2019). ROS generation has been linked to several chronic health issues, including cancer, aging, Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis (Dumont and Beal, 2011). In healthy people, ROS generation could be counteracted by natural antioxidant defense mechanisms. However, in an oxidative stress process, the physiological equilibrium between prooxidants and antioxidants is upset in favor of the former, potentially causing harm to the organism (Unsal et al., 2020).
Dietary antioxidant intake is relevant to disease prevention as it may be a critical method for preventing or postponing the oxidation of vulnerable cellular substrates. Phenolic compounds like tannins, flavonoids, and phenolic acids have drawn consideration due to their potent antioxidant activity. Natural antioxidants are increasingly used to preserve food materials due to health issues (Mirmiran et al., 2022).
A significant number of dietary and therapeutic products nowadays commonly incorporate synthetic antioxidants. These additives, among other actions, prolong the self-life of food products by blocking unsaturated double-bond fatty acids from oxidizing. Antioxidants improve the chemical stability of therapeutic compounds sensitive to oxidative destruction in pharmaceutical goods. The food and pharmaceutical sectors typically utilize synthetic antioxidants, such as propyl gallate, butylated hydroxyanisole (BHA), tert-butylhydroquinone, and butylated hydroxytoluene (BHT) (Mizobuchi et al., 2022). Although there is no evidence that these synthetic antioxidants harm people, the European Food Safety Authority evaluated data on several of these substances in 2012. It developed an appropriate scale for using antioxidants in food by providing updated tolerable daily intakes for human consumption. Due to the toxicity of BHA and BHT, the pharmaceutical industries extensively use ascorbic acid derivatives, thiol derivatives, and sulfuric acid salts as antioxidative agents (Ahmed et al., 2022).
Nigella sativa L., known as black cumin, is a spice from the Ranunculaceae traditionally used to treat eczema, fever, influenza, rheumatism, headache, and bronchitis. It has a long history of using this plant as traditional medicine, particularly in the Arabian Peninsula, North Africa, and the Indian subcontinent (Burdock, 2022). Its fruit comprises 3–7 united follicles and contains some seeds. The seeds are minute, dicotyledonous, angular, tubercular, trigonous, black outer, inner, and mildly aromatic (Goreja, 2003; Warrier et al., 2004).
This plant has numerous therapeutic effects, including diuretics, analgesics, antidiarrheal liver tonics, antihypertensive, digestive, antibacterial, appetite stimulant, and treatment of skin disorders (Ahmad et al., 2013). In addition, it has been reported that the plant seed parts, such as seed oil, essential oil (EO) (Singh et al., 2014), and hexane and acetone extracts (Tiji et al., 2021), possess high antioxidant activity (Bordoni et al., 2019).
Several spectroscopy techniques have confirmed some chemical constituents of N. sativa (Liu et al., 2011). Gas chromatography-mass spectrometry (GC-MS) analysis has revealed some terpenoid components of N. sativa, such as p-cymene, thymoquinone, and γ-terpinene as the dominant volatile component of EO from N. sativa (Albakry et al., 2022). Fatty acids, such as stearic, linoleic, and oleic, have also been detected (Suri et al., 2023). Furthermore, β-sitosterol and Δ5-avenasterol have been reported to be significant sterol components present in N. sativa seed (Rezig et al., 2022). It is yet unknown which phytochemical components play a role in the antioxidant activity of N. sativa.
Thus, this work was carried out to test the antioxidant activity of various extracts and EO of black cumin (N. sativa) seed and to identify their phytochemical profiles using GC-MS and Fourier transform infrared (FTIR). Additionally, to classify the samples and their chemical constituents related to antioxidant properties, a principal component analysis (PCA) was carried out. This study provides important information regarding which extracts or phytochemicals from N. sativa possess antioxidant potential.
MATERIALS AND METHODS
Chemicals and plant materials
Plant materials, black cumin (N. sativa L.) seeds, were bought commercially (CV. Assyifa, Bogor, Indonesia). This plant material was authenticated by Dr. Prayoto Tonoto (Landscape ecologist, Riau Environment and Forestry Office, Indonesia, ORCID ID: 0000-0002-1439-3218). Organic solvents, including methanol, ethanol, distilled water, ethyl acetate, and chloroform, were purchased from Sigma-Aldrich (Singapore, Singapore). Folin–Ciocâlteu reagent, 2,2’-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) aluminum chloride, 2,2-diphenyl-1-picrylhydrazyl (DPPH), acetic acid (CH3COOH), sodium acetate anhydrous, quercetin (QE), and potassium persulfate were also procured from Sigma-Aldrich (Singapore, Singapore).
EO and plant extraction
Black cumin seeds (1,480 g) were immersed and extracted with 80% methanol (3,400 ml) in ambient conditions for 3 days. The liquid phase was filtrated, and it was evaporated by a rotary evaporator under vacuum at 40°C (Q.R. 2005-S, Shimadzu, Tokyo, Japan) and yielded 152.97 g of extract. In contrast, the oil extract was acquired by hydrodistillation the 150 g of black cumin seeds powder in a Clavenger tube following the method reported previously (Andriana et al., 2019). This process yielded around 105 mg of EO, and the EO was kept at 4°C until analysis.
Fractionation of methanol extract
According to a previous report, a 25 g methanol extract of black cumin seeds was fractionated using different polar solvents (Andriana et al., 2023). The methanol extract was placed in a separatory funnel, and then 500 ml of distilled water was added, followed by 500 ml of hexane. The mixture was mixed thoroughly, and then the hexane layer was separated, filtered, and concentrated at 40°C under a vacuum. The same process was conducted on distilled water, chloroform, and ethyl acetate until it yielded 1.18, 0.14, 1.03, and 3.46 g of chloroform, hexane, water, and ethyl acetate extracts. These extracts were stored at 4°C for further analysis.
Antioxidant potentials of N. sativa seed extracts and EO
DPPH assay
With a few minor adjustments, the method reported by Minh et al. (2023) was used to figure out the DPPH free radical scavenging activity of N. sativa extracts and EO. A volume of 1 ml of the sample (10–3,000 ppm) was combined with 1 ml of acetate buffer (0.1 M, pH 5.5) and 0.5 ml of DPPH solution (0.5 mM). The mixture was put at 26°C without light for 30 minutes. After that, the absorbance was read by a spectrophotometer at the wavelength of 517 nm. The inhibition percentage level of the sample on DPPH was calculated by the following formula:
% DPPH = [(AC– AS)] / AC] * 100%, (1)
where AC (absorbance of control) was the reaction’s absorbance when the sample wasn’t present, and AS (absorbance of sample) was when the sample was present.
ABTS assay
The ABTS assay was carried out using a slightly modified version of the method outlined by Minh et al. (2022). The ABTS reagent was obtained by reacting 25 ml of ABTS solution (7 mM) with 25 ml of potassium persulfate (2.45 mM), and then it was kept at ambient conditions in the dark for 16 hours. Methanol was used to dilute the ABTS solution before the test to obtain an absorbance of 0.70 ± 0.05 at 734 nm. A volume of 0.4 ml of extracts or EO (10–3,000 ppm) was combined with 2 ml of the ABTS methanol solution for 30 minutes and then was measured using a spectrophotometer. The antiradical scavenging level was figured out using the following formula:
ABTS Radical Scavenging Activity = [(AC – AS] / AC] *100, (2)
where AC was the reaction’s absorbance when the sample wasn’t present, while AS was the reaction’s absorbance when the sample was present.
Total phenolic contents
With a minor adjustment, the total phenolic contents were adopted from the earlier technique (Iwansyah et al., 2020). Gallic acid was used to make a calibration curve. A volume of 0.2 ml of extract or EO was taken and combined with 1 ml of Folin–Ciocâlteu reagent (10%). A volume of 0.8 ml sodium carbonate (5%) was added after vortexing. The mixture was shaken and rested for 30 minutes in a darkened room. At 750 nm, the absorbances of both the samples and the standards were measured. An mg of gallic acid equivalent (GAE) per 100 g extract represented the total phenolic contents.
Total flavonoid contents
With slight adjustments, the earlier method (Minh et al., 2016) was applied to evaluate the total flavonoid content. Sample (1,000 ppm) and a 2% aluminum chloride-methanol solution were incorporated in 1 ml each. The mixture was then left to rest at room temperature for 15 minutes. A spectrophotometer was employed to measure the mixture’s absorbance at 430 nm. The outcome was mg (QE)/100 g extract.
Identification of functional group by FTIR spectroscopy analysis
The infrared spectra data were collected using FTIR (Bruker Ltd., Vertex-80, Germany). Following the earlier technique, attenuated total reflectance (ATR) was used to gather sample spectra (Iwansyah et al., 2022). The sample was mounted on an ATR crystal and measured at 19°C. The resolution was 8 cm−1 and conducted 32 scans in the mid-infrared range (4,000-650 cm−). Each sample was measured 3 times, and the spectra were recorded in transmittance mode. The obtained spectra were analyzed using OPUS software version 8.5 (Bruker Ltd., Germany).
Identification of chemical components of extracts and EO of N. sativa by GC-MS
Using the prior technique (Andriana et al., 2023) and a GC-MS system (Agilent 7890B Agilent Technology, Inc., Santa Clara, CA, USA), the chemical components of N. sativa were detected. The GC-MS system received an injection of a 1 L sample volume. The column had the following specifications: 30 m in length, 0.25 mm in thickness, and 250 mm internal diameter. It was an HP-5MS Agilent column (19091S-433: 93.92873). Helium was the carrier gas, and the helium inlet was operated in spitless mode at 104 ml/minute total flow, 7.0699 psi of pressure, and a heater temperature of 250°C. The GC oven was maintained at the following temperature: initial hold time was 1 minute at 40°C. The programmed phase is 10°C/minute up to a final temperature of 325°C with a hold time of 4 minutes. The mass scaled between 122 and 1021 amu. Agilent Chem Station software was used to process the data peaks and control the GC-MS system.
Statistical analysis
The statistical analysis of all samples was compared by a one-way analysis of variances (ANOVA) using Metabo Analyst 5.0 Software (https://www.metaboanalyst.ca) with a 1% of significant level. The particle component analysis (PCA) using a correlation matrix was described to evaluate chemical components linked to antioxidant activity. At the same time, the clustering of samples was accomplished using hierarchical cluster analysis. Dendrograms, which are branched structures with a qualitative quality that enable the display of clusters and relationships among samples, are the basis of hierarchical clustering algorithms (Heryanto et al., 2023). The entire linkage method was used to create the clustering patterns. This demonstration performs better when the distance between the samples (points) is calculated using the squared Euclidean approach.
RESULTS
Antioxidant activity of N. sativa seeds
Table 1 shows the antioxidant activity of N. sativa extracts and EO of N. sativa seeds which presented in inhibition concentration (IC50) against ABTS and DPPH free radicals. The EO exhibited the lowest IC50 values for DPPH or ABTS free radicals (189.53 or 17.96 µg/ml, respectively). The IC50 values for DPPH or ABTS free radicals were in order= EO < ethyl acetate < chloroform < water < hexane extracts. The ANOVA revealed that the type of samples affected the radical scavenging activities of N. sativa. The post hoc test by Tukey’s test of each extract was significantly different (p < 0.01) in the DPPH assay. However, in the ABTS assay, IC50 values of chloroform, ethyl acetate, and water extracts were relatively similar. Additionally, the EO presents more antioxidative agents than other samples.
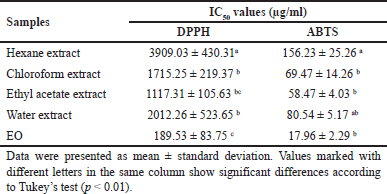 | Table 1. The antioxidant activity (DPPH and ABTS) of N. sativa seed extracts and essential oil. [Click here to view] |
Total phenolic (TPC) and total flavonoid contents (TFC) N. sativa extracts
The TPC and TFC contents of the extracts and EO from N. sativa seeds are displayed in Figure 1. The EO showed the highest amount of total phenolic and flavonoid. The type of samples affected the total phenolic and flavonoid contents of N. sativa extracts and the EO (Tukey’s test, p < 0.05). In line with antioxidant activity, the EO was indicated to contain the most antioxidative agent compared to the other samples. Generally, the hexane extract showed the lowest amount of total phenolic and flavonoid contents (5.95 mg GAE/g extract and 3.02 mg QE/ g extract, respectively).
Identification of functional groups of N. sativa Extracts by FTIR
Figure 2 displays different spectra of N. sativa extracts and EO by a Fourier transport spectrometry spectrum (FTIR). The FT-IR spectrum of EO, ethyl acetate, and chloroform extracts of N. sativa seeds show a strong absorption band in the 3,000–3,700 cm−1 area, indicating the presence of alcohol groups (-OH). Then a strong absorption band is seen in the 2,700–2,900 cm−1 area for hexane, ethyl acetate, and water extracts indicating the presence of the C-H group. The aldehyde group (R-CH = O), characterized by adsorption bands in the area 1,410–1,210 cm−1, were detected for hexane, ethyl acetate, and water extracts with the strongest absorption presence in hexane and chloroform extracts. The strong absorption band near the 1710 cm−1 region is linked to the carbonyl group (C = O). Rohman and Ariani (2013) stated that N. sativa has two peaks responsible for detecting its authenticity, which were 1,750–1,700 cm−1 (carbonyl C = O stretching) and 1,128–1,084 cm−1 (C-O, C-N, and C-C stretching). Figure 3 shows that this carbonyl group is rich in hexane, chloroform, and ethyl acetate extract. The absorption band at wave number 2,924 cm−1 was the absorption of aliphatic C-H, while for 1,657 cm−1, it was the absorption of C = O from carboxylic acids or phenols, strengthened by an absorption band at wave number 1,003 cm−1. Moreover, the absorption band at wave number 1,240 cm−1 presented CH2.
Identification of phytochemical constituents of N. sativa seed extracts and EO by GC-MS
The GC-MS system identified the phytochemical constituents of N. sativa extracts and EO; the results are displayed in Figure 3 and Table 2. In total, 34 compounds, such as monoterpenes, cinnamates, fatty acid methyl ester, alkaloids, and others, have been successfully identified by a GC-MS system. Trans-13-octadecenoic acid, methyl ester, and p-cymene-2,5-diol were the significant components of hexane and chloroform extracts. While for ethyl acetate, water extract, and EO, p-cymene-2,5-diol, 6-octadecenoic acid, methyl ester, (Z)-, and 2,3-dimethylhydroquinone were accounted as the dominant component, respectively.
Chemometric analysis based on GC-MS chemical profiles
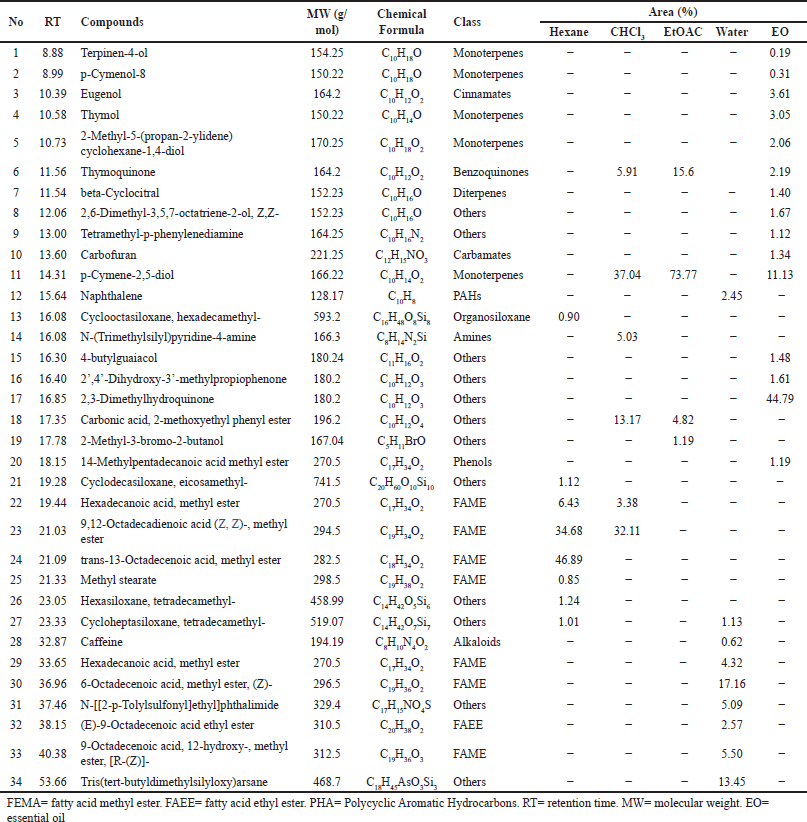
The score plot of PCA analysis based on antioxidant activity, total phenolic, and phytochemical profiles is played in Figure 4. It shows that the cumulative proportion accounted for 99.9% of the overall variation (PC1 = 99.8%, PC2 = 0.1%). This analysis reduced 38 variables observed to become two principal components. The score plot (Fig. 4) indicates the grouping of extract samples and EO of N. sativa. It is shown that the phytochemicals of N. sativa were placed into four quadrants. Water extract was placed in quadrant I, whereas ethyl acetate and chloroform extracts were put together in quadrant II. In contrast, EO and hexane extract were separated in quadrants III and IV. The intersection between the ethyl acetate and chloroform extracts regions was observed due to the similarity in the levels of antioxidant activity of both extracts.
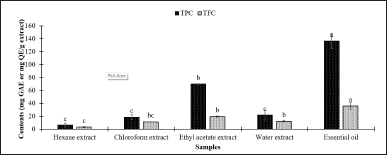 | Figure 1. Total phenolic (TPC) and total flavonoid contents (TFC) of extracts and essential oil N. sativa. Data were presented as means ± standard deviation (SD). Means with different small letters on the same color bar indicated significantly different by Tukey’s test (p < 0.01). [Click here to view] |
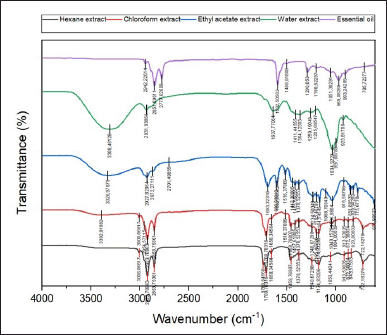 | Figure 2. FTIR spectra of essential oil and extracts of N. sativa seeds. [Click here to view] |
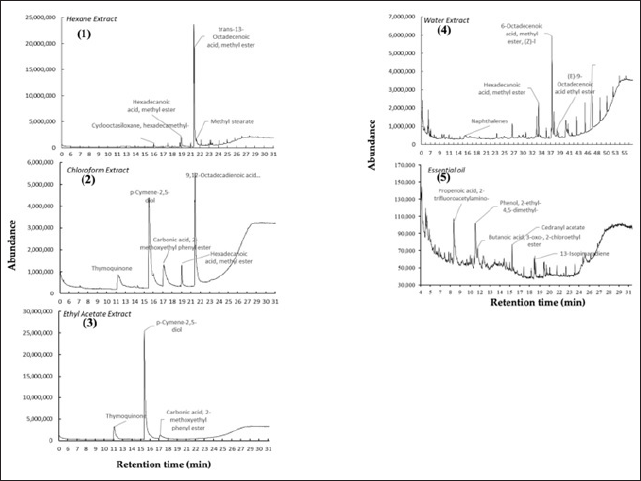 | Figure 3. GC-MS chromatogram of extracts and essential oil N. sativa. (1) = hexane extract, (2) = chloroform extract, (3) = ethyl extract, (4) = water extract, and (5) = essential oil. [Click here to view] |
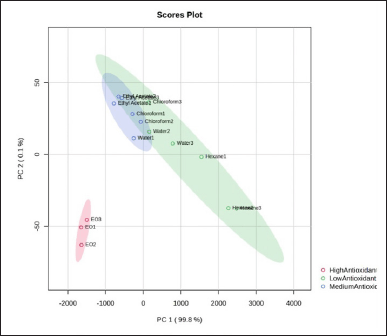
Figure 5 shows the loading plot from PCA analysis of different extracts and EO of N. sativa. Some phytochemical constituents of extracts and EO have a correlation with antioxidant activity, represented by the angle between vectors diverging and forming a large tip (close to 180 degrees). Some compounds belonging to EO such as 2,3-dimethylhydroquinone, thymoquinone, p-cymene2,5-diol, and 2’,4’-dihydroxy-3’-methylpropiophenone were the most discriminating in negative PC1. In contrast, eugenol has the highest discrimination in IC50 values of DPPH and ABTS, meaning the higher the chemicals contents, the higher the antioxidant activity.
Heatmap and hierarchy cluster analysis (HCA) of Extracts and EO of N. sativa
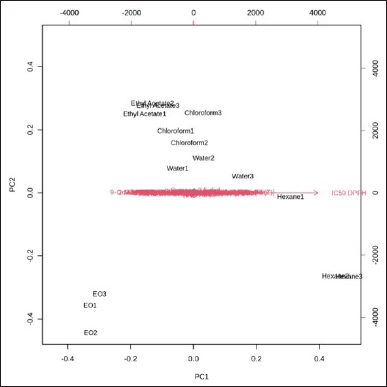
A total of the top 21 compounds with potential antioxidant activity was depicted as a heatmap in Figure 6. The heatmap represented the peak area percentage in GC-MS analysis using the intensity of the color. Based on antioxidant activity assays, EO showed a very high potential for antioxidative agents, presented by low IC50 of DPPH and ABTS values. This result indicated that the antioxidant compounds of N. sativa were more dominant in EO than in the other sample extracts. The compounds of p-cymene-2,5-diol and thymoquinone were recorded to have potent antioxidative agents with medium levels.
An HCA clustering was performed to understand the group of various extracts and EO of N. sativa. Figure 7 shows the HCA dendrogram based on the GC-MS metabolic profiles and their relationship to antioxidant activity. HCA clustered the extracts and EO into three main clusters. The hexane extract was clustered in Cluster I, while EO was clustered in Cluster II. The final cluster, Cluster III, was chloroform and ethyl acetate extracts. The dendrogram confirmed the results obtained from the PCA, revealing the closeness of chloroform and ethyl acetates.
DISCUSSION
This study determined the phytochemical profile and antioxidant activity of different extracts and EO of N. sativa. A PCA was performed to classify extracts or EO related to antioxidant activity. The results showed that the EO of N. sativa possessed the strongest antioxidant activity (IC50 DPPH and ABTS = 189.53 and 17.96 ppm, respectively). The same condition was also found for the total phenolic (123.23 mg GAE/g extract) and flavonoid contents (34.51 mg QE/ 100 g extract) in comparison to other samples (EO> ethyl acetate > chloroform > waster > hexane extracts (Table 1 and Figure 1). Various studies have reported the antioxidant activity of the EO of N. sativa. Burits and Bucar (2000) reported the antioxidant activity of the EO of N. sativa with IC50 DPPH value of 460 ppm and the major component of thymoquinone (27.8%–46.60%) and p-cymene (7.07%–15.53%). Another study conducted by Singh et al. (2014) found that EO and oleoresin of N. sativa exhibited 10–20 ppm in scavenging of IC50 DPPH and thymoquinone (37.6%) and p-cymene (31.4%) was the presence of the dominant component in its EO. In contrast, thymoquinone was the only minor component (2.19%%), and 2,3-dimethylhydroquinone was the significant component 44.79% in this study. Based on PCA results, 2,3-dimethyl hydroquinone might have a role in the antioxidant activity of N. sativa EO. This finding supported the previous study highlighting thymoquinone and 2,3-dimethyl hydroquinone as antioxidative agents (Burner et al., 2000; Kassab and El-Hennamy, 2017). These compounds also exhibit hypolipidemic and hypercholesterolemic effects (Nader et al., 2010).
 | Table 2. Phytochemical constituents of extracts and EO from N. sativa seeds. [Click here to view] |
 | Figure 4. Score plot of PCA analysis based on different samples. [Click here to view] |
 | Figure 5. Loading plot of PCA analysis of phytochemicals constituents of different extracts and EO of N. sativa linked with antioxidant activity. [Click here to view] |
 | Figure 6. Heat map of top 20 phytochemicals from N. sativa in correlation with antioxidant activities. [Click here to view] |
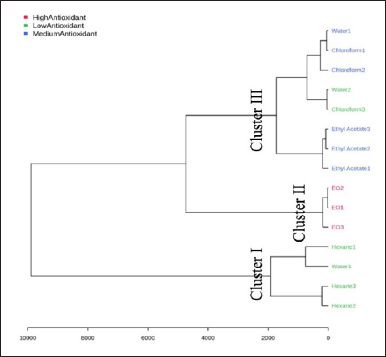 | Figure 7. Dendrogram hierarchy cluster analysis of extracts and essential oil from N. sativa. [Click here to view] |
On the other hand, ethyl acetate and chloroform extracts presented a medium level of antioxidant activity compared to hexane and water extracts, classified as having low antioxidant levels. In GC-MS analysis, ethyl acetate and chloroform extracts contained p-cymene-2,5-diol at a high level (37.04% and 73.77%, respectively). It can be surmised that the level of antioxidant activity of p-cymene-2,5-diol < 2,3-dimethylhydroquinone. Moreover, p-cymene-2,5-diol or hydroquinone is one of the phenol group compounds. Phenol left in the open air quickly changes color due to the formation of oxidation products. The reaction of 1,4-dihydroxybenzene is easily controlled and produces 1,4-benzoquinone, often called quinone (Wall, 1983). However, further investigation is needed to understand better these two compounds’ role in the antioxidant activity of N. sativa.
Furthermore, PCA successfully classified extracts and EO of N. sativa into four groups. EO was placed differently in quadrant II, meaning both antioxidant and phytochemical concentrations of EO differed with hexane, chloroform, ethyl acetate, and water extracts. Ethyl acetate and chloroform were put in the same quadrant due to their similar property, while hexane was arranged in a different quadrant. In this case, the EO of N. sativa was classified as having a high antioxidant property, while hexane was a low one. However, ethyl acetate was still superior to chloroform, water, and hexane extracts. This result agrees with the research conducted by Andriana et al. (2019) that assessed the antioxidant activity of Tridax procumbens and reported that the highest antioxidant activity was found in ethyl acetate compared with n-hexane, chloroform, and water extracts.
CONCLUSION
This research investigated the phytochemical constituents and antioxidant capacity of N. sativa extracts and EO. Compared to chloroform, hexane, water, and ethyl acetate extracts, the EO of N. sativa exhibited the highest antioxidant activity against DPPH and ABTS free radicals and total phenolic and flavonoid contents. The GC-MS analysis revealed that 2,3-dimethylhydroquinone (44.79%), p-cymene-2,5-diol (11.13%), eugenol (3.61%), and thymol (3.05%) were the major constituents of N. sativa EO, which is in line with FTIR analysis that showed terpenoids was the most active group presence in this volatile oil. A PCA successfully classified the tested samples and phytochemical constituents of N. sativa into three groups. EO was classified as a high antioxidant activity group, chloroform and ethyl acetate extracts were medium, and water and hexane were low. Based on the PCA result, 2,3-dimethylhydroquinone might have a role in the antioxidant activity of N. sativa EO. Additionally, PCA revealed that some substances, including 2,3-dimethyl hydroquinone, 6-octadecenoic acid-methyl ester, thymoquinone, and carbonic acid-2-methoxyethyl phenyl ester, positively correlated with the antioxidant activity of N. sativa. However, further study is needed to understand the role of this compound on the antioxidant activity of N. sativa.
ACKNOWLEDGMENTS
The authors thank Advanced Characterization Laboratories Serpong, National Research and Innovation Agency via E- Layanan Sains, and Badan Riset dan Inovasi Nasional for their facilities and scientific and technical support.
AUTHOR CONTRIBUTION
Concept and design were contributed by Y.A.; data acquisition was contributed by L.N.M, N.A.F, N.F., and A.R.S; Data analysis was contributed by J.J., W.S., R.C.E.A., E.A., F.S., and Y.A. preparation and submission of the manuscript were done by Y.A., Y.P.W., and W.S; supervision and final approval were contributed by Y.A., S.D.I, and R.S. All the authors have made a significant contribution to the content of the submitted manuscript; they adjusted it and agree with its publication.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data presented in this study are available on request from the corresponding author.
PUBLISHER’S NOTE
This journal remains neutral regarding jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi, AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed, 2013; 3(5):337–52.
Ahmed MW, Haque MA, Mohibbullah M, Khan MSI, Islam MA, Mondal MHT, Ahmmed R. A review on active packaging for quality and safety of foods: current trends, applications, prospects, and challenges. Food Pack and Shelf Life, 2022; 33:100913.
Albakry Z, Karrar E, Ahmed IAM, OE Proestos C, El Sheikha AF, Oz F, Wu G, Wang X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae, 2022; 8(7):575.
Andriana Y, Xuan TD, Quy TN, Minh TN, Van TM, Viet TD. Antihyperuricemia, antioxidant, and antibacterial activities of Tridax procumbens L., Foods, 2019; 8:21.
Andriana Y, Fajriani NA, Iwansyah AC, Xuan TD. Phytochemical constituents of Indonesian adlay (Coix lacrima-jobi L.) and their potential as antioxidants and crop protection agents. Agrochemicals, 2023; 2(1):135–49.
Bordoni L, Fedeli D, Nasuti C, Maggi F, Papa F, Wabitsch M, De Caterina R, Gabbianelli R. Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes. Antioxidants, 2019; 8(2):51.
Burdock GA. Assessment of black cumin (Nigella sativa L.) as a food ingredient and putative therapeutic agent. Regul Tox Pharm, 2022; 128:105088.
Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res, 2000; 14(5):323–8.
Burner U, Krapfenbauer G, Furtmüller PG, Regelsberger G, Obinger C. Oxidation of hydroquinone, 2, 3-dimethylhydroquinone and 2, 3, 5-trimethylhydroquinone by human myeloperoxidase. Red Report, 2000; 5(4):185–90.
Dumont M, Beal MF. Neuroprotective strategies involving ROS in alzheimer disease. Free Radic Bio Med, 2011; 51(5):1014–26.
Goreja WG. Black seed nature’s miracle remedy. Amazing Herbs Press, New York, NY, 2003.
Heryanto R, Putra CA, Khalil M, Rafi M, Putri SP, Karomah AH, Batubara, I. Antioxidant activity and metabolite profiling of Xylocarpus granatum extracts using gas chromatography–mass spectrometry. Metabolites, 2023; 13(2):156.
Iwansyah AC, Manh TD, Andriana Y, Aiman bin Hessan M, Kormin F, Cuong DX, Xuan HN, Thai HH, Thi YD, Thinh PV, The HL. Effects of various drying methods on selected physical and antioxidant properties of extracts from Moringa oliefera leaf waste. Sustainability, 2020; 12(20):8586.
Iwansyah AC, Andriana Y, Indrianingsih AW, Putri DP. Relationship of antioxidant properties, phytochemical and toxicity profile of wild adlay (Coix lacryma-jobi L. var. Agrotis) extracts: a principal component analysis approach. In AIP Conference Proceedings (Vol. 2493, No. 1, p. 070002). AIP Publishing LLC. 2022, [Online] Available via https://pubs.aip.org/aip/acp/article/2493/1/070002/2827118/Relationship-of-antioxidant-properties (Accessed 23 December 2022).
Kassab RB, El-Hennamy RE. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rats. Egypti J Bas Appl Sci, 2017; 4(3): 160–7.
Liu X, M Abd El-Aty A, Shim JH. Various extraction and analytical techniques for isolation and identification of secondary metabolites from Nigella sativa seeds. Mini Rev. Med Chem, 2011; 11(11):947–55.
Minh TN, Khang DT, Tuyen PT, Minh LT, Anh LH, Quan NV, Ha PTT, Quan NT, Toan NP, Elzaawely AA, Xuan TD. Phenolic compounds and antioxidant activity of Phalaenopsis orchid hybrids. Antioxidants, 2016; 5(3):31.
Minh TN, Van TM, Khanh TD and Xuan TD. Isolation and identification of constituents exhibiting antioxidant, antibacterial, and antihyperuricemia activities in Piper methysticum Root. Foods, 2022; 11(23):3889.
Minh TN, Anh LV, Trung NQ, Minh BQ, Xuan, TD. Efficacy of green extracting solvents on antioxidant, xanthine oxidase, and plant inhibitory potentials of solid-based residues (SBRs) of Cordyceps militaris. Stresses, 2023; 3(1):11–21.
Mirmiran P, Hosseini-Esfahani F, Esfandiar Z, Hosseinpour-Niazi S, Azizi F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci Reports, 2022; 12(1):1504.
Mizobuchi M, Ishidoh K, Kamemura N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem Toxic, 2022; 45(4):1899–906.
Nader MA, El-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res, 2010; 33:637–43.
Rezig L, Martine L, Nury T, Msaada K, Mahfoudhi N, Ghzaiel I, Prost-Camus E, Durand P, El Midaoui A, Acar N, Latruffe, N. Profiles of fatty acids, polyphenols, sterols, and tocopherols and scavenging property of mediterranean oils: new sources of dietary nutrients for the prevention of age-related diseases. J Oleo Sci, 2022; 71(8):1117–33.
Rohman A, Ariani R. Authentication of Nigella sativa seed oil in binary and ternary mixtures with corn oil and soybean oil using FTIR spectroscopy coupled with partial least square. Sci World J, 2013; 2013:740142.
Singh S, Das SS, Singh G, Schuff C, de Lampasona MP, Catalan CA. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). BioMed Res Inter, 2014; 2014:918209.
Suri K, Singh B, Kaur A, Yadav MP. Physicochemical characteristics, oxidative stability, pigments, fatty acid profile and antioxidant properties of co-pressed oil from blends of peanuts, flaxseed, and black cumin seeds. Food Chem Adv, 2023; 2:100231.
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov, GS, Dmitriev AA. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidat Med Cell Long, 2019; 2019; 6175804:17.
Tiji S, Bouhrim M, Addi M, Drouet S, Lorenzo JM, Hano C, Bnouham M, Mimouni M. Linking the phytochemicals and the α-glucosidase and α-amylase enzyme inhibitory effects of Nigella sativa seed extracts. Foods, 2021; 10(8):1818.
Unsal V, Dalk?ran T, Çiçek M, Kölükçü E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv Pharm Bull, 2020; 10(2):184.
Warrier PK, Nambiar VPK, Ramankutty. Indian medicinal plants a compendium of 500 species. Chennai Orient Longman Pvt. Ltd, Chennai, India, 2004.
Wall CR. Approaches to the synthesis of xanthone-1, 4-quinones. The University of Manchester, Manchester, UK, 1983.