INTRODUCTION
Acute lung injury (ALI), is a severe hypoxic respiratory failure allied with substantial morbidity and mortality. It affects approximately 7.2–79 people per 100,000 globally, with a death rate of around 30%–40% (Bian et al., 2021). ALI predominantly affects pulmonic capillary endothelial and epithelial cells resulting from severe infections, traumatic injuries, or major surgeries. ALI develops acute respiratory distress syndrome (ARDS), resulting in persistent pulmonary dysfunction and continued necessity of mechanical ventilation in the intensive care unit, high vulnerability to multi-organ dysfunction, and mortality (Sun et al., 2011). Conventional treatment of ALI includes supportive care methods such as antibiotic treatment, low-tidal-volume ventilation, and fluid restrictions (Lu et al., 2020). In spite of improvements in support measures and ventilator supervision, the death rate from ALI is disappointingly high. Also, the majority of the survivors suffer from ARDS which in turn leads to social and economic burdens (Li et al., 2012). Thus, it is important to explore other effective approaches to improve the immune responses against ALI.
Recently transplantation of mesenchymal stem cells (MSCs) was evolved as a new therapeutic process to reduce mortality and mitigate ALI. MSCs have low immunogenicity and are considered ideal candidates for xenotransplantation. MSCs are pluripotent and can differentiate into several types of other cells and have the potential to restore injured tissues (Jiang et al., 2021). They can be obtained from numerous tissues like bone marrow, umbilical cord blood, umbilical cord tissue, adipose tissue, amniotic fluid, fetal lung, and fat tissue (Brown et al., 2019). The low immunogenic properties and immunoregulatory benefits are not dependent on the source of tissue. Owing to all these properties MSCs have enticed huge consideration as a promising approach in cell therapy for several disorders such as pulmonary injury, myocardial infarction, a few neurodegenerative conditions, and acute kidney failure (Tavakoli et al., 2020). Among the numerous stated stem cell sources, MSCs derived from the human umbilical cord are being preferred for cell therapy due to the following advantages (i) lack of ethical issues, (ii) readily available, (iii) easy isolation, (iv) abundant expansion capability, (v) low-risk of viral contamination, and (vi) painless procurement from donors (Nagamura-Inoue and He, 2014). Also, compared with the BM-MSCs, the UC-MSCs proliferate rapidly and secrete several elements to produce an immunosuppressive environment. Few studies reported the efficacy of UC-MSCs in the abatement of bleomycin-induced pulmonary fibrosis (Zhang et al., 2017), lupus (Cheng et al., 2021), colitis (Li et al., 2020), and arthritis (Vohra et al., 2020) owing to their immunomodulation, anti-inflammation, anti-oxidation, and anti-apoptosis properties. In recent times, few studies reported the efficacy of MSCs in alleviating LPS-induced ALI by repairing lung activity and decreasing mortality. As a multi-factorial ailment, ALI is highly associated with the immune-responsive and inflammatory processes. The key reason for ALI is Gram-negative bacterial infection stimulating the immune response by its component LPS (Wang et al., 2011).
MSCs have been intensely investigated in the last few decades. However, the mainstream listed clinical trials employing MSC treatment for a range of ailments have fallen short of anticipations, despite the promising results in pre-clinical trials in various animal disease models. This is ascribable to inconsistent conditions for MSCs characteristics across studies and their innate heterogeneousness. Additionally, the challenges of industrial scale-up and good manufacturing practice (GMP) compliance affected hassle-free and expedient evolution to clinical developments. In this regard, the therapeutic potential of GMP-complaint pooled UC-MSCs cultured in large-scale in single use bioreactor was evaluated in an LPS-induced rat model of ALI to deliver a basis for employing this intervention in clinical practices. To guarantee safety, thorough donor screening, and testing were carried out. Also, a comprehensive set of GMP-compliant quality and safety measures were practiced throughout the production of hUC-MSCs.
MATERIALS AND METHODS
Isolation and culture of hUC-MSCs
The production of hUC-MSCs was carried out in an advanced clean room following GMP-complaint regulations. The utility system comprised 560 square feet of clean room production area, a quality control lab, storing room, and cold storage area. Human umbilical cord tissues were collected from pregnant females (gestational age: ≥37 weeks) with informed consent from the donors and as per the norms of the institutional ethical committee (Ref: 135/IRB-IBSEC/SIST). All the donors were thoroughly assessed for donor eligibility considering clinical background, physical evaluation, blood tests for transfusion contagious infections along with karyotyping and gene profiling. A complete description of the isolation, culturing, harvesting, and characterization of UC-MSCs is reported in our previous publication (Padhiar et al., 2022). Briefly, UC tissues were washed with VOA antibiotic solution comprising vancomycin (Gland pharma: 50µg/ml), amphotericin-B (Himedia: 250µg/ml), and ofloxacin (Ranbaxy: 100µg/ml). Wharton’s jelly was scraped, cut into 5-7 mm fragments, and cultured in a 60 mm dish (Corning Inc.) containing 5 ml of culture medium, for 3 d at 37°C, and 5% CO2 (ThermoHeracell™-240i incubator). At 70% to 80% confluency, harvesting of cells was carried out by trypsinization (TrypLE express 1X, Gibco), and cryopreserved (P0) in 10% DMSO (Origen) with a minimum density of 1 × 106 cells/ml. Expansion of recovered cells was carried out in T225 culture flasks (Corning Inc.) until 50% to 60% confluency was achieved. At this phase, for cryopreservation pooling of cells from three different donors was done in a 1:1:1 ratio and a master cell bank was prepared with a cell density of 5 × 106 cells/ml (P1). The additional expansion was carried out in hyper flasks (Corning Inc.) and the cells were cryopreserved as working cell banks (WCB) with a density of 5 × 106 cells/ml (P3).
Later, to prepare an investigational medicinal product, cells from WCB were retrieved and inoculated in Eppendorf BioBLU®-3c bioreactor amended with 94 g of Star-Plus microcarriers (Pall Corporation). After inoculation (3,000 cell/cm2), static culture conditions were maintained for 24 hours while shaking intermittently for 1 minute at 20 rpm in 1 hour intervals, and later constant agitation was carried out at the minimum rate for suspension assessed to be 65 rpm. Harvest of cells was performed when the concentration reached 5 to 6 × 105 cells/ml. Separation of UC-MCs was carried out using a 70µ strainer (Thermo Fisher Scientific) and the requisite concentration (30×) was achieved by tangential flow filtration unit (Repligen). Cryopreservation of cells was done at a density of 10 × 106 cells/ml with 45% plasmaLyte (Baxter International), 45% human serum albumin (Baxter International), and 10% DMSO in 20 ml cryovials (Aseptic technologies, Belgium).
ANIMAL WELFARE AND HUSBANDRY PRACTICES
All the practices of this research were in agreement with the guiding principles set by the Committee for the Purpose of Control and Supervision of Experiments on Animals as published in The Gazette of India, December 15, 1998. All the studies were carried out by following Good Laboratory Practices for toxicity test regulations set by National Good Laboratory Practice Compliance Monitoring Authority. The experiments have been REPORTED following/in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments). A total of 20 male Sprague-Dawley rats were acquired from National Institute of Bioscience, Pune. The rats were permitted to acclimatize for a minimum time of 3 days at facility conditions (22°C ± 3°C, 30% to 70% relative humidity, and 12 hours light/dark photoperiod). Maximum 3 animals per cage were housed in clean, sterilized polycarbonate cages (41.0 cm × 28.2 cm × 15.2 cm). For easy identification, rats and cages were tail marked by using different color cage cards. Animals were fed ad libitum standard pelleted laboratory animal diet and reverse osmosis water in autoclaved polypropylene bottles. During the in-life phase of the experimental period air was changed 10-15 times per hour. Rats were inspected at least once every day for medical signs and twice every day for morbidity and mortality.
EXPERIMENTAL DESIGN
To instigate ALI, the rats were assembled into 4 different groups (G1 to G4) with 5 rats per group dependent on body weights through physical zig-zag manner. The mean body weight variation of rats across the groups did not exceed ± 20% of the mean body weight. After randomizing, particular rats were recognized by animal number on the base of the tails. G1 was a control group and the other groups G2, G3, and G4 received Lipo polysaccharide (LPS) (Sigma), low-dose UC-MSCs, and high-dose UC-MSCs, respectively. While administrating the dosage on the day of dose administration, the test item vial (UC-MSCs – 100 million cells/10 ml) was removed from the liquid nitrogen canister and was immediately thawed by using the preheated water bath (37°C) (Biotechnics; BT147), and swirled gently. After thawing, the vial was wiped immediately with disinfectant. For the preparation of stock, 10 ml of the vehicle (20% human normal albumin; Reliance Life Sciences, India) was thawed in preheated water bath (37°C) transferred to the test item vial and was gently mixed to obtain the desired cell concentration of 4 million cells/ml which was then used for dose administration.
The rats of group G2 were induced by intratracheal (IT) administration of LPS (2.5 mg/kg) dissolved in normal saline while maintaining the concentration at 5 mg/ml. Normal saline was instilled via intra-tracheal route in rats of group G1. Four hours after induction of LPS all animals from group G3 and G4 were treated with the test item at a dose of 2 × 106 cells/kg and 10 × 106 cells/kg respectively by intravenous route (IV). Details of the dose formulation to different treatment groups is shown in Table 1.
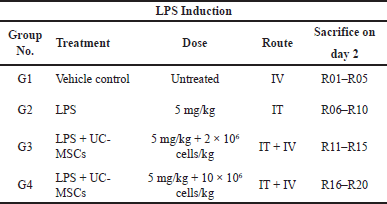 | Table 1. Dose formulation to different treatment groups. [Click here to view] |
OBSERVATIONS
Clinical signs, mortality, body weight, and temperature
Immediately after the dose administration of LPS and test item all the rats were observed carefully for clinical signs and mortality. Furthermore, after 4 hours of test item administration all the rats were examined. Body weights of all the rats were recorded prior to the LPS induction and before the terminal sacrifice. Similarly, the body temperature of all the rats was recorded using digital infrared thermometer gun (Testo 830-TI), before dose administration of LPS as well as before and 4 hours post dose of the test item.
Immunogenicity parameters
After 24 hours of test item dose administration, rats were sedated with the help of ketamine (Themis Medicare) and xylazine (Indian Immunological Limited). Later, blood was collected from retro-orbital sinuses in tubes without any anticoagulant which were processed for separation of serum. Serum samples were evaluated for immunogenicity parameters. Cytokine levels such as Interleukin-6 (IL-6), Interleukin 1 beta (IL-1β), Tumor Necrosis Factor-α (TNF-α), growth factors rat hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1) in the serum were determined in all animals by enzyme-linked immunosorbent assay (ELISA) as per kit instructions. Rat ELISA kits IL-6 (ELR-1L6), IL-1β (ELR-1L1b), TNF-α (ELR-TNFa), HGF (ELR-HGF), and IGF-1 (ELR-IGF1) were procured from RayBio® (RayBiotech Life, Inc, Georgia). To analyze the neutrophil infiltration, myeloperoxidase (MPO) activity in lung homogenates was assayed using the MPO Calorimetric Assay Kit (Elabscience, Hangzhou, China) as per kit instructions. All animals were sacrificed, by using thiopental sodium. Following sacrifice, lungs were collected and weighed.
Broncho-alveolar lavage fluid (BALF) analysis
Broncho alveolar lavage fluid analyses were performed for all surviving animals using right lung.
All the animals were anesthetized using isoflurane and were placed on back to open the abdominal cavity. They were then exsanguinated via aorta abdominalis and the thoracic cavity was dissected to expose the lungs. BALF was collected with PE-90 tubing connected to 19 gauge needle hub after flushing the trachea with 30 ml/kg of cold saline back and forth. Aspirated BALF was collected and subjected to centrifugation at 3,000 rpm at 4°C for 10 minutes. The collected BALF fluid was centrifuged and the supernatant was used for the estimation of differential leucocyte count (alveolar macrophages, neutrophils, lymphocytes, and eosinophils), lactate dehydrogenase (LDH) (Erba LDH-P kit; DGKCH method) and total protein content (Erba Total Protein kit; Biuret method).
Lung histopathology
The remaining lung lobes from all animals were fixed in neutral buffered formalin (Himedia), embedded in paraffin (Histofin), and 3 to 5 µm thickness sections were cut. The sections were stained with hematoxylin and eosin (H&E) and were inspected under a microscope (Labomed LX300). The H&E stained lung sections of all the rats were observed for inflammatory changes such as alveolar congestion, hemorrhage, neutrophil infiltration, and interstitial edema.
Statistical analysis
All the individual data were summarized in terms of groups to obtain mean and standard deviation. The body weight, BALF, immunogenicity evaluation, and organ weight data was analyzed using unpaired t-test when comparing between G1 (vehicle control) and G2 (disease control) group of rats. One-way ANOVA test followed by Dunnett’s test was done while comparing G2 rats with G3 and G4 rats. The analysis was done using Graph Pad Prism (Version 7.03). All analyses and comparisons were evaluated at the 5% (p < 0.05) level in comparison with control.
RESULTS
Development of LPS-induced ALI
Following LPS induction the rats from groups G2, G3, and G4 showed clinical signs of systemic illness such as lethargy, forced expiration and redness of nose, reduction in body weight, increased body temperature, and increased lung weights. No mortality or morbidity was observed in any of the experimental rats from G1 to G4. Immediately after administration of LPS all the rats were observed with forced expiration. 4 hours post dose, all the rats (except from G1 group) were observed with lethargy, forced expiration, and redness of nose at minimal severity. The rats of groups G2 and G3 were continued to be observed with similar findings from 4 hours post dose till terminal sacrifice (24 hours post dose). Before terminal sacrifice animals from G4 were observed with forced expiration and redness of the nose. Induction of LPS also triggered severe systemic inflammatory response as evident from the higher serum concentrations of pro-inflammatory mediators TNF- α, IL-1β, and IL-6 as compared with those of control group animals indicating the successful development of ALI model in rats. Gross pathological observations did not reveal any gross lesions in any of the rat from group G1 to G4.
Body weights, body temperatures and lung weights
The body weights of rats of group G1 were found to be normal throughout the experiment. Group G2 animals showed statistically significant decrease in body weight on day 2 when compared to control (G1) animals. However, both G3 and G4 group animals did not exhibit a significant decrease in body weights when compared to G2 animals (Fig. 1).
The body temperature of G1 group animals did not show any difference in temperature throughout the study period. On the other hand, intra-tracheal instillation of LPS exhibited increased body temperature in the rats of groups G2, G3, and G4. However, animals from G3 and G4 groups treated with UC-MSCs showed a trend towards lowering of body temperature 4 hours post dose (Fig. 2).
Compared to control, there was significant increase in absolute and relative lungs weights in group G2 rats. Whereas compared to G2, there was dose dependent non-significant decrease in absolute and relative lung weights in G3 and G4 groups (Fig. 3)
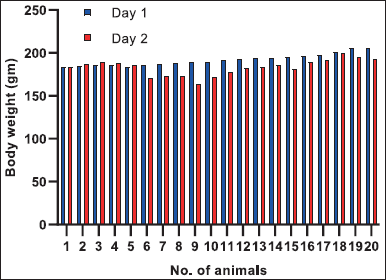 | Figure 1. Individual Body weights of rats. Group 1:1–5 animals, Group 2:6–10 animals, Group 3: 11–15 animals and Group 4:16–20 animals. [Click here to view] |
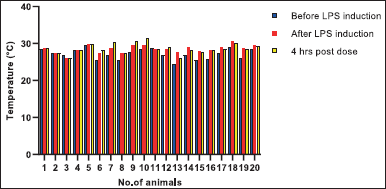 | Figure 2. Individual body temperatures of rats. Group 1:1-5 animals, Group 2:6-10 animals, Group 3 11–15 animals and Group 4: 16–20 animals. [Click here to view] |
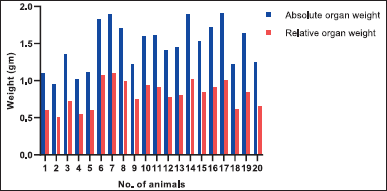 | Figure 3. Absolute organ weights and organ weights relative to terminal body weights of study rats; Group 1:1–5 animals, Group 2:6–10 animals, Group 3 11–15 animals and Group 4: 16–20 animals. [Click here to view] |
Lung histopathology
Histological evaluation of lung sections 24 hours post administration of LPS showed apparent inter-alveolar septal thickening, and inflammatory infiltration in alveolar septa and alveolar space (Fig. 4). Infiltration was not observed in alveolar space of control group. Treatment with a low dosage of UC-MSCs decreased the infiltration of neutrophils in interstitial space. Also infiltration in alveolar space and deposition of proteinaceous debris was minimum. On the other hand treatment with a high dosage of UC-MSCs showed dramatic reduction in the inflammatory infiltration of neutrophils in alveolar space and proteinaceous debris deposition.
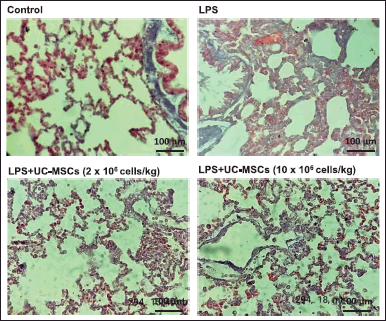 | Figure 4. Histological evaluation of the effect of UC-MSCs on LPS-induced lung injury. Control: No infiltration in alveolar space; LPS treated: Note thickening of alveolar septa, infiltration of neutrophils in alveolar septa and alveolar space; Low Dose: Note reduced infiltration of neutrophils in interstitial space, minimal infiltration in alveolar space and proteinaceous debris; High Dose: Note reduced infiltration of neutrophils in alveolar space and proteinaceous debris. MT, 40×; Scale: 100 µm [Click here to view] |
Immunogenicity parameters
To further assess the anti-inflammatory effects of UC-MSCs pro-inflammatory cytokine and chemokine concentrations were measured in BALF. Pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 are considered markers of acute inflammatory reaction and are known to play a vital role in the pathophysiology of ALI (Bhatia and Moochhala, 2004). Challenging the rats with LPS leads to increased oxidative stress and triggers the discharge of pro-inflammatory cytokines by the airway epithelial cells and alveolar macrophages as a result serves as a model of ALI (Dong and Yuan, 2018). Results indicate that cytokine (IL-6, IL-1β, TNF-α) levels were elevated in the BALF in the animal groups induced with LPS in comparison with untreated naive rats. An apparent decrease in the cytokine levels was observed in the groups treated with UC-MSCs which indicates the attenuation of ALI-induced cytokines by UC-MSCs transplantation. However, no significant difference was noticed in the cytokine levels between the groups G3 and G4 which received low and high doses of UC-MSCs, respectively (Fig. 5).
Also, a significant increase in myeloperoxidase (MPO) activity was observed in group G2 compared to G1 group. MPO is an enzyme that gets secreted from the neutrophils and hence the quantity of MPO positive cells is directly proportional to the buildup of neutrophils and thus serves as an inflammatory index (Rehring et al., 2021). There was a significant decrease in IGF-I concentration in group G2 compared to group G1. Treatment of UC-MSCs revealed that there is dose-dependent decreasing trend in MPO activity and dose-dependent increasing trend in IGF-1 activity compared to group G2 animals. This resembles the probable pharmacological property of test item in reducing the MPO activity (Mashhouri et al., 2020). The level of IGF-1 in the blood is directly proportional to pulmonary fibrosis (Hung et al., 2013). The reduction in IGF-1 level in animals from G3 and G4 compared to G2, is considered to be efficacious property of test item. Similarly, a slight increase in HGF concentration was observed in rats of the G2 group when compared to G1 rats. HGF circulates in the lungs under pathological conditions of ALI and displays persistent barrier-defensive effects on human pulmonary endothelial cells (Yang et al., 2015). Treatment with UC-MSCs (10 × 106 cells) in G4 rats showed a slight decrease in HGF concentration when compared to G2 rats (Fig. 5). Decreased HGF expression is directly related to pulmonary fibrosis (Panganiban and Day, 2011). During pathogenesis, lymphocytes and neutrophils play an important role in the host defense mechanism, however, they are also accountable for pulmonic injury (Tirunavalli et al., 2021). Lymphocytes produce several cytokines, which in turn trigger the endothelial cells and thereby facilitate the movement of inflammatory cells into tissue. LPS causes a shift in hematological factors (Ali et al., 2020). Results indicate a slight increase in lymphocytes, neutrophils, and macrophages in LPS-challenged rats (G2) when compared with control (G1) and the levels were similar in those treated with UC-MSCs (G3 and G4) (Fig. 6). There was no significant difference as the quantification was done after 12 hours of treatment. UC-MSC treatment suppressed the LPS-induced accumulation of neutrophils. Total protein levels were also estimated in the BALF to assess the integrity of the alveolar capillary membrane barrier and evaluate pulmonary vascular leakage as a marker for ALI. Evaluation of total protein and LDH contents showed that there is a marked decrease in total protein content in the G2 group from the control group. In the G3 group animals it still decreased however in the G4 group it showed a trend toward increasing total protein contents. LDH content of the G1 group was higher than those of the G2 group. When it was compared with the G2 group, LDH contents of G3 and G4 were markedly reduced. Evaluation of the smear of BALF for differential cell count did not reveal any significant changes.
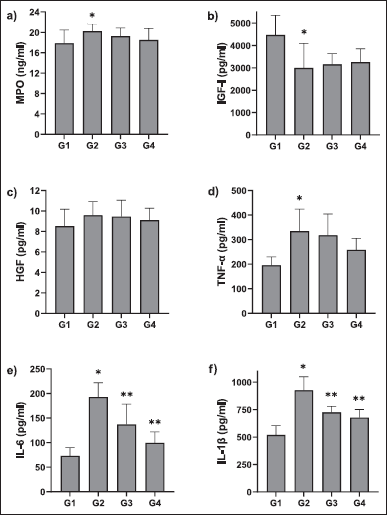 | Figure 5. The effect of UC-MSCs on BALF cytokine levels in rats; *statistically significant from G1; **statistically significant from G2 (p < 0.05); values are means + SD; n = 5 [Click here to view] |
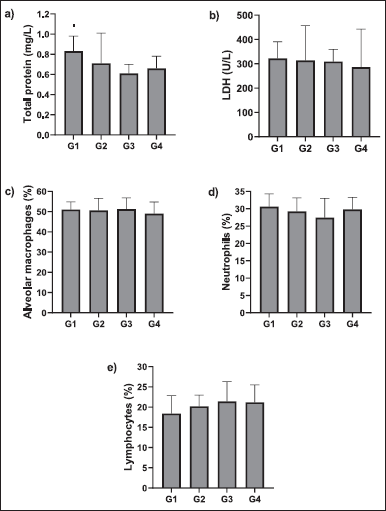 | Figure 6. Effect of UC-MSCs on protein content, LDH, and percentage of macrophages, neutrophils, and neutrophils in BALF; values are means + SD; n = 5 [Click here to view] |
DISCUSSION
In this study, an LPS-induced lung injury model was developed in rats to demonstrate the therapeutic use of UC-MSCs on ALI. Test group rats were administered with LPS to induce lung injury. LPS is the main constituent of the external membrane of Gram-negative bacteria and it stimulates inflammatory reactions by entering the bloodstream (Giordano et al., 2020). LPS triggers the activation of monocytes and macrophages and discharges a huge amount of inflammatory cytokines in several cell types through toll-like receptor 4 signaling which leads to an acute inflammatory response (Tucureanu et al., 2018). The induction of ALI in rats via the intra-tracheal injection of LPS was established in line with previous publications (Li et al., 2022). It simulated the sepsis-induced lung injury which was confirmed by the severe inflammatory responses such as neutrophil accumulation, the release of excess pro-inflammatory cytokines, lung-tissue hyperemia, hemorrhage, alveolar septal thickening, infiltration of inflammatory cells, and MPO activity. These interpretations specified that the animal model was efficacious.
In recent years, human MSCs are being extensively studied in various animal models for their efficacy in the treatment of ALI. MSCs alleviate ALI by normalizing lung function owing to their anti-inflammatory, anti-apoptosis, and immune-regulatory characteristics (Park et al., 2018). The results of this study indicated the efficacy of UC-MSCs in the mitigation of ALI. The lung histology showed a substantial enhancement in UC-MSCs administered rats compared to the control group. The neutrophil and lymphocyte infiltrate diminished in the lung. Earlier studies reported that lung injury caused by LPS instillation is triggered by a rapid influx of neutrophils into the air gaps followed by severe inflammation within a few hours (Grommes and Soehnlein, 2011). Neutrophils and alveolar macrophages are considered to be the most common etiological agents for lung damage. Neutrophils accumulating in the lungs clog the pulmonary capillary bed mechanically, disrupting microcirculation. Furthermore, the metabolic substances of stranded and active neutrophils damage and enhance the permeability of the alveolar-capillary barrier. As a result, pulmonary edema develops from the leakage of protein-rich fluid into the alveolar lumen and interstitial lung (Park et al., 2019). In this study, rats intravenously infused with UC-MSCs had fewer neutrophils in BALF than LPS-injected rats. Furthermore, the findings of the lung weight test revealed that the pulmonary edema had improved.
The immune system generates antibodies, employs inherent inflammatory cells at the site of infection in the presence of LPS, and produces pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which in turn trigger inflammatory reactions cascade, which play a key role in the pathophysiology of the inflammatory process in ALI and contribute to the intensity of lung injury (Potey et al., 2019). Sustained elevations of pro-inflammatory cytokines in persons with ALI or sepsis have been linked to a worse prognosis. In this investigation, TNF-α, IL-1β, and IL-6 plasma concentrations increased considerably following LPS treatment. The plasma levels of pro-inflammatory cytokines and lung inflammation, on the other hand, decreased dramatically 4 hours after UC-MSC delivery. The production of pro-inflammatory cytokines is significantly reduced when UC-MSCs are administered. As a result, it improves the cytokine network homeostasis and keeps the inflammatory and anti-inflammatory processes associated with ALI in check. MSCs have been found in previous research to reduce TNF-α and IL-6 secretions by lung macrophages via a paracrine pathway or direct contact with host cells (Hu et al., 2020).
Though the MSCs mechanism of anti-inflammatory is unclear, previous studies have been reported in renal (Semedo et al., 2007), myocardiac (Ohnishi et al., 2007), and pulmonary injury (Ortiz et al., 2003). In an inflammatory environment, there has been a shift from pro-inflammatory activity to an anti-inflammatory environment due to altering in the secretion of the cytokine profile of T cells and dendritic cells by human bone marrow MSCs (Aggarwal and Pittenger, 2005). The MSCs improve lung injury induced by LPS via a decrease in TNF Alpha, MIP-2 level, and elevated level of IL-10 in BALF. This result shows the anti-inflammatory effect of MSCs in the lung injury model (Lee et al., 2009). In the early stage of ALI, similar result we have shown with decreased levels of TNF alpha, IL-6, and IL 1 β in BALF. Combined immunomodulation activity shows the mechanism of anti-inflammatory effect. Kim et al. (2011) reported a possible mechanism for MSCs anti-inflammatory effects in ALI mice. MSC transplantation resulted in a reduction in inflammation. These findings clearly show a link between the mitigation of LPS-induced ALI mediated by UC-MSCs and the anti-inflammatory actions of these cells.
We also investigated the effects of UC-MSCs on MPO activity to better understand the nature of the inhibitory action of UC-MSCs on cytokine production. MPO is an enzyme found mostly in neutrophil primary granules. When LPS is administered, a large number of polymorphonuclear neutrophils from the peripheral blood are recruited into the lungs, producing large amounts of MPO and reactive oxygen derivatives, resulting in a cascade-like response and tissue damage (Fu et al., 2012). As a result, MPO activity is a sign of lung neutrophil accumulation (Strzepa et al., 2017). The main indicator of neutrophil infiltration is MPO. Lung injury was significantly mitigated by intra-tracheal transplantation of human UC-MSCs into LPS-induced ALI rats in this study, as evidenced by a reduction in MPO activity in lung tissue. LPS stimulation increased lung MPO activity significantly (p < 0.05). With UC-MSCs therapy, MPO activity was reversed in a dose-dependent manner. We can deduce from this that UC-MSC treatment mitigated the LPS-induced surge in neutrophil accumulation in the lungs.
CONCLUSION
In the present study, intra-tracheal instillation of LPS-induced lung injury in rats and the treatment with UC-MSCs showed dose-dependent effects on body temperature, body weights, lung weights, and cytokine profiles indicating that they have beneficial protective effects against LPS-induced lung injury. These results were consistent with the histological evaluation of the lung tissues, where extensive neutrophil infiltration was observed in the rats with LPS-induced ALI. UC-MSCs efficaciously reduced lung inflammation. To conclude, treatment with UC-MSCs decreased the LPS-induced accumulation of neutrophils, lymphocytes, and macrophages as well as reduced the LPS-induced cytokines generation. This indicates that UC-MSCs have a substantial anti-inflammatory activity through LPS-induced ALI. Hence, UC-MSCs can be considered potential therapeutic agents for mitigating ALI.
AUTHORS’ CONTRIBUTIONS
CP: concept and design, admin, technical or material support, drafting the manuscript; MM: data acquisition, data interpretation; MA: revision of draft; WA: critical revision of manuscript, supervision, and final approval.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
All authors declares no conflicts of interest.
ETHICAL APPROVALS
Human umbilical cord tissues were collected from pregnant females (gestational age: ≥37 weeks) with informed consent from the donors and as per the norms of the institutional ethical committee (Ref: 135/IRB-IBSEC/SIST).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aggarwal S, Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 2005; 105(4):1815–22.
Ali H, Khan A, Ali J, Ullah H, Khan A, Ali H, Irshad N, Khan S. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol Toxicol, 2020; 21(1):1–14.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol, 2004; 202(2):145–156.
Bian S, Cai H, Cui Y, Liu W, Xiao C. Nanomedicine-based therapeutics to combat acute lung injury. Int J Nanomed, 2021; 16:2247.
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med, 2019; 13(9):1738–55.
Cheng T, Ding S, Liu S, Li Y, Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics, 2021; 11(2):893.
Dong Z, Yuan Y. Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: inhibition by ST1926. Int J Mol Med, 2018; 41(6):3405–21.
Fu P-K, Wu C-L, Tsai T-H, Hsieh C-L. Anti-inflammatory and anticoagulative effects of paeonol on LPS-induced acute lung injury in rats. Evid-Based Complementary Altern Med, 2012; 2012(1):81.
Giordano NP, Cian MB, Dalebroux ZD. Outer membrane lipid secretion and the innate immune response to Gram-negative bacteria. Infect Immun, 2020; 88(7):e00920–19.
Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med, 2011; 17(3):293–307.
Hu X, Liu L, Wang Y, Li Z, Yu Y, Chai J, Liu Y. Human umbilical cord-derived mesenchymal stem cells alleviate acute lung injury caused by severe Burn via secreting TSG-6 and inhibiting inflammatory response. Stem Cells Int, 2020; 18:2022:8661689.
Hung CF, Rohani MG, Lee S-s, Chen P, Schnapp LM. Role of IGF-1 pathway in lung fibroblast activation. Respir Res, 2013; 14(1):1–12.
Jiang L-L, Li H, Liu L. Xenogeneic stem cell transplantation: research progress and clinical prospects. World J Clin Cases, 2021; 9(16):3826.
Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res, 2011; 12(1):1–11.
Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther, 2009; 9(10):1259–70.
Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm, 2012; 9(1):1–11.
Li Y, Ma K, Zhang L, Xu H, Zhang N. Human umbilical cord blood derived-mesenchymal stem cells alleviate dextran sulfate sodium-induced colitis by increasing regulatory T cells in mice. Front Cell Dev Biol, 2020; 8:1412.
Li Y, Wang S-M, Li X, Lv C-J, Peng L-Y, Yu X-F, Song Y-J, Wang C-J. Pterostilbene pre-treatment reduces LPS-induced acute lung injury through activating NR4A1. Pharm Biol, 2022; 60(1):394–403.
Lu Z, Meng S, Chang W, Fan S, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells activate notch signaling to induce regulatory dendritic cells in LPS-induced acute lung injury. J Transl Med, 2020; 18(1):1–16.
Mashhouri S, Froushani SMA, Tehrani AA. Non-adherent bone marrow-derived mesenchymal stem cells ameliorate clinical manifestations and inflammation in an experimental model of ulcerative colitis in rats. Iranian J Med Sci, 2020; 45(5): 341.
Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cell, 2014; 6(2):195.
Ohnishi S,Yanagawa B, Tanaka K.,Miyahara Y, Obata H.,Kataoka M.,Kodama M.,Ishibashi-Ueda H.,Kangawa K.,Kitamura S,Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol, 2007; 42(1):88–97.
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci. USA, 2003; 100(14):8407–8411.
Padhiar C, Aruni AW, Abhaya M, Muthuchamy M, Dhanraj AK, Ganesan V, Bovas FB, Rajakani SN. GMP compliant clinical grade and xenofree manufacturing of human Wharton’s jelly derived mesenchymal stem cell from pooled donors. Biochem Eng J, 2022; 184:108470.
Panganiban RAM, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmacol Sin, 2011; 32(1):12–20.
Park I, Kim M, Choe K, Song E, Seo H, Hwang Y, Ahn J, Lee S-H, Lee JH, Jo YH. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur Respir J, 2019; 53(3).
Park J, Jeong S, Park K, Yang K, Shin S. Expression profile of microRNAs following bone marrow?derived mesenchymal stem cell treatment in lipopolysaccharide?induced acute lung injury. Exp Thera Med, 2018; 15(6):5495–502.
Potey PM, Rossi AG, Lucas CD, Dorward DA. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding the biological function and therapeutic potential. J Pathol, 2019; 247(5):672–85.
Rehring JF, Bui TM, Galán-Enríquez CS, Urbanczyk JM, Ren X, Wiesolek HL, Sullivan DP, Sumagin R. Released myeloperoxidase attenuates neutrophil migration and accumulation in inflamed tissue. Front Immunol, 2021; 12:1361.
Semedo P, Wang PM, Andreucci TH, Cenedeze MA, Teixeira VP, Reis MA, Pacheco-Silva A, Câmara NO. Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc, 2007; 39(2):421–3.
Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: a new player in autoimmunity. Cell Immunol, 2017; 317:1–8.
Sun J, Han Z-B, Liao W, Yang SG, Yang Z, Yu J, Meng L, Wu R, Han ZC. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+ CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti-and pro-inflammatory factors. Cell Physiol Biochem, 2011; 27(5):587–96.
Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, Zamani M, Javidi K, Naimi A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol, 2020; 235(12):9185–210.
Tirunavalli SK, Gourishetti K, Kotipalli RSS, Kuncha M, Kathirvel M, Kaur R, Jerald MK, Sistla R, Andugulapati SB. Dehydrozingerone ameliorates Lipopolysaccharide-induced acute respiratory distress syndrome by inhibiting cytokine storm, oxidative stress via modulating the MAPK/NF-κB pathway. Phytomed, 2021; 92:153729.
Tucureanu MM, Rebleanu D, Constantinescu CA, Deleanu M, Voicu G, Butoi E, Calin M, Manduteanu I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int J Nanomed, 2018; 13:63.
Vohra M, Sharma A, Bagga R, Arora SK. Human umbilical cord-derived mesenchymal stem cells induce tissue repair and regeneration in collagen-induced arthritis in rats. J Clin Transl Res, 2020; 6(6):203.
Wang B, Gong X, Wan J-Y, Zhang L, Zhang Z, Li H-Z, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther, 2011; 24(4):434–41.
Yang Y, Chen Q-H, Liu A-R, Xu X-P, Han J-B, Qiu H-B. Synergism of MSC-secreted HGF and VEGF in stabilizing endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther, 2015; 6(1):1–14.
Zhang C, Yin X, Zhang J, Ao Q, Gu Y, Liu Y. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe idiopathic pulmonary fibrosis: A case report. Exp Ther Med, 2017; 13(5):1922–6.