INTRODUCTION
Inflammation in the respiratory tract is one of the symptoms of acute respiratory distress syndrome (ARDS). Although inflammation is one of the body’s responses to a type of infection, an excessive (high) inflammatory response can result in fatal things to the body’s physiological system, such as acute infection with severe acute respiratory syndrome virus 2 (SARS-CoV-2), avian influenza, and several other acute infectious diseases. ARDS is a severe respiratory condition in approximately 10% of intensive care unit patients during the global COVID-19 pandemic (Fiala et al., 2020).
Several types of care and treatment for ARDS have high costs, risks, and mortality rates. In addition, the process of accessibility, practicality, and ease of therapy is relatively low. These make the ARDS condition more severe and difficult to treat appropriately in healthcare facilities such as hospitals, clinics, or community health centers in conditions of the COVID-19 pandemic and other infectious causes as it is today (Jo et al., 2020; Kumar and Trivedi, 2021; Mastutik et al., 2022; Matthay et al., 2012; Prakoso et al., 2021; Putra et al., 2021; Randolph and Barreiro, 2020). Patients who failed to improve had significantly higher levels of proinflammatory cytokines at the onset of the disease (Soncini et al., 2018). Various therapeutic interventions or treatments have been sought and developed to increase the cure rate or prevent lung organ injury in patients with ARDS to improve patient survival (Soncini et al., 2018).
Peronema canescens leaves have great potential as anti-inflammatory and immunomodulatory because they have intense antioxidant activity according to previous scientific studies and contain flavonoid compounds, saponins, and polyphenols that strongly associate with anti-inflammatory and immunomodulatory efficacy (Latief et al., 2021a; Maigoda et al., 2022; Purkon et al., 2021). Some scientific studies for preclinical (in vivo) pharmacological activity on P. canescens leaves that have been carried out include the following: anti-inflammatory activity by induction of carrageenan compounds in rats with inflammatory inhibition of 87.78% (Latief et al., 2021a), antidiabetic activity in monohydrate-induced rat (Latief et al., 2021b), antihyperuricemia in rat (Latief et al., 2021c), immunostimulatory in vivo (in rat) and in vitro (Dillasamola et al., 2021), antibacterial, antiplasmodial, and cytotoxic activities in vitro (Ningsih and Ibrahim, 2013; Suwandi et al., 2018). Meanwhile, research results related to anti-inflammatory testing in animal models testing ARDS induced by lipopolysaccharides (LPSs) in rats have not been carried out until now.
Thus, this study aims to determine the effect of giving P. canescens leaves extract (PCLE) (Sungkai/Jati Sabrang/Ki Sabrang leaves for the term in Indonesia) in regulating the immune system (immune system) on the incidence of ARDS as a model of COVID-19 disease (Maigoda et al., 2022). ARDS disease disorders were induced by induction in the test animal model of Wistar strain male rats with LPS compounds (Ilyas et al., 2019; Lee et al., 2019). Some of the parameters observed in this study include clinical parameters (preclinical), complete hematological examination, levels of proinflammatory biomarkers, such as cytokines tumor necrosis factor-alpha (TNF-α), and histological examinations to see the rate of repair/recovery from damage to the lung organs due to LPS induction (Ikawati et al., 2014; Laksmitawati et al., 2017; Shin et al., 2016; Toklu et al., 2010; Zubaidah et al., 2021).
MATERIALS AND METHODS
Materials and dosages
The dose of LPS as an inflammatory inducer in the respiratory system used was 5 mg/kg bw, according to research conducted by Lee et al. (2019). Imboost® is an immunomodulatory drug product produced by PT. Soho Global Health contains 250 mg of Echinacea purpurea extract and 10 mg of zinc picolinate for each tablet (Mahadi et al., 2018; Tristantini et al., 2021). Moreover, P. canescens leaves are obtained from Bengkulu Province, Sumatera Island, Indonesia.
Test animals
The test animals used were 30 Wistar strain rats obtained from the iRATco Veterinary Laboratory, Bogor, Indonesia, randomly grouped into 6 groups, with the details of the group division as shown in Table 1. In originality, the most suitable test animal model for LPS-induced COVID-19 pneumonia or ARDS testing (from Escherichia coli) was in a golden Syrian hamster test animal (Mesocricetus auratus) that had a high degree of similarity between ACE2 hamsters and human ACE2 (hACE2). However, in this study, a slight modification was made to replace the test animal model due to the limitations of obtaining the test animal (Gruber et al., 2022). The test animals were rats of male sex and 8 weeks old with a body weight of about 200 g, which were obtained from the iRATco Veterinary Laboratory Services animal breeding facility. Six test groups consisting of five test groups were induced with LPSs at a dose of 5 mg/kg bw on day 7. Then after the induction process, the five test animal groups were given their respective test preparations for 5 days. In comparison, one more group is a normal control group.
The test animals were placed in individual cages with an area of 625.5 cm2 and a height of 18.7 cm. Each group of test animals containing five rats was placed in one cage. The animal cages were tested using an air handling unit with an air speed of 60 air change per hour. And for temperature control, humidity is regulated with an air conditioner with a temperature range of 22°C ± 3°C and humidity in the range of 55%–68%. The cage’s base is a clean-dry husk that is free of pests and has been irradiated with ultraviolet radiation. The light source comes from a lamp (artificial light) with a duration of 12 hours of light and 12 hours of darkness. This is done because the rat test animal is a nocturnal animal with a 24-hour circadian rhythm with 12 hours of darkness and 12 hours of light. Furthermore, this animal is nocturnal for exploration, eating, and drinking (Poulos and Borlongan, 2000; Soares-Cunha et al., 2018).
During acclimatization, the feed used standard laboratory feed for rodents with 18% crude protein and 5.7% fat. The drinking water provided is in accordance with the drinking water normally drunk by humans, which is available constantly, sufficient, clean, fresh, and uncontaminated (Purkon et al., 2021). Feed and drink were given to the test animals as desired (ad libitum) (Gong et al., 2019; Ilyas et al., 2019). Before the experiment was carried out, the test animals were acclimatized in the animal cages for approximately 7 days (Purkon et al., 2021; Sinam et al., 2016). Animals that die in the middle of the study will be subjected to a necropsy to determine the cause of death of the test animal. Organ samples were stored in 10% neutral buffered formalin and then histopathologically processed using paraffin embedding (Seger et al., 2018).
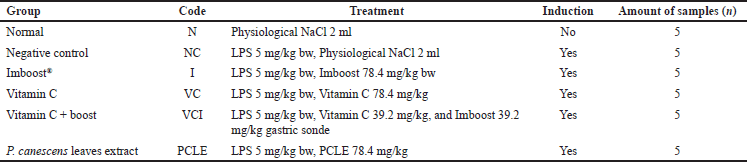 | Table 1. Division of treatment groups in rat test animals. [Click here to view] |
ARDS induction test
The process of induction of ARDS as a model of COVID-19 disease was carried out by administering LPS (E. coli 0111: B4, L 2630 from Sigma-Aldrich) as much as 5 mg/kg bw intratracheal which was carried out by a doctor experienced animal. The protocol carried out is as rats were anesthetized with ketamine 90 mg/kg bw and xylazine 10 mg/kg bw and disinfected on the skin under the neck with 70% alcohol. The skin is slashed, and the muscle is exposed to open the trachea. LPS as much as 5 mg/kg bw in 0.2 ml saline is injected slowly into the trachea. This preclinical (in vivo) testing model was carried out according to Lee et al. (2019).
Examination and clinical analysis process for the respiratory system, complete hematology and TNF-α cytokines, and histopathological analysis in test animals
Preclinical
Clinical analysis was carried out every day both before the induction of ARDS to determine whether there were signs of toxicity from the test material and after the induction process to determine the effectiveness of the test material in reducing the severity of ARDS disease preclinically. The clinical analysis includes behavior, motor activity, respiratory rate, and signs of pneumonia from nostril discharge.
Analysis of complete hematology and TNF-α cytokines (using ELISA)
The sampling process was carried out on day 0, day 7 (before induction), and day 14 (or day 7 after induction). Blood was drawn from the retroorbital vein/plexus of the eye and collected in an ethylenediamine tetraacetic acid tube for hematological analysis and in a tube without anticoagulant for serum collection. The hematological analysis includes red blood cell/RBC (total RBC, hemoglobin/Hb, hematocrit/HCT, mean corpuscular hemoglobin /MCH, mean corpuscular hemoglobin concentration/MCHC), platelet distribution red cell distribution width (RDW), platelet distribution width (PDW), and analysis of leukocytes (total leukocytes, lymphocytes, neutrophils, monocytes, basophils, and eosinophils). Hematology analysis was carried out with a hematology analyzer with the brand/type BC2800Vet Mindray. The serum was isolated by centrifuging blood at a speed of 2,500 rpm for 10 minutes and the serum was separated from the blood object. The enzyme-linked immunosorbent assay (ELISA) analysis process for TNF-cytokines was carried out following the instruction manual of the prepared kit (Laksmitawati et al., 2017).
Histopathological analysis process
The process of taking lung organs was carried out on the 14th day (last day) of treatment. Observations on lung damage, including bleeding, inflammation, and fibrosis as a result of ARDS modeling were performed preclinically (Koksel et al., 2004).
Statistical analysis
Multiple comparison methods on independent data were used to determine data normality, equality variance, and the right type of statistical test. This statistical processing is carried out using R software ver. 3.6.1.
RESULTS AND DISCUSSION
Figure 1 shows that in vitamin C and PCLE, the average percentage increase in body weight of the test animals on the 7th and 14th days was highest compared to the negative control group, which was only given ARDS inducer. This research shows a correlation between maintaining/increasing weight, food intake, and appetite with efficacy from immunomodulators compared to negative control groups that are only infected/induced with certain pathogens. The relative weight percentage (%) was calculated by dividing the lung weight of each treatment group by the final body weight multiplied by 100% (Deyno et al., 2020; Dongmo et al., 2019; Kpemissi et al., 2020). ARDS is a common disease disorder with a serious medical emergency level with many pathological symptoms (Fiala et al., 2020; Layne et al., 2018; Masisi et al., 2021). One of the symptoms/syndromes of ARDS is inflammation of the lung tissue that causes edema around the lung organs and stiffness of the lung tissue, thereby interfering with CO2 elimination and causing hypoxemia (Soncini et al., 2018).
In patients with respiratory system disorders such as COVID-19 patients, vitamin C is one type of supplement that is widely used in the treatment of COVID-19 because it has very strong antioxidant activity and can reduce oxidative stress and inflammation. In addition, vitamin C has the effect of increasing vasopressor synthesis, immune system, and endovascular function and provides epigenetic immunological modification (Muliyani et al., 2022). Vitamin C cannot be produced by the human body but can be obtained/processed from foods in the form of vegetables, fruits, or vitamin C supplements. Excessive consumption of vitamin C will not be absorbed and not metabolized but will be directly excreted through the kidneys (Koomson, 2018). Imboost® is also a type of supplement that is widely used in Indonesia because it contains dried herb extracts of E. purpurea and zinc picolinate in the form of film-coated caplets. This supplement is widely used to increase endurance (immunomodulator), which functions to prevent illness due to infection and accelerate the healing process (Muliyani et al., 2022).
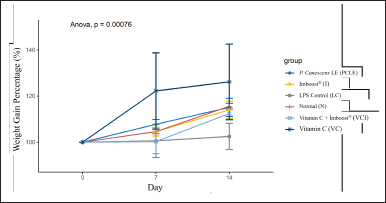 | Figure 1. Graph of the percentage curve for a weight gain of test animals after ARDS induction on day 7 and after administration of test preparations for each group of test animals on day 14. [Click here to view] |
Testing the anti-inflammatory activity of the ethanolic extract of PCLE in animal models of rats with ARDS induced by LPS compounds to determine the rate of recovery in improving clinical conditions, biomarker levels, and histopathological damage to lung organs compared to the negative control group which was induced only with LPS but not given the test preparation. The results of clinical conditions in test animals (preclinical) are summarized in Table 2. In examining the preclinical and locomotor activity abnormalities, all test groups and normal groups looked normal. However, in the negative control group, the test animal looked more inactive, and there was a curvature display on the spine of the test animal in the negative control group, which made the upper back look abnormally rounded/bent (kyphosis). This indicates that the negative control group was seen that the test animals endured the pain/appearance of sepsis symptoms due to the induction process with LPSs which are endotoxins/part of the bacterial membrane, without any significant improvement conditions compared to other test groups (Fiala et al., 2020). Moreover, the results of the examination of pulmonary organs macroscopically can be seen in Figure 2, observing the infiltration of inflammatory cells, congestion, and pneumonia (which refer to exudation at a microscopic level), as well as desquamation in the bronchial epithelium, which is ensifematus especially seen in the negative control group. In contrast, in the test group, it looks relatively normal. This can occur in the lung organs caused by irritant compounds, infectors/pathogens (such as membranes/endotoxins of LPSs), allergen inhalations, and toxic reactions of certain drugs (Revercomb et al., 2020; Swain et al., 2020). Microscopic observation continued with histopathological examination of the lungs in various treatment groups in Figure 5. Then a scoring test was carried out on lung capacity by observing 20 fields of view. In Figure 5, the results of the histological scoring analysis/examination of lung tissue can also be used to confirm the incidence of edema, bleeding, and inflammation in the lungs.
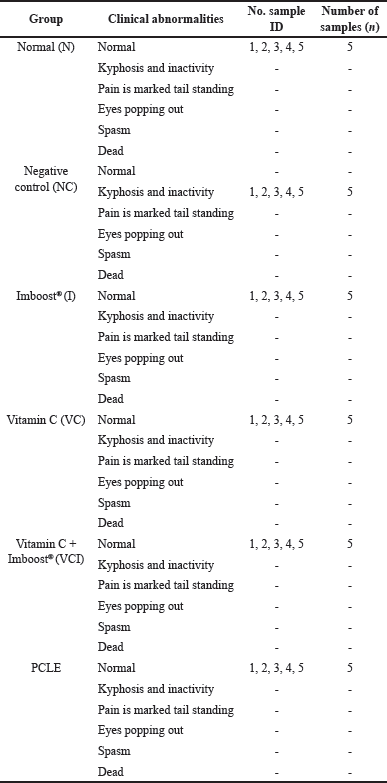 | Table 2. Results of clinical examination analysis process on test animals (preclinical) on PCLE testing. [Click here to view] |
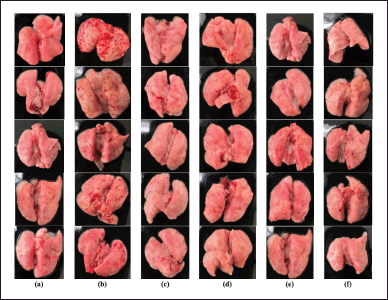 | Figure 2. The results of macroscopic observations of the lungs in each treatment group include: (a) normal group (N), (b) negative control group (NC), (c) Imboost® group (I), (d) group of vitamin C (VC), (e) group vitamin C + Imboost® (VCI), and (f) group PCLE. [Click here to view] |
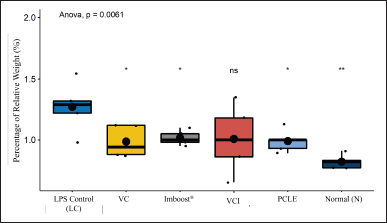 | Figure 3. Percentage of relative weight (%) in all treatment groups. Marking * has a significantly different p value (p 0.05) and ** has a significantly different p value against the negative/NC group (p 0.01). VC = vitamin C, VCI = vitamin C + Imboost®, and PCLE = P. canescens leave extract. [Click here to view] |
Acute mucosa inflammation with a picture of infiltration of inflammatory cells/leukocytes that then form edema, and destruction of the walls of the alveoli with high severity in the negative control group, while in the test group, it looks relatively normal. This is because ARDS can cause symptoms of bilateral pulmonary infiltration radiographically, interfering with progressive respiratory failure, decreased arterial oxygen pressure, and hypoxemia (Lee et al., 2019). The index of pneumonia was measured by weighing the dry and wet weight of the test animal’s lungs, the dry weight divided by the wet weight multiplied by 100%. The results of the observations can be seen in Figure 4. In the results, the percentage of edema index (%), the normal control group, vitamin C (VC), and Imboost (I) differed very significantly (p < 0.01). The PCLE group and the combination of vitamin C and Imboost® (VCI) differed significantly (p < 0.05) compared to the negative control group. This indicates that the entire test group can reduce the incidence of inflammation/anti-inflammatory from the results of the edema index (%) obtained (Latief et al., 2021a; Soncini et al., 2018). Figure 6 shows the results of lung capacity scoring (%) in all groups of test preparations that experienced a very significant difference against the negative control group (p value < 2.2 × 10−16 or p value < 0.00000000000000022). So, the test preparation group for the process of edema, bleeding, and inflammation decreased significantly compared to the negative control group.
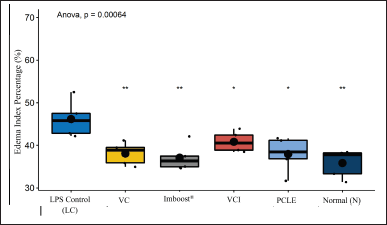 | Figure 4. Percentage of pneumonia index in all treatment groups of test animals. Marking * has a significantly different p value (p 0.05) and ** has a significantly different p value against the negative/NC group (p 0.01). VC = vitamin C, VCI = vitamin C + Imboost®, and PCLE= P. canescens leave extract. [Click here to view] |
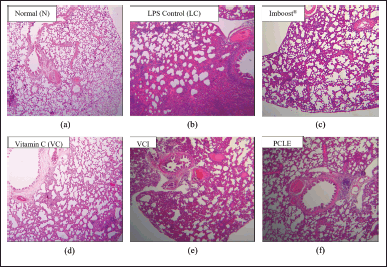 | Figure 5. Comparative description of lung histopathology in various treatment groups, which include (a) normal control group (N), (b) negative control group (NC), (c) Imboost® group (I), (d) group of vitamin C (VC), (e) the combination group of vitamin C and Imboost® (VCI), and (f) PCLE group. [Click here to view] |
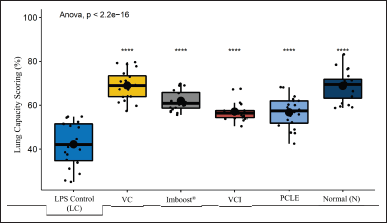 | Figure 6. The results of the percentage of lung capacity scoring in all test treatments (%) with the results of statistical analysis of the negative group (p value < 2.2 × 10−16). [Click here to view] |
Currently, intratracheal induction with LPS in murine animal models is the standard strategy used. This is because LPS is an endotoxin compound that can induce certain immune responses, such as increased neutrophil migration, levels of cytokines TNF-α, IL-6, IL-8, and several other immune response compounds. The use of LPS compounds in this model to induce the formation of respiratory tract inflammation mimics the characteristics of moderate-to-severe ARDS in humans (Henderson et al., 2017; Matthay et al., 2012). In the development of this model, it also induces lung organ damage without causing systemic problems. In addition to inflammation or edema, on ARDS-induced results, animal models experienced hypoxemia, decreased lung volume, and decreased capacity to eliminate CO2 compounds (Fiala et al., 2020). Based on the anatomy and physiology of the respiratory system in rats, which did not differ significantly between the male and female sexes, male test animals with consistent age and weight ranges were used as test samples (Fiala et al., 2020; Sadek, 2012).
Several previous studies reported that SARS-CoV-2 can cause severe symptoms of illness and death due to a hyperinflammatory response. A multiplex rapid cytokine level examination process was carried out to measure serum interleukin (IL)-6, IL-8, TNF-α, and IL-1β in hospitalized patients suffering from COVID-19 (coronavirus disease in 2019) at the Mount Sinai Health System in New York (n = 1,484), which explained that increased serum levels of IL-6, IL-8, and TNF-α had a higher survival rate and shorter hospitalization time. So, serum levels of IL-6 and TNF-α are the correct predictive parameters in the severity and mortality of COVID-19 patients. These findings were validated in a second group of patients (n = 231) (Valle et al., 2020).
In another study, two types of cytokines, namely IL-6 and IL-10 from 13 other tested cytokines, can prove that these two compounds can be predictive diagnostic compounds in increasing IL-6 and IL-10 levels to see a decrease in symptoms of COVID-19 at considerable risk. The two compounds have shown that the standardized mean difference data is significantly different, with an accuracy rate of ~92% as a covariate through various previous tests (Dhar et al., 2021).
As seen in Figure 3, the result of calculating the percentage of edema index (%) has a function as an indirect measurement of edema in the lungs, where a higher percentage of edema index (%) indicates more fluid accumulation in lung tissue as seen in the most negative control group (NC) compared to all test preparations significantly (p < 0.01 and p < 0.05). In the negative group (NC), there is also a correlation between impaired lung function due to decreased gas exchange of O2 and CO2 in the alveolus (Deyno et al., 2020; Qian et al., 2021).
One of the hematological observation parameters for the occurrence of inflammation is increased production and migration of leukocyte cells (including neutrophils) from the blood circulation in the area of inflammation (Latief et al., 2021a). On the results of histological examination, especially in the negative group (NC), all samples of the lung organs of the group experienced bleeding, inflammation, and edema in their lung organs (Tables 3 and 4). This is in accordance with research from Fiala et al. (2020), which showed that after 24–36 hours of the induction process, the test animals, in addition to experiencing significant physical and clinical symptoms of ARDS from moderate-to-severe categories but also histological test results, showed significant pneumonia and moderate-severe inflammation of the lungs (Fiala et al., 2020). The dose of LPS in the induction process must also be precise in its administration because if it is given in excessive doses, it can cause test animals to die, or when LPS is given at low doses, it cannot cause significant ARDS symptoms (Calder, 2020; Lee et al., 2019).
The potential mechanism is the properties of bioactive compounds of PCLE, such as alkaloids, flavonoids, saponins, tannins, and steroids, which play important roles in downregulating the secretion level and protein expression levels of interferon α/ß in macrophages inhibit the proliferation of the virus and improve pulmonary interstitial edema caused by endotoxin, the study of meta-analysis of randomized controlled trials (Liu and Huang, 2016). Furthermore, plant products/herbs demonstrate protective effects in cardiovascular diseases by attenuating damage in cardiomyocytes, endothelial cells, vascular smooth muscle cells (VSMCs), and macrophages/monocytes. In VSMCs, plant products/herbs show the beneficial effect by inhibiting the expression or activity of contractile and structural proteins, modulating the expression of extracellular matrix (ECM) proteins/glycoproteins, regulating calcium levels, attenuating proliferation and migrations, alleviating inflammation, and improving mitochondrial function (Liu and Huang, 2016; Dillasamola et al., 2022).
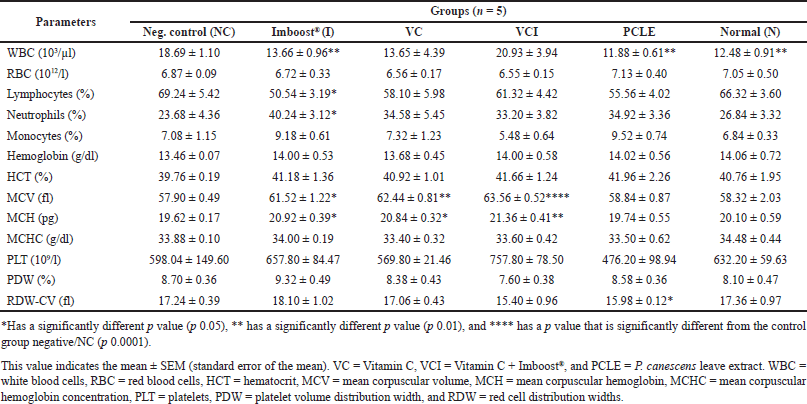 | Table 3. Examination data on hematology and statistical processing of all test treatment groups on day 7. [Click here to view] |
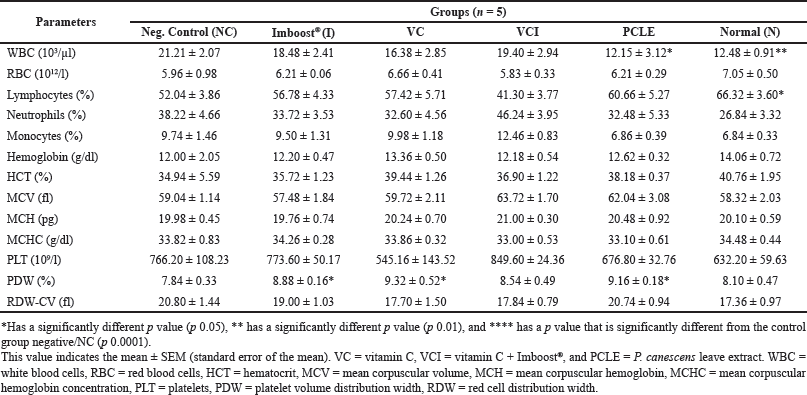 | Table 4. Examination data on hematology and statistical processing of all test treatment groups on day 14. [Click here to view] |
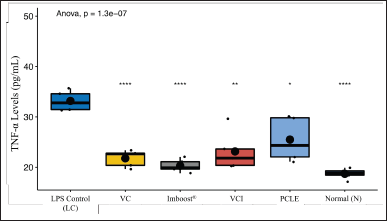 | Figure 7. Analysis of the levels of cytokine TNF-α in rat serum for all treatment groups, which included normal control group (N), negative control (NC), vitamin C (VC), Imboost®, combination vitamin C and Imboost®(VCI), and P. canescens leaves extract (PCLE). [Click here to view] |
The continuous production of inflammatory mediators supported by proinflammatory cytokine compounds such as TNF-α at the tissue level can maintain a longer inflammatory state (Fig. 7). This can result in tissue injury and intra- and extravascular coagulation (in the form of exudation) in previously absent lobules, then undergoing mesenchymal cell proliferation with prior ECM deposition. Affected lobules (interalveolar, interstitial, and endovascular) can result in an adaptive decline in lung tissue repair and end in fibrotic conditions (Kumar et al., 2012; Soncini et al., 2018).
CONCLUSION
The results showed the success of the experimental animal model using the ARDS method with LPS at a dose of 5 mg/kg bw. The ethanol extract of P. canescens leaves effectively treats pneumonia or reduces the level of lung damage due to ARDS, as shown in macroscopic observations, hematological/biochemical examinations, and histology in rats. The administration of PCLE has the same effect as vitamin C and did not show a negative impact during the study based on the clinical symptoms observed. However, the response of inflammatory biomarkers has failed to improve after being given the PCLE test substance.
ACKNOWLEDGMENTS
The team of authors is very grateful to the Directorate General of Health Personnel (Ditjen-Nakes) of the Ministry of Health of the Republic of Indonesia and UPPM Poltekkes of the Ministry of Health Bengkulu for all their financial support.
AUTHORS’ CONTRIBUTIONS
TCM and JJ contributed to the concept of study and performed analysis. DBP and ANEMH contributed to the sample preparation and extraction, and drafted and revised the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
All studies conducted on test animals have been reviewed and are standardized by the approval of the Ethics Committee for the Use of Test Animals by iRATco Veterinary Laboratory Services, CV. Gemawang Putera, Bogor, West Java Province, Indonesia with Number: 4.2.016/KEHI/VI/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Heal, 2020; 3:74–92. doi: https://doi.org/10.1136/bmjnph-2020-000085
Deyno S, Abebe A, Tola MA, Hymete A, Bazira J, Makonnen E, Alele PE. Acute and sub-acute toxicity of Echinops kebericho decoction in rats. BMC Complement Med Ther, 2020; 20:1–12. doi: https://doi.org/10.1186/s12906-019-2794-z
Dhar SK, Vishnupriyan K, Damodar S, Gujar S, Das M. 2021. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon, 2021; 7(e06155):1–9. doi: https://doi.org/10.1016/j.heliyon.2021.e06155
Dillasamola D, Aldi Y, Wahyuni FS, Rita RS, Dachriyanus, Umar S, Rivai H. Study of Sungkai (Peronema canescens, Jack) leaf extract activity as an immunostimulators with in vivo and in vitro methods. Pharmacogn J, 2021; 13:1397–407. doi: https://doi.org/10.5530/PJ.2021.13.177
Dillasamola D, Wahyuni FS, Rita RS, Dachriyanus, Alen Y, Umar S, Aldi Y. immunostimulating study of active agent fraction from Sungkai (Peronema canescens Jack.) leaf from SARS-COV-2 virus antigen exposure to NK and CD8+ T cells. Pharmacogn J, 2022; 14(4):344–51. doi: https://doi.org/10.5530/pj.2022.14.105
Dongmo OLM, Epoh NJ, Tadjoua HT, Yousuf S, Telefo PB, Tapondjou LA, Choudhary MI. Acute and sub-acute toxicity of the aqueous extract from the stem bark of Tetrapleura tetrapteura Taub. (Fabaceae) in mice and rats. J Ethnopharmacol, 2019; 236:42–9. doi: https://doi.org/10.1016/j.jep.2019.02.026
Fiala A, Slagle C, Legband N, Aghabaglou F, Buesing K, Borden M, Harris S, Terry B. Treatment of a rat model of LPS-induced ARDS via peritoneal perfusion of oxygen microbubbles. J Surg Res, 2020; 246:450–6. doi: https://doi.org/10.1016/j.jss.2019.09.017
Gruber AD, Firsching TC, Trimpert J, Dietert K. Hamster models of COVID-19 pneumonia reviewed: how human can they be? Vet Pathol, 2022; 59(4):528–45. doi: https://doi.org/10.1177/03009858211057197
Gong Z, Jiang A, Peng X, Yan M. Typha angustifolia extract reduces diet-induced hyperlipidemia in rats. Chin Herb Med, 2019; 11:98–102. doi: https://doi.org/10.1016/j.chmed.2018.12.001
Henderson WR, Chen L, Amato MBP, Brochard LJ. Fifty years of research in ARDS: respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med, 2017; 196:822–33. doi: https://doi.org/10.1164/rccm.201612-2495CI
Ikawati Z, Yuniarti N, Margono SA. Acute toxicity and suppressive effects of a curcumin analogue gamavuton-0 (Gvt-0) On CFA-induced arthritis in rats. J Appl Pharm Sci, 2014; 4:19–23. doi: https://doi.org/10.7324/JAPS.2014.4114
Ilyas S, Tanjung M, Nurwahyuni I, Pasaribu FY. Effect of Mahkota Dewa ethanolic extracts (Phaleria macrocarpa Scheff.) to hepar histology of female rat (Rattus novergicus) in preeclampsia. IOP Conf Ser Earth Environ Sci, 2019; 305:012075. doi: https://doi.org/10.1088/1755-1315/305/1/012075
Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem, 2020; 35:145–51. doi: https://doi.org/10.1080/14756366.2019.1690480
Koksel O, Cinel I, Tamer L, Cinel L, Ozdulger A, Kanik A, Ercan B, Oral U. N-acetylcysteine inhibits peroxynitrite-mediated damage in oleic acid-induced lung injury. Pulm Pharmacol Ther, 2004; 17:263–70. doi: https://doi.org/10.1016/j.pupt.2004.05.002
Koomson DA. Phytochemical constituents, total saponins, alkaloids, flavonoids and vitamin C contents of ethanol extracts of five Solanum torvum fruits. Pharmacogn J, 2018; 10:946–50. doi: https://doi.org/10.5530/pj.2018.5.160
Kpemissi M, Metowogo K, Melila M, Veerapur VP, Negru M, Taulescu M, Potârniche AV, Suhas DS, Puneeth TA, Vijayakumar S, Eklu-Gadegbeku K, Aklikokou K. Acute and subchronic oral toxicity assessments of Combretum micranthum (Combretaceae) in Wistar rats. Toxicol Rep, 2020; 7:162–8. doi: https://doi.org/10.1016/j.toxrep.2020.01.007
Kumar D, Trivedi N. Disease-drug and drug-drug interaction in COVID-19: risk and assessment. Biomed Pharmacother, 2021; 139:111642. doi: https://doi.org/10.1016/j.biopha.2021.111642
Kumar VS, Navaratnam V, Rajasekaran A, Nair N, Matharasi DSP, Narasimhan S, Ramachandran S. Isolation and characterization of glucosamine from Azadirachta indica leaves: an evaluation of immunostimulant activity in mice. Asian Pac J Trop Biomed, 2012; 2:S1561–7. doi: https://doi.org/10.1016/S2221-1691(12)60453-5
Laksmitawati DR, Widyastuti A, Karami N, Afifah E, Rihibiha DD, Nufus H, Widowati W. Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J Pharmacol, 2017; 12:35–40. doi: https://doi.org/10.3329/bjp.v12i1.28714
Latief M, Fisesa AT, Sari PM, Tarigan IL. Anti-inflammatory activity of Sungkai leaves (Peronema canescens Jack.) ethanol extract in Carrageenan induced mice. J Farm Sains Dan Prakt, 2021a; 7:144–53.
Latief M, Sari PM, Fatwa LT, Tarigan IL, Rupasinghe HPV. Antidiabetic activity of Sungkai (Peronema canescens Jack) leaves ethanol extract on the male mice induced alloxan monohydrate. Pharmacol Clin Pharm Res, 2021b; 6:64. doi: https://doi.org/10.15416/pcpr.v6i2.31666
Latief M, Tarigan IL, Sari PM, Aurora FE. Aktivitas antihiperurisemia ekstrak etanol daun Sungkai (Peronema canescens Jack) pada mencit putih jantan. Pharmacon J Farm Indones, 2021c; 18:23–37. doi: https://doi.org/10.23917/pharmacon.v18i01.12880
Layne K, Passacquale G, Ferro A. The role of platelets in the pathophysiology of atherosclerosis and its complications. In: Cardiovascular thrombus: from pathology and clinical presentations to imaging, pharmacotherapy and interventions. Elsevier, London, UK, pp 51–65, 2018. doi: https://doi.org/10.1016/B978-0-12-812615-8.00004-1
Lee SA, Lee SH, Kim JY, Lee WS. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J Thorac Dis, 2019; 11:1287–302. doi: https://doi.org/10.21037/jtd.2019.04.14
Liu C, Huang Y. Chinese herbal medicine on cardiovascular diseases and the mechanisms of action. Front Pharmacol, 2016; 7:1–21. doi: https://doi.org/10.3389/fphar.2016.00469
Mahadi R, Rasyiid M, Dharma KS, Anggraini L, Nurdiyanti R, Nuringtyas TR. Immunomodulatory and antioxidant activity of green grass jelly leaf extract (Cyclea barbata Miers.) in vitro. J Trop Biodivers Biotechnol, 2018; 3:73. doi: https://doi.org/10.22146/jtbb.33441
Maigoda TC, Judiono J, Purkon DB, Haerussana ANEM, Mulyo GPE. Evaluation of Peronema canescens leaves extract: Fourier transform infrared analysis, total phenolic and flavonoid content, antioxidant capacity, and radical scavenger activity. Open Access Maced J Med Sci, 2022; 10:117–24. doi: https://doi.org/10.3889/oamjms.2022.8221
Masisi K, Masamba R, Lashani K, Li C, Kwape TE, Gaobotse G. Antioxidant, cytotoxicity and cytoprotective potential of extracts of Grewia flava and Grewia bicolor berries. J Pharmacopuncture, 2021; 24:24–31. doi: https://doi.org/10.3831/KPI.2021.24.1.24
Mastutik G, Rohman A, I’tishom R, Ruiz-Arrondo I, de Blas I. Experimental and natural infections of severe acute respiratory syndrome-related coronavirus 2 in pets and wild and farm animals. Vet World, 2022; 15:565–89. doi: https://doi.org/10.14202/vetworld.2022.565-589
Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest, 2012; 122:2731–40. doi: https://doi.org/10.1007/978-3-662-09751-9_48
Muliyani M, Zaini M, Isnani N, Rahmah M. Profil Penggunaan Vitamin Dan Suplemen Pada Pasien COVID-19 Rawat Inap Di Rumah Sakit Bhayangkara Tk. Iii Banjarmasin Pada Tahun, 2020. J Insa Farm Indones, 2022; 5:87–97. doi: https://doi.org/10.36387/jifi.v5i1.926
Ningsih A, Ibrahim A. Aktifitas Antimikroba Ekstrak Fraksi n-Heksan Daun Sungkai (Peronema canescens Jack.) terhadap Beberapa Bakteri dengan Metode KLT-Bioautografi. J Trop Pharm Chem, 2013; 2:76–82. doi: https://doi.org/10.25026/jtpc.v2i2.51
Poulos SG, Borlongan C V. Artificial lighting conditions and melatonin alter motor performance in adult rats. Neurosci Lett, 2000; 280:33–6. doi: https://doi.org/10.1016/S0304-3940(99)00997-0
Prakoso YA, Rini CS, Kristianingrum YP, Hidayah N, Widhowati D, Sigit M. Severe acute respiratory syndrome-coronavirus 2 in domesticated animals and its potential of transmission: a meta-analysis. Vet World, 2021; 14:2782–92. doi: https://doi.org/10.14202/vetworld.2021.2782-2792
Purkon DB, Iwo MI, Soemardji AA, Rahmawati SF, Fadhlillah FM, Nadhifah A. Immunostimulant activity of Marchantia paleacea Bertol. herb liverwort ethanol extract in BALB/c mice. Indones J Pharm, 2021; 32:464–73. doi: https://doi.org/10.22146/ijp.2128
Putra ON, Anggraini ED, Faizah AK. Peresepan Obat “Off-Label” Pada Anak Dengan Penyakit Infeksi Saluran Pernapasan Akut. Lumbung Farm J Ilmu Kefarmasian, 2021; 2:94. doi: https://doi.org/10.31764/lf.v2i1.3729
Qian D, Florenly F, Liena L, Purba DR. Effectiveness of ethanol extract mangosteen peel (Garcinia Mangostana L.) in accelerating post-tooth extraction healing in Wistar rats. Budapest Int Res Exact Sci J, 2021; 4:22–30. doi: https://doi.org/10.33258/birex.v4i1.3473
Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity, 2020; 52:737–41. doi: https://doi.org/10.1016/j.immuni.2020.04.012
Revercomb L, Hanmandlu A, Wareing N, Akkanti B, Karmouty-Quintana, H. Mechanisms of pulmonary hypertension in acute respiratory distress syndrome (ARDS). Front Mol Biosci. 2021;7:624093. doi: https://doi.org/10.3389/fmolb.2020.624093
Sadek KM. Antioxidant and immunostimulant effect of Carica papaya Linn. aqueous extract in acrylamide intoxicated rats. Acta Inform Medica, 2012; 20:180–5. doi: https://doi.org/10.5455/aim.2012.20.180-185
Seger S, Stritt M, Vezzali E, Nayler O, Hess P, Groenen PMA, Stalder AK. A fully automated image analysis method to quantify lung fibrosis in the bleomycin-induced rat model. PLoS One, 2018; 13:1–12.
Shin JS, Jung JY, Lee SG, Shin KS, Rhee YK, Lee MK, Hong HD, Lee KT. Exopolysaccharide fraction from Pediococcus pentosaceus KFT18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J Appl Microbiol, 2016; 120:1390–402. doi: https://doi.org/10.1111/jam.13099
Sinam G, Sahu V, Pandey N, Asthana AK. Effect of arsenic on biochemical and antioxidant enzymes in two species of Marchantia L. (Marchantiophyta): role of enzymes in stress acclimatization. J Environ Agric Sci, 2016; 8:79–85.
Soares-Cunha C, Coimbra B, Borges S, Domingues AV, Silva D, Sousa N, Rodrigues AJ. Mild prenatal stress causes emotional and brain structural modifications in rats of both sexes. Front Behav Neurosci, 2018; 12:1–15. doi: https://doi.org/10.3389/fnbeh.2018.00129
Soncini R, Vieira J, Ramos Lopes AC, Ruginsk SG, Incerpi EK, Barchuk AR. Glucocorticoid receptor gene expression in a CLP-induced ARDS-like rat model treated with dexamethasone and metyrapone. Mol Cell Endocrinol, 2018; 474:151–7. doi: https://doi.org/10.1016/j.mce.2018.03.001
Suwandi JF, Wijayanti MA, M. In vitro antiplasmodial and cytotoxic activities of a Sungkai (Peronema Canescens) leaf extract. Int J Pharm Pharm Sci, 2018; 10:109. doi: https://doi.org/10.22159/ijpps.2018v10i10.25124
Swain SS, Singh SR, Sahoo A, Hussain T, Pati S. Molecular docking-simulation edge assessment of potential and less-toxic ‘anti- HIV-drug and phyto-flavonoid’ combination against COVID-19 2020. [Preprint]. doi: https://doi.org/10.21203/rs.3.rs-28225/v1
Toklu H, ?ehirli Ö, ?ahin H, Çetinel ?, Ye?en BC, ?ener G. Resveratrol supplementation protects against chronic nicotine-induced oxidative damage and organ dysfunction in the rat urogenital system. Marmara Pharm J, 2010; 14:29–40. doi: https://doi.org/10.12991/201014462
Tristantini D, Wahidin W, Feliana F, Widigarka M, Santoso LL. Immunomodulatory and antioxidant activity from Indonesian anti-degenerative herbs water extract. J Complement Integr Med, 2021; 18:695–700. doi: https://doi.org/10.1515/jcim-2020-0223
Valle DMD, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med, 2020; 26:1636-43. doi: https://doi.org/10.1038/s41591-020-1051-9
Zubaidah E, Iastika AR, Widyaningsih TD, Febrianto K. Immunomodulatory activity of black tea Kombucha (Camellia sinensis) and Arabica coffee leaves tea Kombucha (Coffee arabica) for Salmonella typhi-infected mice. IOP Conf Ser Earth Environ Sci, 2021; 733:012128;1–8. doi: https://doi.org/10.1088/1755-1315/733/1/012128