INTRODUCTION
The excessive use of antibiotics and intrinsic changes of (gene expression changes) in bacteria has increased antimicrobial resistance (AMR). In 2019, The World Health Organization released that AMR is 1 of 10 warnings to global health. It can affect clinical, economic, and death losses, especially in developing countries (Aslam et al., 2018; Foekh et al., 2019). Infection with AMR causes severe illnesses, prolonged length of stay in the hospital, increases in healthcare costs, and the higher cost of second-line drugs and treatment failures. Centers for disease control and prevention released that AMR adds a 20 billion dollar surplus in health costs in the United States and caused 23,000 deaths per year and 2.5 months extra hospital days, while in Europe, more than 9 billion euros per year spent on AMR and caused more than 25,000 deaths per year and 2.5 months additional hospital days (Dadgostar, 2019).
AMR has triggered researchers to find new alternative drug ingredients that can act as antibiotics from nature. One of the natural materials that could be used as an antibiotic is earthworms. Earthworms have been used as a traditional medicine for 100 years. Only a few species can be used as medicine. Lumbricus rubellus is one of them. Lumbricus rubellus extract comprises antibacterial characteristics and could inhibit Gram-positive and Gram-negative bacteria (Foekh et al., 2019; Sun, 2015). Lumbricus rubellus has an antimicrobial peptide (AMP) called lumbricin-I, which plays a vital role in natural defense against pathogenic microbes. Lumbricin-I was a proline-rich AMP of 62 amino acids. Lumbricin-I represented antimicrobial action in vitro against a broad spectrum of microorganisms and fungi without hemolytic side effects. This peptide leads to the establishment of multimeric pores in the bacterial cell wall. It causes the cytoplasm of bacterial cells to be exposed to the outside environment causing bacterial death. Lumbricin-I found in adult L. rubellus (Cho et al., 1998). Several studies on the use of L. rubellus extract as an alternative drug to treat infectious diseases such as typhoid fever (Lestari et al., 2019; Purwitanto et al., 2013), periapical infection (Andayani et al., 2016) periodontitis (Dharmawati et al., 2019), and pullorum disease in poultry (Damayanti et al., 2009). There has been no systematic review to date to evaluate the evidence of the antibiotic property of earthworm extract. We, therefore, conducted a systematic review of the available literature on L. rubellus as an antibacterial. We analyzed the effect of L. rubellus as an antibacterial on a comprehensive and heterogenous range of bacteria to evaluate its effectiveness and safety.
MATERIALS AND METHODS
Objectives
The objective of this study was to assess the evidence from previous studies on the use of L. rubellus as an antibacterial agent.
Protocol
We followed the preferred reporting items for systematic reviews and meta-analyses guidelines (Liberati et al., 2009).
Data sources and search strategy
Searches were performed in Science Direct (1998–2022), Wiley Online Library (1892–2022), Academic Search Complete (1983–2022), PubMed (1973–2022), Directory of Open Access Journal (1990–2022), Cochrane Library (2018-2022), Indonesian Publication Index (2012–2022), and direct contact the specific researcher with keywords “Lumbricus rubellus”. We included all research types (in vitro study, animal study or clinical trial) published in English and Indonesian up to October 2022. A selection of relevant studies based on title and abstract, then the selected article is downloaded and reviewed by the author. The available data were extracted and tabled (Table 1). We excluded literature on L. rubellus as other agents such as antipyretic, antithrombotic, antiaging, and duplicate records.
Outcome
Our primary outcome of this systematic review was the effect of L. rubellus as an antibacterial.
Risk of bias assessment
The risk of bias was assessed by the Cochrane Risk of Bias tools. There was only one clinical trial evaluation performed.
RESULTS
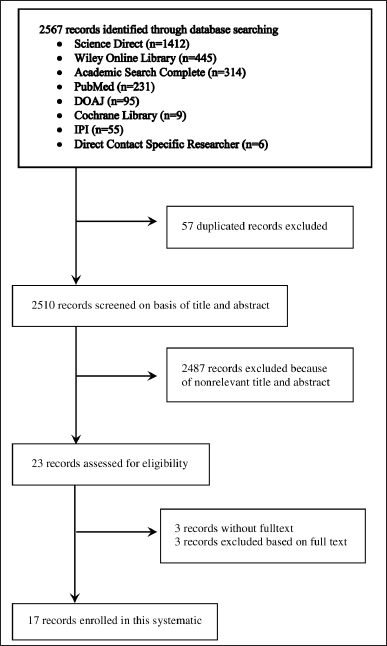
The literature searches identified 2,567 studies, of which 57 articles were excluded as duplicates between databases. The title and abstract were reviewed and yielded 23 articles that were reviewed in full text; of these, six were excluded, and 17 articles met the inclusion criteria (Fig. 1).
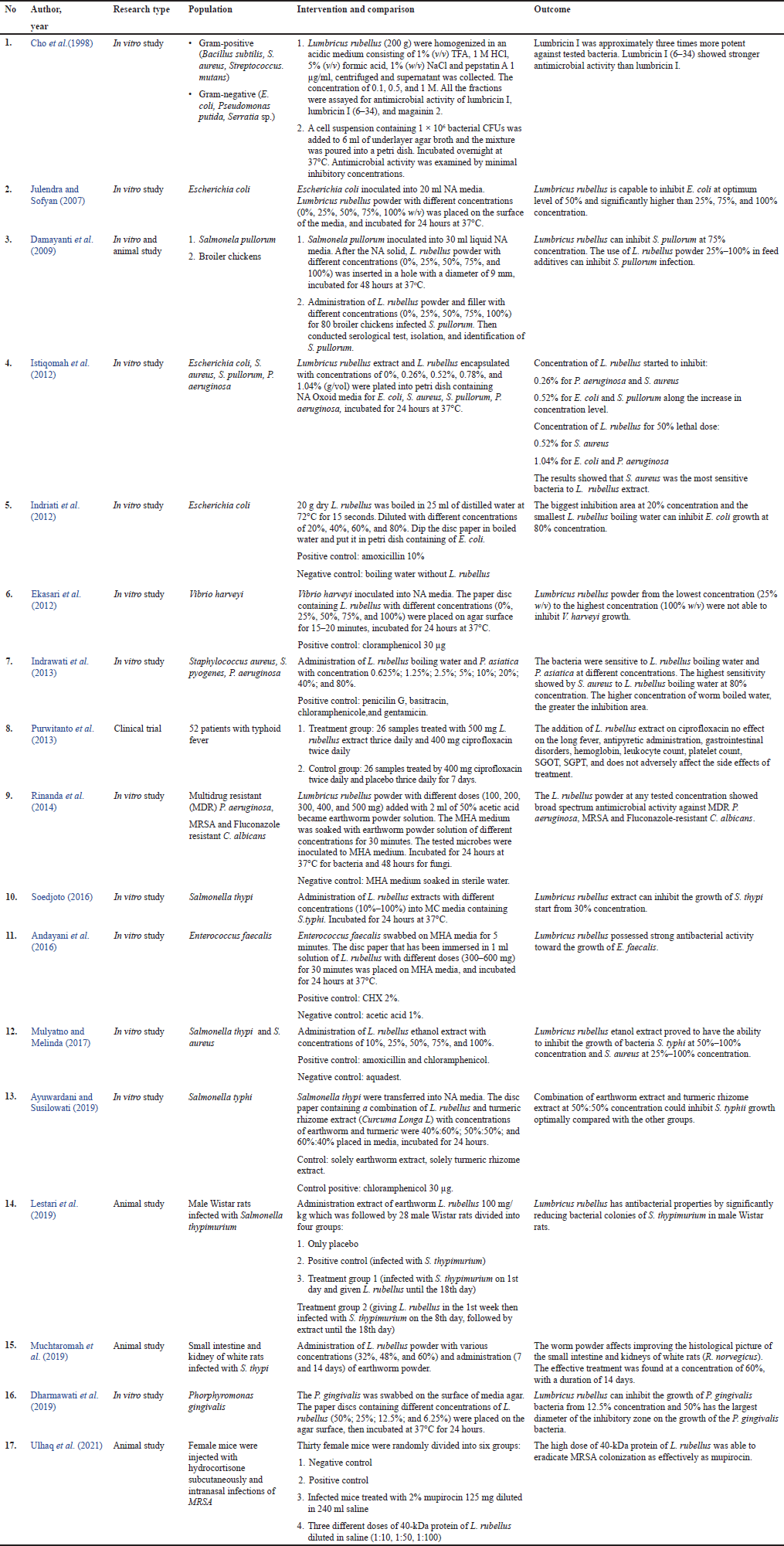
Table 1 describes the microorganisms tested, the type of the study, the methodologies used, and the study’s outcome. Among all the eligible studies, one article was a clinical trial (Purwitanto et al., 2013), four articles were animal studies (Damayanti et al., 2009; Lestari et al., 2019; Muchtaromah et al., 2019; Ulhaq et al., 2021) while the remaining 13 studies were in vitro studies (Andayani et al., 2016; Ayuwardani and Susilowati, 2019; Cho et al., 1998; Damayanti et al., 2009; Dharmawati et al., 2019; Ekasari et al., 2012; Indrawati et al., 2013; Indriati et al., 2012; Istiqomah et al., 2012; Julendra and Sofyan, 2007; Mulyatno and Melinda, 2017; Rinanda et al., 2014; Soedjoto, 2016). Eleven studies used L. rubellus extract as an active ingredient (Andayani et al., 2016; Cho et al., 1998; Dharmawati et al., 2019; Ekasari et al., 2012; Istiqomah et al., 2012; Julendra and Sofyan, 2007; Lestari et al., 2019; Muchtaromah et al., 2019; Rinanda et al., 2014; Soedjoto, 2016; Ulhaq et al., 2021). Two studies used L. rubellus boiling water (Indrawati et al., 2013; Indriati et al., 2012). Lumbricus rubellus as an additive in poultry feed (Damayanti et al., 2009), combination therapy with ciprofloxacin (Purwitanto et al., 2013), and combination with turmeric rhizome extract (Ayuwardani and Susilowati, 2019), and combination with Pheretima asiatica earthworm (Indrawati et al., 2013).
The tested microorganisms varied: Gram-negative, Gram-positive, and fungi. Five studies determine antibacterial activity of L. rubellus in Salmonella typhi (Ayuwardani and Susilowati, 2019; Muchtaromah et al., 2019; Mulyatno and Melinda, 2017; Purwitanto et al., 2013; Soedjoto, 2016), four studies against Staphylococcus aureus (Cho et al., 1998; Indrawati et al., 2013; Istiqomah et al., 2012; Mulyatno and Melinda, 2017), four studies against Escherichia coli (Cho et al., 1998; Indriati et al., 2012; Istiqomah et al., 2012; Julendra and Sofyan, 2007), three studies against Pseudomonas aeruginosa (Indrawati et al., 2013; Istiqomah et al., 2012; Rinanda et al., 2014), two examinations against Salmonela pullorum (Damayanti et al., 2009; Istiqomah et al., 2012), two studies against methicillin resistant S. aureus (MRSA) (Rinanda et al., 2014; Ulhaq et al., 2021) and Fluconazole resistant Candida albicans (Rinanda, et al., 2014), one study against Vibrio harveyi (Ekasari et al., 2012), one study against Enterococcus faecalis (Andayani et al., 2016), and one study against Porphyromonas gingivalis (Dharmawati et al., 2019).
The concentration and dose of L. rubellus vary depending on the dosage form used. Five studies used L. rubellus extract as an active ingredient with concentrations of 0% to 100% (Damayanti et al., 2009; Ekasari et al., 2012; Julendra and Sofyan, 2007; Mulyatno and Melinda, 2017; Soedjoto, 2016), two studies used L. rubellus extract as an active ingredient with dose 500 and 100 mg/kg, respectively (Lestari et al., 2019; Purwitanto et al., 2013), two studies used L. rubellus powder with dose 100 till 600 mg (Andayani et al., 2016; Rinanda et al., 2014). Among 14 studies, 9 studies concluded that the greater the dose or concentration of L. rubellus used, the greater the antibacterial effect found (Andayani et al., 2016; Damayanti et al., 2009; Dharmawati et al., 2019; Indrawati et al., 2013; Istiqomah et al., 2012; Mulyatno and Melinda, 2017; Rinanda et al., 2014; Soedjoto, 2016; Ulhaq et al., 2021)
Only one clinical trial established the effect of L. rubellus extract in typhoid fever patients treated with ciprofloxacin. It was a double-blind study with a pretest-posttest with the control group. The treatment group consisted of 26 samples treated with 500 mg L. rubellus extract thrice daily and 400 mg ciprofloxacin twice daily. In the control group, 26 samples were treated with 400 mg of ciprofloxacin twice daily and a placebo thrice daily. In both groups treated for 7 days, there was no significant difference in loss of fever (p = 0.896), using antipyretic (p = 0.159), amount of leukocytes (p = 0.484), amount of hemoglobin (p = 0.984), and the total of thrombocytes (p = 0.657) (Purwitanto et al., 2013).
 | Table 1. Main characteristics from the included studies. [Click here to view] |
 | Figure 1. Flow diagram. [Click here to view] |
DISCUSSION
This study aimed to review the antibacterial effect of L. rubellus and to gain further and specific conclusions about the mechanism of action of L. rubellus as an antibacterial agent. Most researchers have reported promising results for L. rubellus as an antibacterial agent from a literature review. However, we still found two studies that did not support this finding (Ekasari et al., 2012; Purwitanto et al., 2013).
Eathworm of L. rubellus contains peptides with board spectrum antimicrobial activity known as lumbricin-I (formed by 62 amino acids and having a measurement of 7,231 Da). It has a proline that includes a conformational structure that influences the secondary system and then represents the mechanism of action. Besides lumbricin-I, certain AMPs have the same design with proline-rich, such as apidaecins, drosocin, metchikowin, bactenecins, and PR-39 but show various mechanisms of action. The mechanism of action of lumbricin-I in inhibiting pathogens remains unclear. However, there was one hypothesis that presents a different tool from other proline-rich AMPs (Cho et al., 1998). The mechanism of action of AMPs begins with the first interaction between the peptide and the target cell (bacteria or fungi) due to the impact of electrostatic power. Cationic is an essential factor that plays a vital role in electrostatic interactions between AMPs and the negatively charged phospholipid membrane of bacteria. The permeability of the cell membrane is assumed can be influenced by the exchange of cation (AMP) and anions (bacteria and fungi cell surface) and cause cell damage so that lumbricin-I can pierce the cytoplasmic membrane (Epand and Vogel, 1999; Jenssen et al., 2006). AMP cationic has a positive correlation with antimicrobial activity. The higher cationicity strongly influences antimicrobial activity (Yeaman and Yount, 2003). Another effectiveness of lumbricin-I as an antimicrobial is evidenced by the interaction between the hydrophobic surface and the hydrophilic surface of the bacterial cell membrane. It causes an increase in membrane permeability so that the lumbricin-I can enter the hydrophilic lipid layer. The entry of lumbricin-I into the intracellular cell membrane causes intracellular instability and inhibits bacterial growth (Pasupuleti et al., 2009).
Overall, the majority of the studies have reported antibacterial properties of L. rubellus in the form of extract, powder, or boiling water against Gram-negative bacteria, Gram-positive bacteria, and fungi (Andayani et al., 2016; Ayuwardani and Susilowati, 2019; Cho et al., 1998; Damayanti et al., 2009; Dharmawati et al., 2019; Indrawati et al., 2013; Indriati et al., 2012; Istiqomah et al., 2012; Julendra and Sofyan, 2007; Lestari et al., 2019; Muchtaromah et al., 2019; Mulyatno and Melinda, 2017; Rinanda et al., 2014; Soedjoto, 2016; Ulhaq et al., 2021). Only two studies did not report this finding (Ekasari et al., 2012; Purwitanto et al., 2013). Lumbricus rubellus is unable to inhibit the growth of V. harveyi bacteria because V. harveyi is resistant to the active ingredients in earthworms through several mechanisms: 1) bacteria produce proteolytic enzymes that can degrade AMPs; 2) proline in earthworms was used by V. harveyi; 3) positive charge of lumbricin-I is only +1, which is very low to interaction through electrostatic force, while the bacterial cell membrane of V. harveyi is negatively charged. That mechanism of action that causes damage to bacterial membrane cells cannot occur (Cho et al., 1998; Ekasari et al., 2012).
The study by Purwitanto et al. (2013) reported that the addition of L. rubellus extract on ciprofloxacin had no effect on leukocyte count in typhoid fever patient (p = 0.484). This study did not assess the effect of pure L. rubellus extract as antibacterial but was seen from the addition of L. rubellus extract in the primary therapy, ciprofloxacin. The antibacterial activity of L. rubellus can cause a negative result from this study against S. typhi, not synergy with ciprofloxacin, and the dose of L. rubellus maybe not be the optimal dose because an optimal dose of L. rubellus remains unclear. Lumbricus rubellus has antithrombotic and thrombolytic effects in several studies. In this study, there were no reports of bleeding manifestations in the treatment group and no significant changes in hemoglobin and thrombocyte levels. The addition of earthworm extract to antibiotic treatment did not affect the duration of fever, gastrointestinal disturbances, leucocytes, liver function in typhoid patients, and incidence of side effects of treatment. The risk of bias in this clinical trial study is high because the sample is not entirely homogenous between the treatment and control groups. It can affect the results (Purwitanto et al., 2013).
Additionally, in this review, we found that S. aureus was the most sensitive bacteria in L. rubellus extract (Indrawati et al., 2013; Istiqomah et al., 2012; Mulyatno and Melinda, 2017). Staphylococcus aureus is a Gram-positive bacteria and more sensitive to bacterial compounds than Gram-negative bacteria due to the structure of the bacteria’s cell walls. Gram-positive bacteria have a single layer of peptidoglycan arranged by tissue with many pores, so the lumbricin-I easy to enter the intracellular cell membrane and causes intracellular instability and inhibits bacterial growth (Istiqomah et al., 2012).
Finally, although the broad-spectrum antibacterial activity demonstrated by L. rubellus shows that lumbricin-I can be possibility developed as a potent antimicrobial agent. Still, this review cannot make definitive conclusions about the compelling form of L. rubellus nor the safety of L. rubellus.
CONCLUSION
Studies show the potential use of L. rubellus for various antibacterial agents. However, investigations that determine the effect of L. rubellus as an antibacterial are still limited. Future clinical studies are required to examine the impact of L. rubellus as an antibacterial agent.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was funded by Universitas Syiah Kuala (The Ministry of Education, Culture, Research and Technology)-Doctoral Dissertation Research Scheme Financial Year 2021 (56/SP2H/LT/DPRM/2021).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Andayani R, Mubarak Z, Rinanda DR. Aktivitas antibakteri tepung cacing tanah (Lumbricus rubellus) terhadap Enterococcus faecalis secara in vitro. J Syiah Kuala Dent Soc, 2016; 1(2):201–10.
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist, 2018; 11:1645–58.
Ayuwardani N, Susilowati AA. Antibacterial activity of Salmonella typhi in combination of earth-worms extract (Lumbricus rubellus) and turmeric rhizoma extract (Curcuma longa L.) in vitro. Aloha Int J Health Adv, 2019; 2(7):165–9.
Cho JH, Park CB, Yoon YG, Kim SC. Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: purification, cDNA cloning and molecular characterization. Biochim Biophys Acta (BBA) Mole Basis Dis, 1998; 1408(1):67–76.
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist, 2019; 12:3903–10.
Damayanti E, Julendra H, Sofyan A, Untari T. Pemanfaatan tepung cacing tanah (Lumbricus rubellus) sebagai agensia anti-Pullorum dalam imbuhan pakan ayam broiler. JITV, 2009; 14(2):83–9.
Dharmawati IGAA, Mahadewa TGB, Widyadharma IPE. Antibacterial activity of Lumbricus rubellus earthworm extract against Porphyromonas gingivalis as the bacterial cause of periodontitis. Open Access Maced J Med Sci, 2019; 7(6):1032–6.
Ekasari, Cahyoko Y, Tjahjaningsih W. Daya antibakteri tepung cacing tanah (Lumbricus rubellus) terhadap pertumbuhan bakteri Vibrio harveyi secara in vitro. J Ilm Perikan Kelaut, 2012; 4(1):1–6.
Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta (BBA) Biomembr, 1999; 1462(1–2):11–28.
Foekh NP, Sukrama IDM, Lestari AAW. The ability of earthworm Lumbricus rubellus extract in slowing down the activation of NFkB and TNF-α in lipopolysaccharide-induced Rattus norvegicus. Bali Med J, 2019; 8(2):439.
Indrawati I, Ratningsih N, Djajasupena S. Uji sensitivitas bakteri Staphylococcus aureus, Streptococcus pyogenes dan Pseudomonas aeruginosa terhadap air rebusan cacing tanah Lumbricus rubellus dan Pheretima asiatica dan antibiotik secara in vitro. J Kajian Islam Sains Dan Teknol, 2013; 7(2):89–105.
Indriati G, Sumitri M, Widiana R. Pengaruh air rebusan cacing tanah (Lumbricus rubellus) terhadap pertumbuhan bakteri Escherichia coli. In: Prosiding Semirata BKS PTN-B MIPA 2012-Biologi, Medan, Indonesia, 2012.
Istiqomah L, Herdian H, Damayanti E, Hayati SN, Julendra H. Inhibitory of encapsulated earthworm extract (Lumbricus rubellus) on pathogenic bacteria in vitro. Media Peternakan, 2012; 35(1):1–8.
Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev, 2006; 19(3):491–511.
Julendra H, Sofyan A. Uji in vitro penghambatan aktivitas Escherichia coli dengan tepung cacing tanah (Lumbricus rubellus). Media Peternakan, 2007; 30:41–7.
Lestari AAW, Made Sukrama ID, Nurmansyah D. The earthworm (Lumbricus rubellus) extract decreased amino transaminase enzyme level and number of bacterial colony in male wistar rats infected with Salmonella typhimurium. Biomed Pharmacol J, 2019; 12(1):325–32.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ, 2009; 339(jul21 1):1–28.
Muchtaromah B, Maslikah SI, Mufarrichah L, Fitriasari PD. Histological description of small intestine and kidney of white rats (Rattus norvegicus) infected with Salmonella typhi by giving earthworm flour. AIP Conf Proc, 2019; 2120(1): 1–7.
Mulyatno, Melinda FS. Uji aktivitas antibakteri ekstrak cacing tanah (Lumbricus rubellus) terhadap bakteri Salmonella thyphi dan Staphylococcus aureus secara in vitro. Muhammadiyah Surakarta, 2017 :1–14.
Pasupuleti M, Schmidtchen A, Chalupka A, Ringstad L, Malmsten M. End-tagging of ultra-short antimicrobial peptides by W/F stretches to facilitate bacterial killing. PLoS One, 2009; 4(4):e5285.
Purwitanto, Datau AE, Nugroho A. Pengaruh penambahan ekstrak Lumbricus rubellus pada pengobatan pasien demam tifoid. J Indon Med Assoc, 2013; 63(2):58–62.
Rinanda T, Sakdiah Oktavia R, Da Fhonsa M, Fitra M. Broad spectrum antimicrobial activity of Lumbricus rubellus powder against drug resistant microbes. In: Proceedings of The 4th Annual International Conference Syiah Kuala University (AIC Unsyiah), Banda Aceh, Indonesia, 2014.
Soedjoto L. Pengaruh konsentrasi ekstrak cacing tanah (Lumbricus rubellus) terhadap pertumbuhan bakteri Salmonella thypi. J Muhammadiyah Med Lab Technol, 2016; 2(2):40–9.
Sun Z. Earthworm as a biopharmaceutical: from traditional to precise. Eur J Bio Med Res, 2015; 1:28
Ulhaq ZS, Atmaja WPS, Putri ASK, Santosaningsih D. The 40-kDa protein of Lumbricus rubellus eradicates methicillin-resistant Staphylococcus aureus in a long-term nasal carriage model. Enferm Infecc Microbiol Clin (Engl Ed), 2021; 39(6):310–1.
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev, 2003; 55(1):27–55.