INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of illness and death in both developed and developing countries (Adriance and Murphy, 2013). Evaluated, 17.9 million people die from CVDs each year, accounting for 32% of all fatalities worldwide, 85% of which are heart attacks or strokes (Hu et al., 2022). Because of the high morbidity, mortality, and rehospitalization rates, researchers have been compelled to seek the most effective methods of diagnosing, risk-stratifying, and managing patients with suspected cardiovascular illnesses (Garcia and Spyropoulos, 2008). CVDs are a set of conditions that affect the heart, the blood vessels (capillaries, arteries, and veins), or both (Behera et al., 2015; Buller et al., 2005; Hyers 1999). These include congenital heart disorders, rheumatic heart disorders, coronary heart disorders, cerebrovascular disorders, peripheral arterial disorders, and venous thromboembolism (VTE), of which we emphasize the VTE in this review (Wang et al., 2020; Wells et al., 2014).
According to comprehensive autopsy studies, VTE in coronavirus disease 2019 (COVID-19) individuals may be the cause of 10% of COVID-19 individual fatalities (Voicu et al., 2021). VTE is a term used to define a multifactorial condition that includes everything from deep vein thrombosis (DVT) to pulmonary embolism (PE) (Cross and Boettner, 2009). After myocardial infarction and stroke, VTE is the third most prevalent cardiovascular condition (Bajaj et al., 2015; Dewar and Panpher, 2010; Goldhaber, 2012; Streiff et al., 2016). It starts with DVT. DVT is characterized by the development of blood clots (thrombi) in the deep veins (Ageno et al., 2008). Deep pelvic or deep leg veins (calf veins, femoral veins, or popliteal veins) are usually affected by DVT (Kesieme et al., 2011; Wadajkar et al., 2013). In certain cases, a thrombus from a deep vein will split and move toward the right side of the heart and eventually become lodged as a blockage in the pulmonary arteries, causing PE. The schematic diagram of VTE is depicted in Figure 1 (Blann and Lip, 2006). Though DVT and PE are traditionally considered separate disorders, growing evidence points to parallels in their origins, incidence, diagnosis, and therapy (Abad Rico et al., 2010; Lavorini et al., 2013) Approximately two-thirds of VTE are caused by DVT alone, with the remaining one-third caused by PE with or without DVT (Lapner and Kearon, 2013). The morbidity and death rates linked with thromboembolic events are significant, with the 28-day mortality rates for DVT and PE reported to be 9% and 15%, respectively (Heit et al., 2016; Leitner et al., 2010; Rali and Criner, 2018). Several advances have been made in the prevention, diagnosis, and treatment of VTE over the last several decades (Khan et al., 2021).
This review emphasizes the developments in the diagnostic and treatment methods and briefly examines the origins and consequences of VTE.
MATERIALS AND METHODS
This systematic review was conducted and reported in accordance with the preferred reporting items for systematic reviews. An electronic search on Cochrane, Google Scholar, Embase, CINAHL, Scopus, Medline, clinicaltrials.gov, PubMed, and International Pharmaceutical Abstracts was conducted using various combinations of key phrases related to VTE, COVID-19, DVT, and PE. Furthermore, the references to the studies that fulfilled the criteria for inclusion were searched for further articles and included.
Data extraction outcomes
A total of 590 articles were selected. After duplicates were removed, 450 articles were evaluated for selection. 50 articles were found for full-text examination, 400 of which were discarded because there was no study population and they were only abstracts. This review finally contained 40 papers. Figure 2 represents the findings of the literature study and screening.
RESULTS AND DISCUSSION
Pathophysiology
The human body’s homeostasis is maintained by the flow of blood. Blood cells perform a variety of functions, including providing oxygen to tissues, fighting infection, and stopping bleeding quickly. Platelet cells circulate and interact with endothelial cells. Platelets do not interface with endothelial cells when they perform a physiological role (Myers, 2015). When platelets contact the vascular wall, they immediately return to blood circulation without becoming activated. On the contrary, endothelial cells lose their capacity to maintain antithrombotic activity when their normal function is disrupted by physical or chemical stimuli. Following that, platelets begin interacting with endothelial cells right away (Auger et al., 2009). Von Willebrand factor (VWF) is now expressed on activated endothelial cells’ cell surfaces. The endothelium-specific storage cells known as Weibel–Palade bodies (WPBs) are responsible for the controlled release of VWF and P- and E-selectins over the membrane of endothelial cells. P- and E-selectins, cell adhesion molecules, play critical roles in thrombogenesis (Wakefield et al., 2008).VWF is taken up by platelets using glycoprotein (GP) Ib. Without regard to whether or not they are active, platelets express GPIb on their surface (Bruni-Fitzgerald, 2015; Stone et al., 2017; Turpie, 2002). The most prevalent leukocytes are neutrophils, which create a neutrophil trap (NET) made of antimicrobial proteins, histones, and DNA. Procoagulant chemicals, platelets, and red blood cells can form thrombus on NETs, which act as a scaffold. In individuals with VTE, quantities of circulating tissue factor (TF) and NET indicators (such as extracellular DNA) are high. Therefore, the existence of anomalies in blood clotting components, the blood vessel wall, and blood flow plays a crucial role in thrombus development and spread (Flinterman et al., 2008).
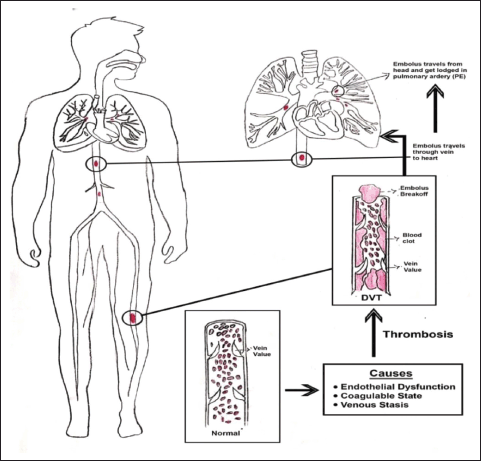 | Figure 1. Schematic diagram of VTE. [Click here to view] |
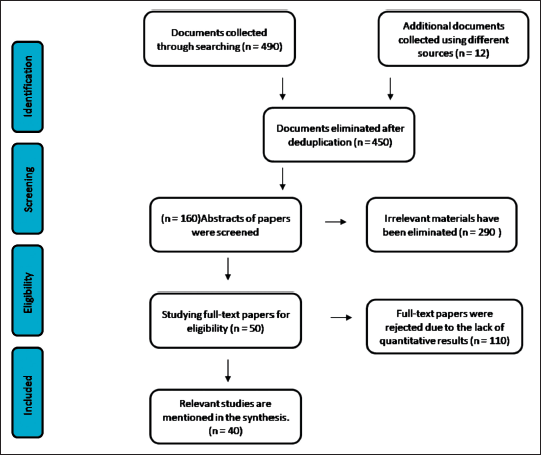 | Figure 2. A flow diagram represents the findings of the literature study and screening. [Click here to view] |
The vascular endothelium, leukocytes, red blood cells, platelets, and fibrin are significant cells that are possible targets for mediating “venous thrombosis” (Lowe, 2001; Montoro-García et al., 2016). Sub-endothelial TF is produced when endothelial cells become disturbed and tight connections get loosened by intracellular phosphorylation processes (Page and Ariëns, 2021). Vein thrombosis frequently develops in conditions involving vascular wall damage or stasis (such as after surgery, trauma, or cancer), systemic inflammation (such as lupus or sepsis), and a procoagulant state (such as antiphospholipid antibodies in inherited thrombophilia) (Zhang et al., 2021).
The stimulation of the coagulation cascade takes place on the surface of the cell in four primary stages such as initiation, amplification, propagation, and termination, according to a more modern cell-based concept of coagulation (Key, 2013). Vascular damage and other diseases cause the release of TFs and collagen from the vessel wall, which activates the coagulation cascade and allows blood to flow. The clotting cascade develops when a sequence of proenzymes or zymogens are sequentially activated into active enzymes, which then activate the next dormant clotting factor (Obi et al., 2021). The intrinsic and extrinsic routes of the coagulation cascade have traditionally been distinguished. Injuries that harm the blood vessel endothelium release the factor VII enzyme, which causes platelet activation (Furie and Furie, 2008). Vascular TFs, Coagulation factor VII, and calcium ions work together to produce a complex that converts factor X into factor Xa, releasing thromboplastin into the circulation to activate the extrinsic coagulation pathway (Yau et al., 2015). The intrinsic coagulation pathway is started when endothelial damage accesses the subendothelial fiber wall and activates factor XII, leading to its transition to factor XIIa. Prothrombin (FII) is converted to thrombin (FIIa) in the presence of phospholipid, Ca2+, and factor V by factor X, which is activated by both separate pathways ultimately. Under the influence of factor XIII, thrombin catalyzes the transition of fibrinogen (Fgn) (factor I) into a fibrin clot. Pathogenesis of venous thrombosis is depicted in Figure 3. The strong inflammatory response, aided by high levels of interleukin 6 (IL-6), fgn, and C-reactive protein (CRP) substantially increases thrombosis. Aside from the rise in fibrinogen level, another one of the most notable laboratory attributes of the hypercoagulability in corona patients is the abnormally high VWF levels; high factor VIII level and factor VIII are transported by VWF. VWF and factor VIII are both stored in the WPBs of endothelial cells, particularly in specific vascular areas such as the lungs and liberated during COVID-19 illness, resulting in high blood levels. This secretion may be due to the virus invading the endothelium cell, which can bind toward the angiotensin-converting enzyme 2 (ACE2) and cause endothelial dysfunction or harm, as well as the release of clotting factors. Other particular pathways, including anticoagulant protein C, may potentially be involved. The endothelial CRP helps thrombin and thrombomodulin build a compound on the surface of the endothelium that activates CRP physiologically. Therefore, interruption of the CRP pathway may be caused by endothelial injury. Due to the pathway’s saturation or overloading by factor V and VIII levels, it was shown to have normal CRP activity in some investigations, reduced in others, or seemed inadequate despite normal CRP levels (Voicu et al., 2021). When taking into account the drastically raised levels of VWF, the VWF-specific protease also appears to be somewhat diminished. Hypoxemia caused by COVID-19-related bronchitis may also promote hypercoagulability by increasing the production of anoxia activating factor-1α, which promotes the expression of procoagulant. Mechanism of action of SARS-COV-2 on thrombosis depicted in Figure 4.
Clinical presentation
VTE can cause reddening of the skin, pain in a part of the body, stiffness that makes it challenging to move the muscles, and a minimal discomfort sensation, which are very general symptoms. The deepest veins of the lower leg are where DVT most frequently develops (Hirsh and Lee, 2002). DVT most usually occurs in the lower limbs, but it can also develop in the upper limbs or in the visceral veins. DVT symptoms and clinical indications are commonly linked to increased intravenous pressure caused by thrombotic venous blockage and inflammation caused by thrombus development (Hirsh et al., 1986). Lower extremity DVT is classified as proximal or distal. Popliteal veins, iliac veins, and femoral veins are involved in proximal lower extremity DVT. The tibial and peroneal veins are associated with distal lower extremity DVT. Upper extremity–DVT is further classified as proximal and distal (Guo et al., 2015). The axillary veins and subclavian veins are involved in proximal upper extremity DVT, whereas the brachial veins, ulnar veins, and radial veins are involved in distal upper extremity DVT (Musil and Ková?ik, 2021).
Distal DVTs, which remain there, are typically asymptomatic and never become clinically relevant; symptoms appear only after the proximal leg veins are involved. People with symptomatic DVT may exhibit discomfort, stiffness, and soreness, along with the spread of the deep leg veins erythema or blue disease (Thomas and Scully, 2022). On the other hand, many individuals with symptomatic DVT may also have asymptomatic PE. PE can manifest in a variety of ways, from no symptoms to cardiac shock. PE is typically characterized by a rapid onset of shortness of breath with spitting out blood or coughing up blood-stained mucus, shallow and erratic breathing, and pain in the chest with pleurisy or collapsing with shock (Kim, 2022). Coughing is a less common clinical symptom, but when it does happen, it typically has little effect. Such individuals should be evaluated and treated as soon as possible since PE is associated with a significant risk of death and morbidity. The majority of patients with PE had no leg symptoms at the time of diagnosis, with just around one-third exhibiting signs or symptoms of DVT.
VTE is a complex disorder caused by environmental and genetic factors. The risk of VTE throughout one’s lifetime is the same for both sexes; however, women between the ages of 20 and 40 are more at risk because of their reproductive risk factors, while males are more at risk in any other age group. Age, cancer and its treatments, prolonged bed rest, stroke, prior VTE, congestive heart failure, acute infection, hormonal therapy, pregnancy, acute inflammatory bowel disorder, dehydration, varicose veins, long-distance air travel, rheumatological disorder, and renal disease are all acquired risk factors for VTE (Bauersachs, 2012; Chopard et al., 2022). There are other acquired variables that have now been linked to a higher incidence of VTE diseases.
There are three categories of genetic risk factors, such as “strong factor,” “moderate factor,” and “weak factor.” Antithrombin deficiency, protein C, and protein S are all strong factors. Factor V Leiden (FVL), fibrinogen, prothrombin, and non-O blood group are moderate factors. Table 1 summarised risk factors for VTE. The incidence and influence of genetic risk factors on VTE risk vary greatly. Prothrombin gene and FVL mutations are linked to a 3–5 fold increase in the chance of developing a first episode of VTE, respectively. Factor XI, Factor XIII, and fibrinogen are examples of weak factors. Long-term hospitalization is the most significant risk factor for VTE. 60% of all VTE patients manifest themselves within 90 days of treatment (Kumar and Likhitha, 2022). VTE risk factors have a cumulative effect. A person with a phospholipid antibody is highly susceptible during and after a surgical treatment, which involves immobilization and bed rest. Another example of cumulative risk is an elderly patient with FVL who takes a long car trip.
Epidemiology
Virchow’s triad is named after Rudolph Virchow, who established three characteristics that predispose to thrombus (Correia et al., 2022; Subhani et al., 2022; Tan et al., 2022). This triad involves endothelial damage, blood flow stasis or turbulence, and hypercoagulability (Chen and Baker, 2022; Gade et al., 2017). Figure 5 shows Virchow’s triad. In DVT caused by trauma or surgery, stasis, and endothelial damage are crucial, whereas hypercoagulability is responsible for the majority of instances of spontaneous DVT. Inflammatory mechanisms are also involved in thrombosis, according to later research. Other linked factors include immune system component activation, blood oxygen concentration, microparticle status, and potential platelet activation. Upon damage to the endothelial vessel wall, platelets attach to exposed collagen on the surface of the vessel wall and become activated, releasing chemicals that in turn generate nearby platelets (Baiges et al., 2023; Keokgale et al., 2022). The development of a fibrin network connecting activated platelets also aids in the formation of clots.
A number of research studies have been conducted expressly to investigate the epidemiology of VTE. Anderson and Spencer (2003) examined the hospital discharge documents of all patients diagnosed with VTE, including both recurring and first-time episodes, over an 18-month period in Worcester, Massachusetts, in the mid-1980s (Coon, 1977). The number of instances was minimal, and 97% of the people were Caucasian (Carter, 1994; Heit, 2005). Silverstein et al. (1998), examined the hospital documents of all Olmsted county individuals identified with VTE between 1966 and 1990 (Kearon, 2001; Kim and Spandorfer, 2001). Importantly, whether or not the PE was clinically symptomatic, a considerable majority of instances of PE discovered at postmortem were classified as definitive VTE (Nair et al., 2022; Semrad et al., 2007; White, 2003). Every year, nearly 10 million people of all races are affected by VTE, which contributes significantly to global illness. Acute VTE occurs in 1–2 occurrences per 1,000 people per year. For both men and women, the incidence rises dramatically with age (Danwang et al., 2017; Diaz and Jo, 2022). VTE is associated with a significant mortality rate; in Europe, the predicted yearly number of VTE-related fatalities is more than double that of prostate cancer, AIDS, and breast cancer.
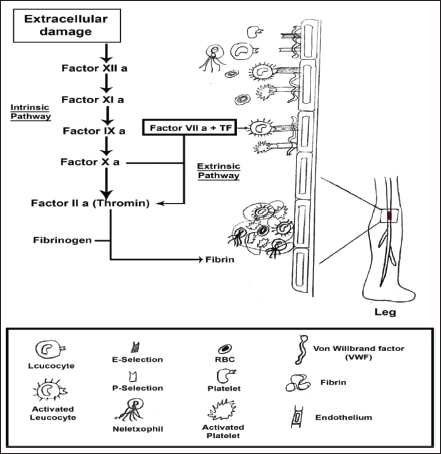 | Figure 3. Pathogenesis of venous thrombosis. [Click here to view] |
Diagnosis and prevention of VTE
Persons at risk of VTE must initially be identified to minimize its occurrence. The best therapy for VTE depends on an objective diagnosis. Despite the fact that VTE is difficult to diagnose clinically, a variety of methods based on clinical characteristics have been shown to be useful and reliable in estimating the likelihood of VTE. The American College of Physicians and American Academy of Family Physicians have produced guidelines for identifying VTE (Fraser et al., 2002; Kearon et al., 2022; Mazzolai et al., 2021; Omar Mohamed Ozaal and Fernando, 2022). Although clinical pretest probability and D-dimer testing can effectively screen out VTE, imaging studies are needed to confirm the diagnosis (Schafer et al., 2022; Silveira Vieira et al., 2022). Since the signs and symptoms of DVT and PE are nonspecific, only around 20% of patients who are evaluated for suspected VTE get their diagnosis confirmed (Behrouzi and Punter, 2018; Martens et al., 2022; Rindi et al., 2022). As a result, imaging all patients suspected of having VTE is difficult. Since diagnostic imaging techniques are time-consuming, expensive, and linked with radiation exposure (e.g., CT) and side effects. Compression ultrasonography is currently the only noninvasive study option for a clinically considered VTE diagnosis. Impedance plethysmography is less sensitive and specific than ultrasonography; however, it is still useful in pregnant women and those with suspected recurrent VTE. Noninvasive blood tests, such as fibrin D-dimer, help with diagnosis. For individuals with a very low pretest chance of DVT, a high-sensitivity D-dimer assay is suggested, and for people who have a medium-to-high pretest risk of DVT, an ultrasound is suggested. Similarly, diagnostic imaging is seen as critical for individuals with a pretest likelihood of PE that is medium or high (Schaefer et al., 2017). The agency for healthcare research and quality has identified effective VTE prevention as a high priority in hospital safety procedures. The risk of VTE can be reduced with either mechanical or pharmaceutical prevention. These techniques are effective VTE prevention strategies. Patients should be categorized based on risk to utilize the best preventative approach throughout therapy (Kesieme et al., 2011; Nicholson et al., 2020). Intermittent pneumatic compression (IPC) devices, graded compression stockings, and venous foot pumps are examples of mechanical prophylaxis against VTE. Mechanical techniques improve blood flow in the deep veins of the leg, avoiding venous stasis and venous thrombosis (Guo et al., 2022; Mishra et al., 2022; Xue et al., 2022). Effective pharmacological treatments for the prevention of VTE include unfractionated heparin (UFH), low-molecular-weight heparins (LMWH), and novel oral direct-selective thrombin inhibitors and factor Xa inhibitors. Low-risk people receiving general surgery really do not require any special prophylaxis. Then, the fixed low dosages of UFH or LMWH are enough for those with intermediate risk. LMWH should be used at higher dosages for orthopedic and general surgeries with a high degree of risk. When combined with anticoagulants, IPC, and compression elastic stockings (CES) may provide high-risk patients with safety (Augustinos and Ouriel, 2004; McRae and Ginsberg, 2004; Sachdeva et al., 2010). Combining pharmacological and mechanical methods to prevent VTE may be more effective than the mechanical method alone. When anticoagulation is contraindicated, CES and IPC may be utilized for moderate- or high-risk individuals. When anticoagulation and mechanical methods are contraindicated in patients at high risk of VTE, inferior vena cava filter (IVCF) installation should be used (Agnelli and Sonaglia, 2000; Hu et al., 2022; Velmahos et al., 2000).
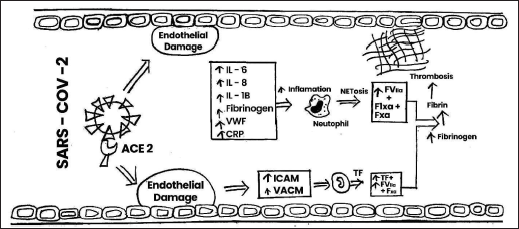 | Figure 4. Mechanism of action of SARS-COV-2 on thrombosis vascular cell adhesion molecule, intercellular adhesion molecule, ACE-2, IL, VWF, CRP, TF. [Click here to view] |
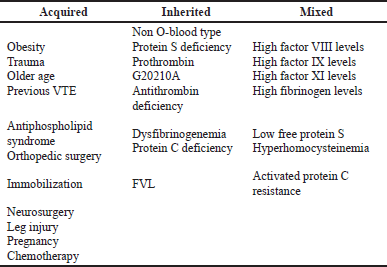 | Table 1. Risk factors for VTE. [Click here to view] |
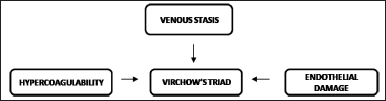 | Figure 5. Virchow’s triad. [Click here to view] |
DISCUSSION
The results of this systematic analysis of the evidence indicate that the treatment for VTE seeks to reduce the risk of long-term problems, thrombus extension, embolization, cardiac collapse, mortality, and recurrent VTE. For patients with proven VTE, anticoagulation is the initial therapy (Sagris et al., 2022; Scarvelis and Wells, 2006; Vedantham et al., 2016). Anticoagulation with heparin followed by a vitamin K antagonist was the mainstay of therapy for VTE in patients without malignancy (Flumignan et al., 2022; Nazario et al., 2002). LMWH has a reduced risk of recurrent venous thrombosis and severe hemorrhage compared with UFH and is the recommended heparin drug in all patients with a glomerular filtration rate of 30 ml/minute or above (Fujioka et al., 2022; Nutescu et al., 2016). In the United States, three LMWHs have recently become available such as enoxaparin, tinzaparin, and dalteparin. These LMWHs have distinct pharmacological properties with distinct Food and Drug Administration (FDA)-approved uses and dose regimes. Fondaparinux, a synthetic pentasaccharide, was recently created and authorized by the FDA (Ashrafi et al., 2022; Weimar et al., 2012; Yoo et al., 2022). Anticoagulation with rivaroxaban, edoxaban, apixaban, and dabigatran is equally effective as LMWH followed by vitamin K antagonist treatment in lowering the risk of recurrent VTE and is linked with a decreased risk of significant bleeding (Diavati et al., 2022; Lastimosa, 2022; Murphy et al., 2022). LMWH is preferred over vitamin K antagonist treatment in individuals with VTE and cancer due to its greater efficacy and equivalent safety (Comerota, 2012; Fleck et al., 2017; Gross and Weitz, 2008). In addition to anticoagulation, thrombolysis is used in patients with proximal DVT to quickly resolve thrombi, maintain venous function, and avoid post-thrombotic syndrome (Hann and Streiff, 2005). Systemic thrombolysis seeks to quickly achieve reperfusion and stop mortality in individuals with PE (Albertson et al., 2023). Tissue plasminogen activator, urokinase, and streptokinase are some of the thrombolytic drugs that are readily accessible. Finally, for individuals with recently diagnosed proximal DVT or PE, permanent or recoverable IVCF may be used when anticoagulant medication is contraindicated (e.g., recent bleeding, upcoming surgery) (Alhassan et al., 2017; Sheahan et al., 2023). There have been few efficacy and safety studies conducted on the use of filters in patients. Permanent filters should not be used and should be replaced as soon as the patient begins receiving anticoagulation.
CONCLUSION
VTE is a complicated condition that comprises DVT to PE. The use of either technical or medicinal protection has been found to lower the occurrence of VTE. Endothelial impairment, thrombophilia, stagnant, and reduced venous blood flows could all describe the elevated prevalence of thrombotic occurrence in COVID-19 patients. The development of Novel oral anticoagulants (NOACs) has expanded the anticoagulant options for the diagnosis and management of VTE. Even though the enormous advancements gained in anticoagulant management with the arrival of NOACs, many fundamental obstacles remain, notably the absence of specialized antidotes and the unavailability of a precise hemorrhage are the most problematic adverse effects. Anticoagulants nearly always raise the probability of bleeding, thus customized therapeutic approaches that include risks, expense, effectiveness, and patient characteristics are necessary. Even though substantial advancements are made, additional study is required to pinpoint patient risk specifically in hopes of providing optimal care. The capacity of nanomaterials to transfer pharmacotherapeutics to illness regions has exhibited remarkable possibilities in the management of a broad spectrum of illness problems. Several of these medicines’ distinctive features have the ability to manage VTE. It will need further investigation to ascertain how nanomaterials will operate and which combos of target chemicals, forms, and therapies will benefit VTE sufferers.
AUTHORS’ CONTRIBUTIONS
PP: Methodology, data extraction, and writing original paper. JPD: methodology and editing. VPM: conceived the review, methodology, data extraction, editing, and supervision.
FINANCIAL SUPPORT
There is no financial support received in this work.
CONFLICT OF INTEREST
The authors declare no relevant conflicts of interest or financial relationships.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abad Rico JI, Llau Pitarch JV, Rocha E. Overview of venous thromboembolism. Drugs, 2010; 70:3–10.
Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci, 2013; 3(2):143.
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation, 2008; 117(1):93–102.
Agnelli G, Sonaglia F. Prevention of venous thromboembolism. Thromb Res, 2000; 97(1):V49–62.
Albertson N, Rice M, Schmitz A, Eskew J, Haste P, Johnson MS. Clinical and imaging outcomes of option ELITE vena cava filter placement procedures. Vasc Surg Venous Lymphat Disord, 2023; 11(2):310–7.
Alhassan S, Pelinescu A, Gandhi V, Naddour M, Singh AC, Bihler E. Clinical presentation and risk factors of venous thromboembolic disease. Crit Care Nurs Q, 2017; 40(3):201–9.
Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation, 2003; 107.
Ashrafi M, Ahmad SB, Antoniou SA, Khan T, Antoniou GA. Treatment strategies for proximal deep vein thrombosis: a network meta-analysis of randomised controlled trials. J Vasc Surg, 2022; 75(3):1120.
Auger J, Munnix I, Cosemans J, Heemskerk J. Platelet response heterogeneity in thrombus formation. Thromb Haemost, 2009; 102(12):1149–56.
Augustinos P, Ouriel K. Invasive approaches to treatment of venous thromboembolism. Circulation, 2004; 110(9_suppl_1):I–27.
Baiges A, Reverter JC, Pagán JC. Reply to:“factor VIII as a potential predictor of recurrent thrombosis in patients with non-cirrhotic portal vein thrombosis”. J Hepatol, 2023; 78(3):e111–2.
Bajaj A, Sethi A, Rathor P, Suppogu N, Sethi A. Acute complications of myocardial infarction in the current era. J Investig Med, 2015; 63(7):844–55.
Bauersachs RM. Clinical presentation of deep vein thrombosis and pulmonary embolism. Best Pract Res Clin Haematol, 2012; 25(3):243–51.
Behera S, Pramanik K, Nayak M. Recent advancement in the treatment of cardiovascular diseases: conventional therapy to nanotechnology. Curr Pharm Des, 2015; 21(30):4479–97.
Behrouzi R, Punter M. Diagnosis and management of cerebral venous thrombosis. Clin Med, 2018; 18(1):75–9.
Blann AD, Lip GY. Venous thromboembolism. BMJ, 2006; 332(7535):215–9.
Bruni-Fitzgerald KR. Venous thromboembolism: an overview. J Vasc Nurs, 2015; 33(3):95–9.
Buller HR, Sohne M, Middeldorp S. Treatment of venous thromboembolism. J Thromb Haemost, 2005; 3(8):1554–60.
Carter CJ. The natural history and epidemiology of venous thrombosis. Prog Cardiovasc Dis, 1994; 36(6):423–38.
Chen C, Baker L. Unlikely cause of primary upper limb venous thrombosis. J Med Imaging Radiat Oncol, 2022; 66(8):1087–8.
Chopard R, Nielsen P, Ius F, Cebotari S, Ecarnot F, Pilichowski H, Schmidt M, Kjaergaard B, Sousa-Casasnovas I, Ghoreishi M, Narayan RL. Optimal reperfusion strategy in acute high-risk pulmonary embolism requiring extracorporeal membrane oxygenation support: a systematic review and meta-analysis. Eur Respir J, 2022; 60(5):2102977.
Comerota AJ. Thrombolysis for deep venous thrombosis. J Vasc Surg, 2012; 55(2):607–11.
Coon WW. Epidemiology of venous thromboembolism. Ann Surg, 1977; 186(2):149–64.
Correia R, Gião N, Bento R, Garcia R, Camacho N, Ferreira ME. Cystic adventitial disease of the popliteal vein, a rare cause of lower limb deep vein thrombosis. EJVES Vasc Forum, 2022; 54:75–8.
Cross MB, Boettner F. Pathophysiology of venous thromboembolic disease. Semin Arthroplasty, 2009; 20(4):210–6.
Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J Thromb Haemost, 2017; 15(9):1770–81.
Dewar C, Panpher S. Incidence of deep vein thrombosis in patients diagnosed with superficial thrombophlebitis after presenting to an emergency department outpatient deep vein thrombosis service. Emerg Med J, 2010; 27(10):758–61.
Diavati S, Sagris M, Terentes-Printzios D, Vlachopoulos C. Anticoagulation treatment in venous thromboembolism: options and optimalduration. Curr Pharm Des, 2022; 28(4):296–305.
Diaz M, Jo J. Venous thrombotic events and anticoagulation in brain tumor patients. Curr Oncol Rep, 2022; 24(4):493–500.
Fleck D, Albadawi H, Shamoun F, Knuttinen G, Naidu S, Oklu R. Catheter-directed thrombolysis of deep vein thrombosis: literature review and practice considerations. Cardiovasc Diagn Ther, 2017; 7(Suppl 3):S228.
Flinterman L, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost, 2008; 6(8):1262–6.
Flumignan CD, Nakano LC, Baptista-Silva JC, Flumignan RL. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database Syst Rev, 2022; 7(7):CD012369.
Fraser DGW, Moody AR, Morgan PS, Martel AL, Davidson I. Diagnosis of lower-limb deep venous thrombosis: a prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med, 2002; 136(2):89.
Fujioka S, Kitamura T, Shikata F, Mishima T, Onishi Y, Araki H, Goto H, Sasahara A, Fukuzumi M, Torii S, Miyaji K. Outcomes after rivaroxaban treatment of extensive deep vein thrombosis. Ann Vasc Surg, 2022; 85:246–52.
Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med, 2008; 359(9):938–49.
Gade IL, Brækkan S, Næss IA, Hansen JB, Rosendaal F, Cannegieter S, Overvad K, Jensvoll H, Hammerstrøm J, Gran OV, Tjønneland A. Epidemiology of venous thromboembolism in hematological cancers: the Scandinavian thrombosis and cancer (STAC) cohort. Thromb Res, 2017; 158:157–60.
Garcia D, Spyropoulos A. Erratum “update in the treatment of venous thromboembolism.” Semin Respir Crit Care Med, 2008; 29(3):319.
Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haemat, 2012; 25(3):235–42.
Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Bio, 2008; 28(3):380–6.
Guo PC, Li N, Zhong HM, Zhao GF. Clinical effectiveness of a pneumatic compression device combined with low-molecular-weight heparin for the prevention of deep vein thrombosis in trauma patients: a single-center retrospective cohort study. World J Emerg Med, 2022; 13(3):189.
Guo F, Shashikiran T, Chen X, Yang L, Liu X, Song L. Clinical features and risk factor analysis for lower extremity deep venous thrombosis in Chinese neurosurgical patients. J Neurosci Rural Pract, 2015; 06(04):471–6.
Hann CL, Streiff MB. The role of vena caval filters in the management of venous thromboembolism. Blood Rev, 2005; 19(4):179–202.
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost, 2005; 3(8):1611–7.
Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis, 2016; 41(1):3–14.
Hirsh J, Hull RD, Raskob GE. Clinical features and diagnosis of venous thrombosis. J Am Coll Cardiol, 1986; 8(6):114B–27B.
Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood, 2002; 99(9):3102–10.
Hu Q, Fang Z, Ge J, Li H. Nanotechnology for cardiovascular diseases. Innovation (Camb), 2022; 3(2):100214.
Hu J, Geng Y, Ma J, Dong X, Fang S, Tian J. The best evidence for the prevention and management of lower extremity deep venous thrombosis after gynecological malignant tumor surgery: a systematic review and network meta-analysis. Front Surg, 2022; 9:841275.
Hyers TM. Venous thromboembolism. Am J Respir Crit Care Med, 1999; 159(1):1–14.
Kearon C. Epidemiology of venous thromboembolism. Semin Vasc Med, 2001; 01(01):007–26.
Kearon C, de Wit K, Parpia S, Schulman S, Spencer FA, Sharma S, Afilalo M, Kahn SR, Le Gal G, Shivakumar S, Bates SM. Diagnosis of deep vein thrombosis with D-dimer adjusted to clinical probability: prospective diagnostic management study. BMJ, 2022; 376:e067378.
Keokgale T, van Blydenstein SA, Kalla IS. Evaluation of the modified Wells score in predicting venous thromboembolic disease in patients with tuberculosis or HIV in a South African setting. S Afr J HIV Med, 2022; 23(1):1349.
Kesieme E, Kesieme C, Jebbin N. Deep vein thrombosis: a clinical review. J Blood Med, 2011; 2:59.
Key NS. Bench to bedside: new developments in our understanding of the pathophysiology of thrombosis. J Thromb Thrombolysis, 2013; 35(3):342–5.
Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet, 2021; 398(10294):64–77.
Kim SM. Clinical presentation of isolated calf deep vein thrombosis in inpatients and prevalence of associated pulmonary embolism. J Vasc Surg Venous Lymphat Disord, 2022; 10(5):1037–43.
Kim V, Spandorfer J. Epidemiology of venous thromboembolic disease. Emerg Med Clin North Am, 2001; 19(4):839–60.
Kumar DKL, Likhitha DU. A hospital based study on etiology and clinical features of varicose veins. Int J Surg, 2022; 6(1):151–4.
Lapner ST, Kearon C. Diagnosis and management of pulmonary embolism. BMJ, 2013; 346:f757.
Lastimosa H. Direct oral anticoagulant versus low molecular weight heparin for the treatment of venous thromboembolism associated with malignancy: a meta-analysis. Eur Heart J, 2022; 43(Supplement_1):ehab849–118.
Lavorini F, Di Bello V, De Rimini ML, Lucignani G, Marconi L, Palareti G, Pesavento R, Prisco D, Santini M, Sverzellati N, Palla A. Diagnosis and treatment of pulmonary embolism: a multidisciplinary approach. Multidiscip Respir Med, 2013; 8(1):1–8.
Leitner JM, Jilma B, Spiel AO, Sterz F, Laggner AN, Janata KM. Massive pulmonary embolism leading to cardiac arrest is associated with consumptive coagulopathy presenting as disseminated intravascular coagulation. J Thromb Haemost, 2010; 8(7):1477–82.
Lowe GD. Factor IX and thrombosis. Br J Haematol, 2001; 115(3):507–13.
Martens ES, Huisman MV, Klok FA. Diagnostic management of acute pulmonary embolism in COVID-19 and other special patient populations. Diagnostics, 2022; 12(6):1350.
Mazzolai L, Ageno W, Alatri A, Bauersachs R, Becattini C, Brodmann M, Emmerich J, Konstantinides S, Meyer G, Middeldorp S, Monreal M. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC working group on aorta and peripheral vascular diseases and the ESC working group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol, 2021; 29(8):1248–63.
McRae SJ, Ginsberg JS. Initial treatment of venous thromboembolism. Circulation, 2004; 110(9_suppl_1):I–3.
Montoro-García S, Schindewolf M, Stanford S, Larsen O, Thiele T. The role of platelets in venous thromboembolism. Semin Thromb Hemost, 2016; 42(03):242–51.
Mishra A, Sharma S, Goel P. Deep vein thrombosis: prophylaxis and management. Knee Arthroplasty, 2022:745–55.
Murphy AC, Koshy AN, Farouque O, Yeo B, Raman J, Kearney L, Yudi MB. Factor Xa inhibition for the treatment of venous thromboembolism associated with cancer: a meta-analysis of the randomised controlled trials. Heart Lung Circ, 2022; 31(5):716–25.
Musil D, Ková?ik F. Delay between clinical presentation and treatment of deep venous thrombosis in the lower limbs and regression of thrombosis. Phlebology, 2021; 37(2):120–4.
Myers DD. Pathophysiology of venous thrombosis. Phlebology, 2015; 30(1_suppl):7–13.
Nair V, Singh S, Ashraf MZ, Yanamandra U, Sharma V, Prabhakar A, Ahmad R, Chatterjee T, Behera V, Guleria V, Patrikar S. Epidemiology and pathophysiology of vascular thrombosis in acclimatized lowlanders at high altitude: a prospective longitudinal study. Lancet Reg Health Southeast Asia, 2022; 3:100016.
Nazario R, Delorenzo LJ, Maguire GP. Treatment of venous thromboembolism. Cardiol Rev, 2002; 10(4):249–59.
Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of venous thromboembolism in 2020 and beyond. J Clin Med, 2020; 9(8):2467.
Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Erratum to: pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis, 2016; 42(2):296–311.
Obi AT, Barnes GD, Napolitano LM, Henke PK, Wakefield TW. Venous thrombosis epidemiology, pathophysiology, and anticoagulant therapies and trials in severe acute respiratory syndrome coronavirus 2 infection. J Vasc Surg Venous Lymphat Disord, 2021; 9(1):23–35.
Omar Mohamed Ozaal AM, Fernando T. Deep vein thrombosis in an elderly patient with chronic limb-threatening ischaemia presented with limb swelling: the role of diagnostic tools and surgical dilemma. SAGE Open Med Case Rep, 2022; 10:1–5.
Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res, 2021; 200:1–8.
Rali PM, Criner GJ. Submassive pulmonary embolism. Am J Respir Crit Care Med, 2018; 198(5):588–98.
Rindi LV, Al Moghazi S, Donno DR, Cataldo MA, Petrosillo N. Predictive scores for the diagnosis of pulmonary embolism in COVID-19: a systematic review. Int J Infect Dis, 2022; 115:93–100.
Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev, 2010; 7:CD001484.
Sagris M, Tzoumas A, Kokkinidis DG, Korosoglou G, Lichtenberg M, Tzavellas G. Invasive and pharmacological treatment of deep vein thrombosis: a scoping review. Curr Pharm Des, 2022; 28(10):778–86.
Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. CMAJ, 2006; 175(9):1087–92.
Schaefer JK, Jacobs B, Wakefield TW, Sood SL. New biomarkers and imaging approaches for the diagnosis of deep venous thrombosis. Curr Opin Hematol, 2017; 24(3):274–81.
Schafer K, Goldschmidt E, Oostra D, Kaminski B, Mattin M, Lurie F. Defining the role of risk stratification and duplex ultrasound in the diagnosis of acute lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord, 2022; 10(5):1021–7.
Semrad TJ, O’Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg, 2007; 106(4):601–8.
Sheahan KP, Tong E, Lee MJ. A review of inferior vena cava filters. Br J Radiol, 2023; 96(1141):20211125.
Silveira Vieira AL, Muniz PazeliJúnior J, Silva Matos A, Marques Pereira A, Rezende Pinto I, Esteves de Oliveira Silva L, SiqueiraGuilherme L, Archângelo e Silva SL. Ultrasonographic evaluation of deep vein thrombosis related to the central catheter in hemodialytic patients. Ultrasound J, 2022; 14:1–6.
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism. Arch Intern Med, 1998; 158(6):585.
Stone J, Hangge P, Albadawi H, Wallace A, Shamoun F, Knuttien MG, Naidu S, Oklu R. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther, 2017; 7(Suppl 3):S276.
Streiff MB, Agnelli G, Connors JM, Crowther M, Eichinger S, Lopes R, McBane RD, Moll S, Ansell J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis, 2016; 41(1):32–67.
Subhani M, Sheth A, Ahmed J, Wijayasiri P, Gardezi SA, Enki D, Morling JR, Aithal GP, Ryder SD, Aravinthan AD. Incidence and prevalence of venous thromboembolism in chronic liver disease: a systematic review and meta-analysis. Thromb Res, 2022; 215:19–29.
Tan R, Daneshmand A, Parys S, Watanabe Y, Sieunarine K. Splanchnic venous thrombosis: aetiologies and a review of the literature. ANZ J Surg, 2022; 92(9):2224–8.
Thomas MR, Scully M. Clinical features of thrombosis and bleeding in COVID-19. Blood, 2022; 140(3):184–95.
Turpie AG. ABC of antithrombotic therapy: venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ, 2002; 325(7369):887–90.
Vedantham S, Piazza G, Sista AK, Goldenberg NA. Guidance for the use of thrombolytic therapy for the treatment of venous thromboembolism. J Thromb Thrombolysis, 2016; 41(1):68–80.
Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based report—part I: analysis of risk factors and evaluation of the role of vena caval filters. J Trauma Acute Care Surg, 2000; 49(1):132–9.
Voicu S, Ketfi C, Stépanian A, Chousterman BG, Mohamedi N, Siguret V, Mebazaa A, Mégarbane B, Bonnin P. Pathophysiological processes underlying the high prevalence of deep vein thrombosis in critically ill COVID-19 patients. Front Physiol, 2021; 11:1680.
Wadajkar AS, Santimano S, Rahimi M, Yuan B, Banerjee S, Nguyen KT. Deep vein thrombosis: current status and nanotechnology advances. Biotechnol Adv, 2013; 31(5):504–13.
Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol, 2008; 28(3):387–91.
Wang XY, Zhang F, Zhang C, Zheng LR, Yang J. The biomarkers for acute myocardial infarction and heart failure. Biomed Res Int, 2020; 2020:1–14.
Weimar C, Masuhr F, Hajjar K. Diagnosis and treatment of cerebral venous thrombosis. Expert Rev Cardiovasc Ther, 2012; 10(12):1545–53.
Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA, 2014; 311(7):717.
White RH. The epidemiology of venous thromboembolism. Circulation, 2003; 107(23_suppl_1):I–4.
Xue ZQ, Tu WJ, Gao JQ, Dong ZT, Yuan JD, Lang JZ. Optimal preoperative timing for prevention of deep vein thrombosis (DVT) in patients over 60 years of age with intertrochanteric fractures. Eur J Trauma Emerg Surg, 2022; 48(5):4197–203.
Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord, 2015; 15(1):1.
Yoo HHB, Nunes-Nogueira VS, Fortes Villas Boas PJ, Broderick C. Outpatient versus inpatient treatment for acute pulmonary embolism. Database Syst Rev, 2022; 5(5):CD010019.
Zhang L, Li Z, Ye X, Chen Z, Chen ZS. Mechanisms of thrombosis and research progress on targeted antithrombotic drugs. Drug Discov Today, 2021; 26(10):2282–302.