INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA), a Gram-positive bacteria (GPB), is at the forefront of the current worldwide health crisis of antimicrobial resistance (AMR) (Prestinaci et al., 2015). The spread of MRSA from hospital to community settings, together with increasing resistance to non-β-lactam antibiotics, has precipitated the crisis (Lohan et al., 2021). Methicillin resistance in S. aureus was encountered soon after methicillin was approved for clinical use against penicillinase-producing S. aureus in 1961. Subsequently, MRSA was encountered in Australia, Europe, the United States (US), and Japan (Udo, 2013). The global prevalence of MRSA is difficult to determine, whereas national surveillance data and publications from all World Health Organization (WHO) regions reported a prevalence ranging from 0% to 100%: African Region (0%–100%), Region of America (21%–90%), Eastern Mediterranean region (10%–53%), European region (0.3%–55%), South-east Asia region (10%–26%), Western Pacific region (4%–70%) (Hassoun et al., 2017; Prestinaci et al., 2015; Wangai et al., 2019). One report from India showed the prevalence ranging between 13% and 74% in different parts of the world (Pradhan et al., 2021). MRSA is one of the most common causes of surgical site infections in tertiary care hospitals in North America, accounting for more than 60% of all infections in these units (Chatterjee et al., 2018). MRSA is a common emerging pathogen in India, with an overall prevalence rate ranging from 26% to 59% and 13% to 47% in intensive care units (ICUs) (Mehta et al., 2020; Taneja and Sharma, 2019). A systematic review and a meta-analysis reported a 37% overall prevalence of MRSA (2015 to 2019) in India. State-by-state stratified results of MRSA prevalence varied from 55% in Jammu & Kashmir to 21% in Maharashtra (Fig. 1) (Patil et al., 2022).
MRSA is linked to significant morbidity and mortality, in addition to significant economic and societal costs, underlining the importance of accurate surveillance. MRSA infects the skin, soft tissues, bone, and joints, urinary tract in addition to triggering metastatic infections such as septic arthritis, infective endocarditis (IE), osteomyelitis, and device-associated infections associated with prosthetic devices and indwelling catheters (Tong et al., 2015). MRSA is a leading cause of bacteremia in developed nations, potentially leading to complications such as sepsis and septic shock (Hassoun et al., 2017; Kwiecinski and Horswill, 2020). Due to the more frequent use of different urinary catheters such as indwelling or condom catheters in debilitated patients, the incidence of urinary tract infection (UTI) caused by MRSA is rising globally. A multicentric study from India showed 55% of MRSA were isolated from urine samples (Mendem et al., 2016; Mitiku et al., 2021). In the pre-antibiotic period, S. aureus bacteremia faced more than 80% mortality. Despite the discovery of penicillin G, improving the prognosis substantially in the early 1940s, resistant strains were identified as early as 1942 (Peacock and Paterson, 2015). MRSA is associated with poorer clinical outcomes than methicillin-sensitive S. aureus (MSSA) (van Hal et al., 2012). WHO reported that MRSA infections are 64% more likely to kill than drug-sensitive infections. A systematic review and meta-analysis showed that the societal cost for one case of community-acquired (CA) MRSA from the Asia-Pacific region was estimated to be $7,070–$20,489 (Wong et al., 2018).
Implementing an effective treatment for MRSA infections requires identifying the pathogen. Diagnostic and treatment delays adversely impact clinical outcomes. While standard techniques for microbial identification take 48 to 72 hours, newer rapid diagnostic tests provide results in 3 hours, enabling optimized antimicrobial therapy, and thereby reducing mortality, hospitalization, and healthcare costs (Bauer et al., 2010; Palavecino, 2014).
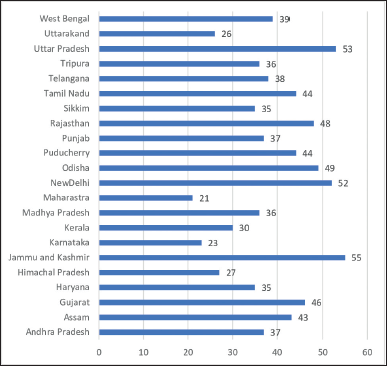 | Figure 1. Prevalence of MRSA (%) from different states of India between 2015 and 2020. [Click here to view] |
This review outlines epidemiological trends and factors affecting the incidence of MRSA infections, resistance patterns, current diagnostic tools, treatments, prevention strategies, and associated healthcare costs in India.
EPIDEMIOLOGY
Carrier status
MRSA can colonize the normal body flora, particularly in the nose, axillae, anomalous skin (eczema, wounds), urine, rectum, and throat, and act as a reservoir. MRSA can infect, particularly those undergoing prolonged hospitalization in a high-risk unit (critical care, renal unit, etc.) or suffering from underlying diseases, or after antibiotic use. MRSA can colonize the body following trauma, wounds, surgical incisions, and indwelling medical devices (Bradley, 2015). Cutaneous and nasal colonization of MRSA is estimated to be around 7% in US hospitals. Numerous reports suggest that the prevalence of MRSA nasal carriage among healthcare workers (HCWs) was between 5.5% and 34%. (Goes et al., 2021). Contact transmission from HCWs to patients is the chief mode of MRSA transmission. Prevalence of MRSA nasal carriers ranges from 1% to 52.3% including children as per multiple reports from India. The highest incidence of MRSA nasal carrier was reported in Brazil, at 74.6% (Chatterjee et al., 2009; George et al., 2016; Goes et al., 2021). A study from India reported the overall prevalence of MRSA carriers among healthcare professionals as 6.5% of whom 28.4%, 21.1%, 9%, and 5.4%, and, 37.5% were physicians, nursing interns, MBBS interns, nurses (5.4%), and others (physiotherapist, housekeeping staff, and helping staff), respectively (Deepashree et al., 2021). Another Indian study found that the overall MRSA transmission rate among HCWs who worked with critically ill patients was only 2.5% with female housekeeping staff (13.3%) accounting for the majority of the cases, followed by female nursing staff (2.7%), much lower than the 4.6% identified in a global meta-analysis (Radhakrishna et al., 2013). Although the colonized patient (or staff member) does not require treatment, a course of decolonization therapy (e.g., povidone Iodine, chlorhexidine-neomycin nasal cream, mupirocin nasal ointment, and systemic antibiotics) may be administered to eradicate carriage and prevent future infections (Lepelletier et al., 2020).
The overall global (reported in 2010) and Indian prevalence between (2015 and 2019) was found to be 13%–74% and 37%, respectively (Patil et al., 2022; Pradhan et al., 2021).
MRSA is commonly classified as either Hospital (HA) or CA. HA-MRSA generally manifests as a nosocomial infection, often acquired during a surgical or invasive medical procedure when a hospital length of stay (LOS) is more than 48 hours. CA-MRSA is found in people who have not recently been hospitalized or had contact with the healthcare system or those who underwent less than 48 hours of hospitalization (Sutton and Steiner, 2016). The phenotypic and genotypic differences between CA-MRSA and HA-MRSA are represented in Table 1 (Bukharie, 2010; George et al., 2016).
MRSA used to be primarily restricted to hospitals but has dramatically increased its spread among people without risk factors or healthcare exposure. Discovery of novel MRSA strains (CA-MRSA), over the last decade has been linked to the spread. CA-MRSA strains appear to have spread swiftly among the general population around the world, affecting people with and without healthcare exposure (Lohan et al., 2021). India currently reports 3.89% to 74% of CA-MRSA isolates (Chatterjee et al., 2018; Joshi et al., 2013). A systematic review and meta-analysis showed that the global pooled prevalence of CA-MRSA and HA-MRSA ranged from 0% to 23.5% and 0.7% to 10.4%, respectively, with maximum prevalence in India (16.5% to 23.5%) (Wong et al., 2018). A study from Finland showed the overall prevalence of CA-MRSA and HA-MRSA as 32% and 68%, respectively (Junnila et al., 2020). Figures 2a and b depict the overall prevalence of CA-MRSA and HA-MRSA across different states of India and countries.
Risk factors
The most frequently associated risk factors for MRSA infection are prolonged hospitalization, ICUs, recent hospitalization, admission to nursing homes, co-morbidities, recent antibiotic use, MRSA colonization in HCWs or patient parties, invasive procedures, HIV infection, hemodialysis, open wounds, and discharge with long-term central venous catheters or indwelling urinary catheter (Lee et al., 2018).
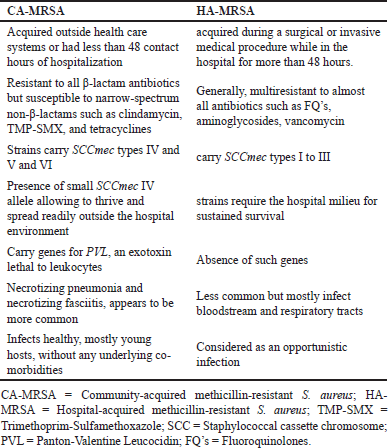 | Table 1. Phenotypic and genotypic differences between CA-MRSA and HA-MRSA. [Click here to view] |
Although growing older is not considered a risk factor in and of itself for MRSA infection, elderly people (>65 years) are at substantial risk for hospitalization, implying an indirect link between advancing age and MRSA infection. A major risk factor for MRSA colonization includes living in a high prevalence zone of CA-MRSA or admission to a hospital with a high incidence of HA-MRSA (National Nosocomial Infections Surveillance System, 2004). Numerous studies have shown that risk factors for MRSA infection differ around the globe. An Indian study reported that prolonged hospitalization, surgery, recent hospitalization, presence of a tracheostomy tube, and pressure/venous ulcer were significant independent risk factors for MRSA infection in hospitalized patients (Thimmappa et al., 2021). Staphylococcus aureus is still the most prevalent bacterium that infects wounds, and surgery increases the risk of infection. Patients with open fractures are more likely to be infected than those with closed fractures or open injuries (Zalavras, 2017).
Molecular characterization of MRSA
Methicillin resistance in clinical isolates entails detecting a methicillin-hydrolyzing-lactamase and mecA-encoded protein-binding protein2a (PBP2a), which reduces the penicillin-binding affinity and increases the rate of release from the bound drug (Stapleton and Taylor, 2002).
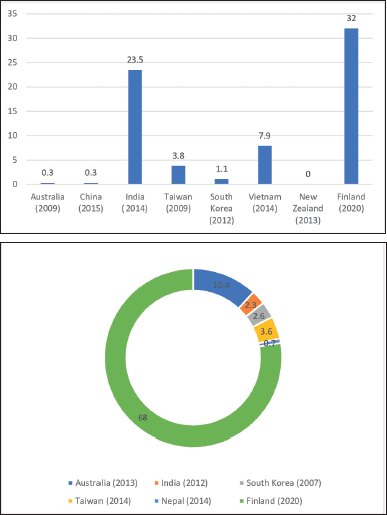 | Figure 2. (a) Prevalence of CA-MRSA (%) from different studies across the globe. (b) Prevalence of HA-MRSA (%) from different studies across the globe. [Click here to view] |
The acquisition of the mecA gene, which is carried by a mobile genetic element called the staphylococcal cassette chromosome (SCCmec), confers methicillin resistance. According to the International Working Group on the classification of staphylococcal cassette chromosome elements, there are currently 13 recognized SCCmec types (I–XIII), based on the combinations of five mec complexes (A, B, C1, C2, and E) (Urushibara et al., 2020). SCCmec types I, II, or III are found in the majority of HA-MRSA strains, whereas SCCmec types IV or V are found in CA-MRSA. Among the mecA-positive strains isolated from Mumbai (2010), 25% were SCCmec III and all were multidrug-resistant (MDR) strains. Others were SCCmec IV and SCCmec V with 34% and 41%, respectively. Seventy-five percent of the strains were susceptible to antimicrobials. The multidrug susceptibility of strains with SCCmec IV and SCCmec V demonstrates the susceptible nature of CA-MRSA. Multiple Asian studies using multilocus sequence typing (MLST) showed clonal expansion of multidrug-susceptible sequence type (ST) 22 (SCCmec IV) and ST 772 (SCCmec V), which may be slowly replacing the multidrug-resistant ST 239 (SCCmec III) in hospitals (D’Souza et al., 2010). HA-MRSA has larger SCCmec types than CA-MRSA, which confers resistance to more non-β-lactam antibiotics. Consequently, CA-MRSA is sensitive to a wider variety of antibiotics than HA-MRSA (Loewen et al., 2017). In addition, Panton-Valentine Leucocidin (PVL) is a pore-forming toxin encoded by the lukSF-PV genes that encode a potent cytotoxin that is mainly found in CA-MRSA. USA300 is the most common PVL-positive clone in the US. USA300 is an MRSA clone within (MLST) clonal complex 8 that harbors the PVL genes. The prevalence of PVL-positive clones from 2004 to 2008 ranges between 16% and 40% (Brown et al., 2012). At present, there are no USA300 clones reported from India. There are very few studies on genotyping MRSA strains from India. The worldwide distribution of PVL among MRSA isolates varies. Kaur et al. (2012), Bouchiat et al. (2015), and D’souza et al. (2010) from India reported the highest prevalence of 85.1%, 68.8%, and 64%, respectively, of PVL-positive clones (Bouchiat et al., 2015; D’Souza et al., 2010; Kaur et al., 2012). Another Indian study found that the PVL gene was present in 70% and 7.8% of CA-MRSA and HA-MRSA, respectively (Preeja et al., 2021). The prevalence of PVL-positive clones from different countries is shown in Figure 3 (Afroz et al., 2008; Brown et al., 2012; Darboe et al., 2019; Holmes et al., 2005; Lina et al., 1999; Moussa and Shibl, 2009).
The major clone of MRSA present in Indian hospitals is ST772-MRSA-V (also called Bengal Bay clone). Originally identified in India, ST772 is noted for its global distribution. Complete genome sequencing showed that ST772 carried several toxin genes such as PVL, staphylococcal enterotoxin (sea) genes, and β-haemolysin (hlb). When compared to prophage-cured strains, virulence studies revealed that ST772 strains induce substantial neutrophil proliferation and cytotoxicity, implying that a novel prophage may contribute to ST772 virulence (Blomfeldt et al., 2017; Sunagar et al., 2016). Furthermore, there was a complex relationship between the distribution of virulence genes and the source and location of isolation. For instance, a study from India showed that 84% of MRSA strains isolated from patients with pharyngitis carried the pathogenic gene icaADBC. The same study also found that icaA/icaD-positive MRSA and MSSA were isolated from wound and ocular infections, respectively (Gowrishankar et al., 2016). The MRSA ST239 clone, another significant HA infection (HAI)-causing clone discovered in India, exhibits a range of global resistances and susceptibilities to mupirocin, aminoglycosides, and trimethoprim-sulphamethoxazole (TMP-SMX). Despite the large number of genotypes that exist, comparative genomic investigations have revealed that epidemic strains of MRSA seem to be constrained to specific genotypes, some of which are also geographically restricted (Sunagar et al., 2016). There is a dearth of information on MRSA ST239 from India, with a significant MRSA prevalence. According to a study conducted in India, ST 239 primarily contained SCCmec V (50%), followed by SCCmec III (32%), SCCmec I (16%), and SCCmec IV (2%) (Abimanyu et al., 2012).
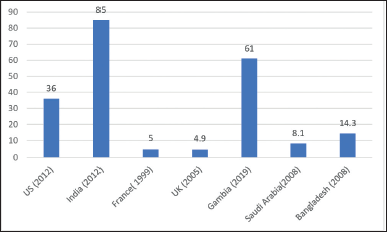 | Figure 3. Prevalence (%) of PVL-positive clones from different countries. [Click here to view] |
DIAGNOSTIC METHODS
Detection of the mecA gene by polymerase chain reaction (PCR) is currently the gold standard test for detecting MRSA. Additionally, Food and drug administration (FDA)-approved assays for molecular identification of the mecA gene and commercially accessible chromogenic agars for MRSA detection are available. Finally, MRSA can be detected via latex agglutination or immunochromatographic membrane testing for PBP2a. Clinical and Laboratory Standards Institute (CLSI) recommends the oxacillin disc diffusion (ODD) test, oxacillin screen agar [mannitol salt agar (MSA)],, and cefoxitin disc diffusion (CDD) test to detect methicillin resistance by phenotypic approaches. CHROMagar (color-based differentiation method), another phenotypic method, uses a chromogenic medium for identifying MRSA. Cefoxitin induces mec-A gene expression more powerfully than other compounds. Because of its extended shelf life, oxacillin is chosen over methicillin (Alipour et al., 2014; Kali et al., 2014; Lohan et al., 2021). Studies have been compared for different parameters like speed, cost of treatment, sensitivity, and specificity with PCR for the mecA gene. A study from India found that the ODD method had a sensitivity and specificity of 93.5% and 83.5%, respectively whereas MSA with the oxacillin method had a sensitivity and specificity of 87.1% and 89.3% respectively (Pillai et al., 2012). A study from Iran, comparing different MRSA detection methods found that the sensitivity and specificity, of the Antimicrobial susceptibility testing (AST) (ODD) method, were lesser than the CDD method and PCR (Table 2) (Pourmand et al., 2014). PCR, on the other hand, was found to be faster and less expensive than other procedures. For instance, the sensitivity of “n%” indicates among N true positives, only “n” will be diagnosed as positives and the remaining “N-n” will be misdiagnosed. Misdiagnosing MRSA isolates is unacceptable since the treatment pattern changes. Instead of getting vancomycin, the patient will be recommended another line of treatment for MSSA, compromising the cure rate. That MRSA would have spread to other patients or HCWs by this time is much more disconcerting. Finally, vancomycin will be forced upon the patient, raising the cost of therapy, culminating in the spread of MRSA in both the hospital and the community. Thus costs of a misdiagnosis (missing MRSA) will be significantly greater than the cost of PCR (Pillai et al., 2012).
A study from Malaysia developed a unique approach termed monoplex PCR assay for the identification of the non-protein-coding RNA gene (Sau-02) in MRSA, which displayed good sensitivity and specificity (Soo Yean et al., 2016).
Susceptibility pattern of MRSA
Methicillin resistance in S. aureus is defined as an oxacillin minimum inhibitory concentration (MIC) of ≥4 μg/ml based on antibiotic susceptibilities (Siddiqui and Koirala, 2023). Except for glycopeptide drugs, most MRSA strains are resistant to multiple antibiotics including methicillin, amikacin, tobramycin, gentamicin, ciprofloxacin, norfloxacin, tetracycline, erythromycin, TMP-SMX, and cefoperazone/sulbactam.
Antibiotic sensitivity profiles can be used to categorize MRSA as either healthcare-associated or community-associated. Macrolide–lincosamide–streptogramin B (MLSB) antibiotics such as clindamycin susceptibility, for example, has a 95% sensitivity, 80% specificity, and a likelihood ratio of 4.86 in predicting CA-MRSA. Isolates resistant to three or more non-β-lactam antibiotics are categorized as HA-MRSA (Loewen et al., 2017). Resistance to MSLB antibiotics can be either constitutive (cMLSB) or inducible (iMLSB). The cMLSB resistance mechanism is mediated via msrA genes, i.e., efflux of antibiotics in which S. aureus strains are resistant to erythromycin but sensitive to clindamycin, both in vitro and in vivo. The constitutively resistant strains do not develop clindamycin resistance during the therapy. The iMLSB-resistant isolates show resistance against erythromycin but are susceptible to clindamycin. iMLSB resistance develops in the presence of a powerful methylase enzyme inducer like erythromycin. The erm genes encode enzymes that confer inducible or constitutive resistance to MLSB agents by methylating the 23S ribosomal RNA, thereby lowering Macrolide–lincosamide–streptogramin agent binding to the ribosome (Sasirekha et al., 2014). Unlike cMLSB resistance, iMLSB resistance cannot be detected by standard susceptibility testing. The inducible clindamycin resistance can be detected by the D-zone test (D-shaped distorted inhibition zone around clindamycin under the in-vitro effect of erythromycin) (Fig. 4). iMLSB resistance should be determined for the effective management of S. aureus, without which, the administration of clindamycin may result in treatment failure from the emergence of constitutive resistance (Thapa et al., 2021). Previous studies (cross-sectional investigations) from India have shown that the prevalence of iMLSB among S. aureus ranges between (7% and 94%) (Patel et al., 2006).
Vancomycin was first employed as an empirical therapy for nosocomial sepsis in the 1980s, due to the high incidence of MRSA. Vancomycin use in the US increased in the early 1990s because of the growing incidence of coagulase-negative staphylococci (CoNS) infections and clostridium difficile in healthcare institutions (Rubinstein and Keynan, 2014). Consequently, selection pressure mounted, resulting in the establishment of S. aureus and other staphylococci strains with increased resistance to vancomycin and other glycopeptides (Szymanek-Majchrzak et al., 2018). In 1997, Japan reported the first S. aureus strain non-susceptible to vancomycin and teicoplanin, followed by the US in 2002 (Howden et al., 2010; Spagnolo et al., 2014). Tiwari and Sen (2006) reported the first instance of Vancomycin-resistant S. aureus/Vancomycin-resistant S. aureus (VRSA/VISA) in India in 2006, despite the absence of the vanA/vanB gene. Therefore, the absence of vanA/vanB genes in these isolates does not rule out the possibility of vancomycin resistance. Vancomycin resistance in GPB was thought to be rare until recently, but vancomycin resistance in S. aureus, CoNS, and Enterococcus spp., has been reported across the globe. Before 2010, and between 2010 and 2019, the global distribution of VRSA was reported to be 1.2% and 2%, respectively (Fig. 4) (Shariati et al., 2020).
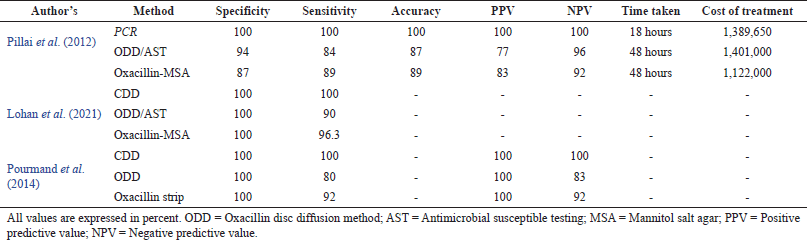 | Table 2. Sensitivity and specificity of different methods used in the detection of MRSA. [Click here to view] |
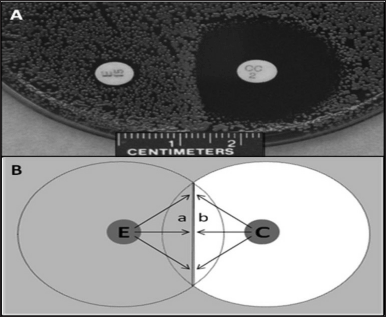 | Figure 4. A positive D-test. A positive D-shaped inhibitory zone is visible around clindamycin in Figure A. The left and right discs are for erythromycin and clindamycin, respectively. The projected O-shaped clindamycin zone of growth inhibition in all negative D-test is blunted on the side that faces the erythromycin disc, resulting in a D-shaped zone. Figure B is a stylized representation of the D-test. Erythromycin molecules diffuse into the area of the clindamycin zone denoted “a” prior to clindamycin molecules, triggering the methylase, granting resistance, and permitting microbiological growth despite the arrival of clindamycin concentrations that would otherwise be inhibitory. Before erythromycin molecules can get there to cause resistance, quantities of clindamycin that impede growth get to the area marked “b.” Gray areas represent microbial growth on the agar surface. White areas denote growth inhibition; E stands for the erythromycin disc; C stands for the clindamycin disc. The image is adapted from the study by Charles et al. (2009). [Click here to view] |
Table 3 provides the susceptibility data from various parts of India resistance patterns of CA-MRSA and HA-MRSA have been combined because most studies do not report them separately. Various Indian investigations found that MRSA is responsive to last-resort antibiotics like linezolid and teicoplanin. Vancomycin and doxycycline are still effective in treating MRSA infections in Indian clinical settings.
SUPERINFECTION WITH MRSA IN COVID-19 INDIVIDUALS
Hospitalized patients with COVID-19 frequently develop acute pneumonia and other life-threatening conditions. There is considerable variation in methodologies and outcomes between research, which may contribute to the elusiveness of the precise burden of MRSA pulmonary infection in individuals with COVID-19. Frequent use of vancomycin (one of the last resorts) as an empirical therapy, has raised concerns over the efficacy of vancomycin against MRSA for the past decade (Punjabi et al., 2020). In relation to research revealing the prevalence of MRSA lung infection along with other complications A study from the US reported patients with COVID-19 superinfected with MRSA (45%) who had pneumonia also developed additional serious illnesses such as bacteremia in 19% of cases, and 30-day mortality of 67%. The most commonly used empiric therapy was the vancomycin-cefepime combination (Cusumano et al., 2020). The WHO “Watch list of antibiotics” for 2019 includes agents like vancomycin as critical stewardship priorities (Punjabi et al., 2020). There is a severe lack of information on bacterial secondary infections in India, and only a very small number of studies—out of hundreds—report secondary infections, and even those do not provide specific information on the pathogens that caused the infections, or their drug susceptibility profiles. The fact that almost all of the studies are from various Asian countries, such as China, may restrict the generalizability of the conclusions. There are no studies that provide data on MRSA superinfection and its management. India needs such prospective studies, which should contain epidemiological, clinical, and microbiological data on superinfections. These data can be utilized to create successful antimicrobial stewardship policies, which can be extremely important for administering the right amount of antibiotics.
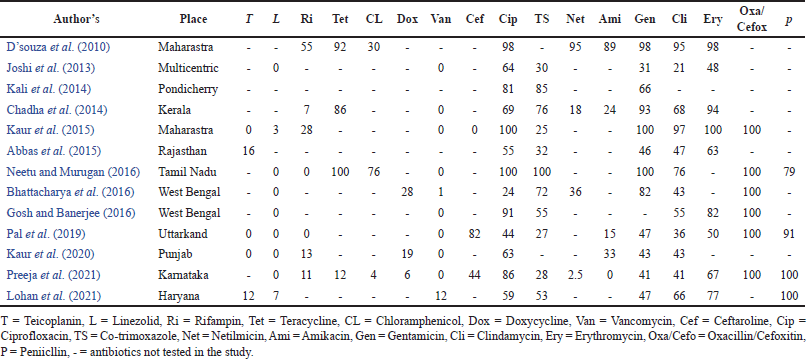 | Table 3. Resistance pattern of MRSA from different Indian studies. [Click here to view] |
MORTALITY
Clinically, MDR HA-MRSA infections are linked to high mortality and morbidity, limiting the choice of appropriate antibiotics (Moosavian et al., 2017). Table 4 shows the global case fatality rate due to MRSA infection-induced complications. HA-MRSA bacteremia increased ICU and LOS, antibiotic prescription length, and attributable mortality in multiple investigations (Chatterjee et al., 2018). This could be because MRSA caused more invasive infections, with multiple co-morbidities and complications, delaying the proper antibiotic therapy, all of which retard recovery. The global mortality due to MRSA ranges from 15% to 60% (Siddiqui and Koirala, 2023). An Indian study showed a 27% case fatality rate due to MRSA bacteremia, with the presence of the PVL gene in most of the strains (Table 4) (Eshwara et al., 2013). An Iranian study showed that SCCmec type III was responsible for MRSA’s lower sensitivity to various antibiotics (Moosavian et al., 2017). An Indian study found SCCmec type II behind higher LOS, poor antibiotic susceptibility, and death (Chatterjee et al., 2018).
COST OF MRSA INFECTION
The economic implications of MRSA infections have been studied extensively. Lee et al. (2015) reported the overall cost of SSTIs caused by MRSA in the US at $13.8 billion in 2012, with per capita cost of $22 706. Frequent hospitalizations and expensive second-line antibiotics increase treatment costs substantially. A study from the US showed that intravenous (IV) antibiotic administration is associated with 42% of SSTIs hospital admissions in the US, suggesting that shifting healthcare delivery away from the inpatient setting, as well as the use of longer half-life antibiotics (e.g., oritavancin, dalbavancin) could help to reduce costs (Wiseman et al., 2015).
 | Table 4. Global mortality rate due to MRSA infections. [Click here to view] |
Similarly, LOS in SSTI also influences healthcare costs. A study from the US showed that surgical wound infections resulted in increased LOS (5.81 days) and the highest overall expenses ($9388). Co-morbidities such as diabetes, renal insufficiency, and immunological compromise, increase SSTI costs by prolonging LOS (Kaye et al., 2019, 2015).
Furthermore, MRSA infections have been linked to poorer patient outcomes and higher healthcare expenses than MSSA infections. A study from China found that MRSA colonization or infection involves higher total hospital costs ($3,220 to $9,606), as well as extra LOS of 6–14 days (Zhen et al., 2020). There is a dearth of data on the cost of MRSA infections in India. A study (2011) from India found that the median overall cost of anti-MRSA therapy was roughly $124 ($45–$484), excluding the expense of treating coexisting diseases. The usage of additional antibiotics to treat MRSA infection from the date of infection diagnosis to the date of discharge or death, it was found that the median cost of therapy per day was roughly $17. Based on World Bank data from 2005, Chen et al. (2010) stated that the median cost of anti-MRSA therapy alone (including drug preparation and administration) was more expensive than the quarterly incomes of more than 40% of the 1.2 billion inhabitants living in India (Chen and Ravallion, 2008; Christopher et al., 2011).
India needs more data on rising MRSA infections to help hospitals and regulatory bodies plan resource allocation, frame regulations, and guide insurance companies to create budgetary plans.
AVAILABLE TREATMENT FOR MRSA INFECTIONS
Since 2005, the FDA has granted fast-track approval to several novel antibiotics for MRSA, especially for SSTIs. These medications consist of omadacycline, ceftaroline, delafloxacin, dalbavancin, tedizolid, oritavancin, and telavancin. However, these medications are only taken into account as alternatives or are completely absent from the current Infectious Diseases Society of America (IDSA) guidelines for MRSA infection and SSTIs (Hindy et al., 2022). Currently, vancomycin or daptomycin is the approved empirical therapy for MRSA infections (Siddiqui and Koirala, 2023). Telavancin, ceftaroline, and linezolid are utilized as second-line therapy (Choo and Chambers, 2016).
Vancomycin and teicoplanin (slower bactericidal activity than vancomycin) are the gold standards for treating drug-resistant GPBs. However, concerns about declining susceptibility and sluggish bactericidal effects may be partly associated with clinical failures in IE and bacteremia. Vancomycin’s limitations have prompted the development of other antibiotics (Choo and Chambers, 2016). Limitations include the antibiotic’s low penetration into infected tissues in the lungs, heart, and meninges, which has been linked to poor treatment outcomes in serious infections such as pneumonia, IE, and meningitis. Second, numerous S. aureus strains produce biofilms, restricting vancomycin’s antimicrobial action. Third, enterococci and staphylococci strains have started developing vancomycin resistance. An increase in the MICs of vancomycin against MRSA was linked to worse outcomes. The CLSI lowered vancomycin’s breakpoint from 4 to 2 g/ml for susceptible strains, from 8–16 to 4–8 g/ml for intermediately susceptible strains, and from 32 to 16 g/ml for resistant strains (Dunbar et al., 2008).
Vancomycin is effective when coupled with a wide range of β-lactam antibiotics, possibly because of the “see-saw” effect, in which lower vancomycin susceptibility suppresses mecA transcription, thereby increasing β-lactam susceptibility (Barber et al., 2014). A retrospective study and a randomized clinical trial showed an increased rate of clearance of bacteremia in patients on a combination of vancomycin and β-lactam than vancomycin alone (Dilworth et al., 2014). However, there is little evidence in favor of vancomycin coupled with other antistaphylococcal drugs. In another retrospective study, in which vancomycin was continued in 12 patients, with an aminoglycoside added in 6, rifampin in 4, and both aminoglycoside and rifampin added in 2, only two cases were cleared of bacteremia after 72 hours (Jang et al., 2009).
The synergistic combination of daptomycin and β-lactam is significantly better than daptomycin alone in lowering the risk of clinical failure in MRSA bloodstream infection (Jorgensen et al., 2020). Table 5 provides the IDSA treatment guidelines for managing different types of MRSA infections in adults and children.
Reports of growing resistance to vancomycin, linezolid, and dalfopristin have raised doubts about their effectiveness. Clindamycin, a reserved option, was chosen by clinicians to treat MRSA isolates because of the drug’s superior pharmacokinetics. However, repeated use of MRSA is developing clindamycin resistance with time (Thapa et al., 2021).
Clindamycin as monotherapy or in combination with other antibiotics has not presented sufficient evidence, despite being advised as a second-line agent for MRSA pneumonia. Staphylococcus aureus susceptibilities to clindamycin have dropped below 40% over the past few years in the US, therefore, it is crucial to make sure the isolate is susceptible. D-testing can rule out any inducible resistance. As for the tetracyclines, support for minocycline was based on data from limited retrospective studies (Liu et al., 2011; Park, 2019).
Although clindamycin, doxycycline, minocycline, and TMP-SMX have good bioavailability and lung penetration (ideal characteristics), there is a paucity of evidence to employ them in MRSA pneumonia. Therefore, physicians should consider each case carefully and base their choice to use these medications on the findings of susceptibility tests. To establish the effectiveness of these medications, more clinical study is necessary (Hong et al., 2019).
Telavancin, a lipoglycopeptide, is used in the management of serious clinical infections caused by GPBs (MRSA, VISA), such as complicated skin and skin structure infections (cSSSI) and pneumonia. Phase II (FAST trial) and phase III trials suggest that telavancin can treat cSSSI rapidly in a concentration-dependent manner, with bactericidal properties and excellent efficacy. Comparing IV telavancin 10 mg/kg od to standard therapy (IV nafcillin 2 g qid or IV vancomycin 1 gm bd), it was found that the telavancin-treated group had a higher clinical success rate with 96% versus 94%, and 88% and 87%, in Phase II and Phase III trials, respectively (Polyzos et al., 2012). Another trial revealed that telavancin-treated patients experienced adverse events (AEs) more frequently than vancomycin (90% vs. 72%), with a 7% drug discontinuation rate in both treatment groups. Additionally, the telavancin group had a higher prevalence of clinically significant elevations (1.5 mg/dl or at least 50% greater than baseline) in blood creatinine (20% vs. 7%) (Stryjewski et al., 2014).
Linezolid has been found effective against cSSSIs, including diabetic foot infections (DFIs) without accompanying osteomyelitis, caused by MRSA and MSSA, simple SSSIs caused by MSSA, Community-acquired pneumonia (CAP), and bacteremia caused by MSSA have all been observed to respond favorably to linezolid (Hashemian et al., 2018).
According to the most recently published recommendations for treating MRSA pneumonia, linezolid is a first-line antibiotic. Linezolid has also been demonstrated in multiple tests to be more effective than vancomycin in treating various diseases. In treating situations like SSSIs and nosocomial pneumonia, linezolid may still be preferable to vancomycin, but this is still up for discussion. Recent research has confirmed the clinical effectiveness of linezolid in cSSSIs, including DFIs without osteomyelitis (Liu et al., 2011; Rodvold and McConeghy, 2014; Wunderink et al., 2012).
Linezolid and vancomycin are equally effective in treating HAP, according to a systematic review and meta-analysis. Additionally, the findings revealed that linezolid, comparator vancomycin, and teicoplanin did not differ statistically from one another in the study of infection eradication. The incidence of AE, such as gastrointestinal problems and thrombocytopenia, was higher with linezolid than with glycopeptides (Hashemian et al., 2018).
In another study, the effectiveness of linezolid against vancomycin for treating burns, abscesses, cellulitis, infected ulcers, or deeper soft tissues was examined. When treating MRSA-infected SSTIs, found linezolid therapy was superior to vancomycin and the drug-related AE was similar in both the linezolid and vancomycin groups (Fu et al., 2013; Yue et al., 2014).
Studies have shown that linezolid administration is more cost-effective than vancomycin in the treatment of MRSA infection due to early hospital discharge In general, compared to vancomycin, linezolid may lower patient mortality (Hashemian et al., 2018; Reveles et al., 2015).
In addition to appropriate antibiotic therapy, consultation for infectious diseases lowers the death rate due to MRSA bacteremia (Lahey et al., 2009). This improved result is probably at least partially attributable to the adoption of a number of quality practices advised by consultants, such as follow-up blood cultures, and echocardiography, to ensure clearance and a thorough search for additional infection foci requiring surgical management. American Heart Association recommends surgery for IE because MRSA is associated with valve dysfunction, potentially leading to heart failure, anatomic complications (e.g. heart blocks, valve perforations, and perivalvular extension), or with a high risk of embolization (Punjabi et al., 2020).
Prevention of MRSA infection in hospital settings
Centers for disease control and prevention recommends contact precautions in acute care settings for inpatients known to be colonized or infected with MRSA or any other MDR organisms. In the US, between 2005 and 2014, the estimated incidence of invasive MRSA infections from normally sterile sources (pleural fluid, blood, etc.) decreased by 40%, while the estimated incidence of invasive HA-MRSA infections decreased by 65%. Both contact precautions and hand hygiene likely played a role in such declines (CDC, 2020).
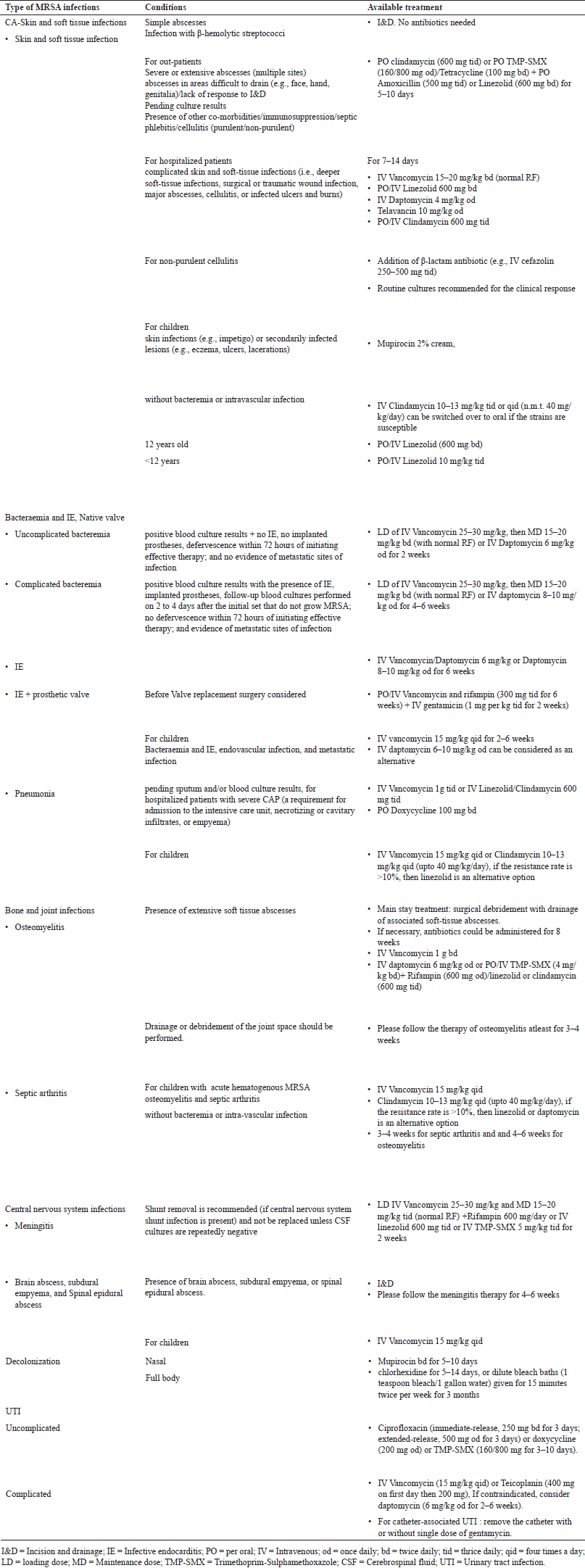 | Table 5. Antibiotic treatment guidelines for different types of MRSA infections in adults and children based on IDSA treatment guidelines. [Click here to view] |
Although debated, there is minimal concrete evidence for an environmental role in S. aureus transmission, except in burns units. Dust, environmental surfaces, and medical devices (e.g. curtains, switches or buttons such as in ventilators, feeding and infusion pumps, phones, computer keyboards, touch panel screens, door handles, light switches, bed tables, bed rails, mattresses, and even pens act as reservoirs for MRSA and other GPBs in general, which easily transfer to hands upon touch (Price et al., 2017). Experts agree that preventing MRSA transmission by hand is the most important aspect of MRSA control. Hand hygiene should be ensured before and after each physical contact with a patient or their immediate environment, including before aseptic procedures, handling or manipulating invasive devices, injections through venous catheters, emptying drains or catheters, entering and exiting critical care units, isolation rooms, and open rooms where MRSA cases are cohorted (Mathur, 2011).
All HCWs and administrative personnel with clinical involvement must comprehend the significance of hand hygiene, and follow the national recommendations of “NATIONAL GUIDELINES FOR INFECTION PREVENTION AND CONTROL IN HEALTHCARE FACILITIES” India (NCDC, 2020). The use of protective clothing such as long-sleeved apron, gowns, and gloves are an important component of the control of healthcare-associated infection. Emerging evidence suggests that nurses’ uniforms readily get contaminated in high-risk environments like critical care units. Before leaving the patient’s environment, the protective apron/gown is removed (WHO, 2014).
Prevention of MRSA infection in the community settings
For patients with skin and soft-tissue infections, clinicians should provide recommendations on personal hygiene and wound care. Clean, dry bandages should be used to cover draining wounds. Hand cleaning with soap and water or an alcohol-based hand gel is recommended on a regular basis, especially after encountering diseased skin or an item that has come in. Recurrent infections despite appropriate personal hygiene and wound care can be remedied by decolonization. Nasal decolonization with mupirocin and topical body decolonization with a skin antiseptic solution (e.g., chlorhexidine) are all options for decolonization. Oral antibiotic medication should only be used to treat active infections; it is not indicated for decolonization. Asymptomatic household contacts may also benefit from decolonization initiatives (Creech et al., 2015).
Population surveillance program for CA-MRSA and HA-MRSA in India
Indian Council of Medical Research (ICMR) and the National Centre for Disease Control have established a nationwide network of laboratories to monitor AMR. Infection control systems are in place at many private hospitals and autonomous institutes. Network laboratories conduct surveillance on AMR trends in different geographical regions of India. Surveillance also includes documenting the emergence of methicillin resistance among community isolates of S. aureus to inform empirical therapy; describing the occurrence and impact of severe S. aureus disease in a community, regardless of resistance pattern; and facilitating timely identification of potential outbreaks. HAI surveillance is available in NABH-accredited hospitals. The All India Institute of Medical Sciences and the ICMR have collaborated to build an HAI surveillance network with 35 public and private sector centers. ICMR has developed web-based tools such as ICMR’s Antimicrobial Resistance Surveillance system, ICMR’s Data import app, and ICMR’s Antimicrobial Resistance Surveillance system using integrative technologies to track HAIs and CAIs. The one-stop AMR data repository has collected over 0.4 million patient records thus far. The entire system is currently being used to collect human susceptibility testing data; but, utilizing the ‘One Health’ paradigm, it can be extended for AMR surveillance (Kaur et al., 2022; NCDC, 2020; Walia et al., 2019).
CONCLUSION
MRSA has become a pervasive infectious agent. MRSA, which is becoming more virulent than ever, continues to aggravate morbidity and mortality. MRSA can also become resistant to new therapeutic agents, with the exception of vancomycin, which has been regarded as effective for the past 40 years. The current circumstance emphasizes the importance of ongoing MRSA and antibiogram surveillance in tertiary care settings as well as outlying hospitals. Regular MRSA surveillance of HCWs, strong hand hygiene compliance, and the framing of antibiotic policies with efficient infection control procedures are the most effective ways to avoid MRSA infection. Studies on COVID-19 superinfections and the cost of MRSA infection should be undertaken to assist hospitals in allocating healthcare resources and making appropriate medical decisions, as well as patients and insurance companies in budgeting. We need to get the information out loud and clear: we’re running out of antibiotics against S. aureus, and unless we stop abusing antibiotics, we’ll be left with no way to fight this dreadful pathogen. All HCWs must practice good hand hygiene. If put into practice, these control measures might help prevent the spread of this dreaded bacterium both in hospitals and among the general population.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas A, Nirwan PS, Srivastava P. Prevalence and antibiogram of hospital acquired-methicillin resistant Staphylococcus aureus and community acquired-methicillin resistant Staphylococcus aureus at a tertiary care hospital National Institute of Medical Sciences. Community Acquir Infect, 2015; 2(1):13–5. doi: https://doi.org/10.4103/2225-6482.153857
Abimanyu N, Murugesan S, Krishnan P. Emergence of methicillin-resistant Staphylococcus aureus ST239 with high-level mupirocin and inducible clindamycin resistance in a tertiary care center in Chennai, South India. J Clin Microbiol, 2012; 50(10):3412–13. doi: https://doi.org/10.1128/JCM.01663-12
Afroz S, Kobayashi N, Nagashima S, Alam MM, Hossain ABMB, Rahman MA, Islam MR, Lutfor AB, Muazzam N, Khan MAH, Paul SK, Shamsuzzaman AKM, Mahmud MC, Musa AKM, Hossain MA. Genetic characterization of Staphylococcus aureus isolates carrying panton-valentine leukocidin genes in Bangladesh. Japan J Infect Dis, 2008; 61(5):393–96.
Alipour F, Ahmadi M, Javadi S. Evaluation of different methods to detect methicillin resistance in Staphylococcus aureus (MRSA). J Infect Public Health, 2014; 7(3):186–91. doi: https://doi.org/10.1016/j.jiph.2014.01.007
Barber KE, Ireland CE, Bukavyn N, Rybak MJ. Observation of ‘Seesaw effect’ with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther, 2014; 3(1):35–43. dai: https://doi.org/10.1007/s40121-014-0023-0
Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program’s impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis, 2010; 51(9):1074–80. doi: https://doi.org/10.1086/656623
Bhattacharya S, Pal K, Jain S, Chatterjee SS, Konar J. Surgical site infection by methicillin resistant Staphylococcus aureus–on decline? J Clin Diagn Res, 2016; 10(9):DC32–36. doi: https://doi.org/10.7860/JCDR/2016/21664.8587
Blomfeldt A, Larssen KW, Moghen A, Haugum K, Steen TW, Jørgensen SB, Aamot HV. Bengal bay clone ST772-MRSA-V outbreak: conserved clone causes investigation challenges. J Hosp Infect, 2017; 95(3):253–58. doi: https://doi.org/10.1016/j.jhin.2016.12.006
Bouchiat C, El-Zeenni N, Chakrakodi B, Nagaraj S, Arakere G, Etienne J. Epidemiology of Staphylococcus aureus in Bangalore, India: emergence of the ST217 clone and high rate of resistance to erythromycin and ciprofloxacin in the community. New Microb New Infect, 2015; 7:15–20. doi: https://doi.org/10.1016/j.nmni.2015.05.003
Bradley SF. MRSA colonisation (Eradicating colonisation in people without active invasive infection). BMJ Clin Evid, 2015; 2015:0923.
Brown ML, Patrick O’Hara F, Close NM, Mera RM, Miller LA, Suaya JA, Amrine-Madsen H. Prevalence and sequence variation of panton-valentine leukocidin in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains in the United States. J Clin Microbiol, 2012; 50(1):086–90. doi: https://doi.org/10.1128/JCM.05564-11
Bukharie HA. A review of community-acquired methicillin-resistant Staphylococcus aureus for primary care physicians. J Fam Community Med, 2010; 17(3):117–20. doi: https://doi.org/10.4103/1319-1683.74320
CDC. Preventing infections in healthcare MRSA CDC. 2020. Available via https://www.cdc.gov/mrsa/healthcare/inpatient.html (Accessed 5 May 2023)
Chadha T, Syeda NK, Shashikant A, Prabhakar CM. Comparison of antibiotic susceptibility pattern of community-and hospital-acquired methicillin-resistant Staphylococcus aureus with special reference to inducible clindamycin resistance in a tertiary care hospital in Southern India. Med J D Y Patil Univ, 2014; 7(4):439–42. doi: https://doi.org/10.4103/0975-2870.135257
Chatterjee A, Rai S, Guddattu V, Mukhopadhyay C, Saravu K. Is methicillin-resistant Staphylococcus aureus infection associated with higher mortality and morbidity in hospitalized patients? A cohort study of 551 patients from South Western India. Risk Manag Healthc Policy, 2018; 11:243–50. doi: https://doi.org/10.2147/RMHP.S176517
Chatterjee SS, Ray P, Aggarwal A, Das A, Sharma M. A community-based study on nasal carriage of Staphylococcus aureus. Indian J Med Res, 2009; 130(6):742–48.
Chen S, Ravallion M. The developing world is poorer than we thought, but no less successful in the fight against poverty.Q J Econ, 2008; 125(4):1577–625. doi: https://doi.org/10.1596/1813-9450-4703
Chen S, Ravallion M. The developing world is poorer than we thought, but no less successful in the fight against poverty. Q J Econ, 2010; 125(4):1577–625. doi: https://doi.org/10.1162/qjec.2010.125.4.1577
Choo EJ, Chambers HF. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect Chemother, 2016; 48(4):267–73. doi: https://doi.org/10.3947/ic.2016.48.4.267
Christopher S, Verghis RM, Antonisamy B, Sowmyanarayanan TV, Brahmadathan KN, Kang G, Cooper BS. Transmission dynamics of methicillin-resistant Staphylococcus aureus in a medical intensive care unit in India.PLos One, 2011; 6(7):e20604. doi: https://doi.org/10.1371/journal.pone.0020604
Creech CB, Al-Zubeidi DN, Fritz SA. Prevention of recurrent Staphylococcal skin infections. Infect Dis Clin North Am, 2015; 29(3):429–64. doi: https://doi.org/10.1016/j.idc.2015.05.007
Cusumano JA, Dupper AC, Malik Y, Gavioli EM, Banga J, Caban AB, Nadkarni D, Obla A, Vasa CV, Mazo D, Altman DR. Staphylococcus aureus bacteremia in patients infected with COVID-19: a case series. Open Forum Infect Dis, 2020; 7(11):ofaa518. doi: https://doi.org/10.1093/ofid/ofaa518
Darboe S, Dobreniecki S, Jarju S, Jallow M, Mohammed NI, Wathuo M, Ceesay B, Tweed S, Roy RB, Okomo U, Kwambana-Adams B, Antonio M, Bradbury RS, de Silva TI, Forrest K, Roca A, Lawal BJ, Nwakanma D, Secka O. Prevalence of panton-valentine leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an Urban Gambian Hospital: a 11-year period retrospective pilot study. Front Cell Infect Microbiol, 2019; 9:170. Available via https://www.frontiersin.org/article/10.3389/fcimb.2019.00170
Deepashree R, Khanum S, Sujatha SR, Tejashree A, Prasad N, Ramya BV. Methicillin-resistant Staphylococcus aureus (MRSA) carriage among health care personnel in nonoutbreak settings in tertiary care hospital in Mysore. Am J Infect Control, 2021; 49(12):1499–502. doi: https://doi.org/10.1016/j.ajic.2021.06.013
Dilworth TJ, Ibrahim O, Hall P, Sliwinski J, Walraven C, Mercier RC. β-lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother, 2014; 58(1):102–9. doi: https://doi.org/10.1128/AAC.01204-13
D’Souza N, Rodrigues C, Mehta A. Molecular characterization of methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. J Clin Microbiol, 2010; 48(5):1806–11. doi: https://doi.org/10.1128/JCM.01867-9
Dunbar LM, Tang DM, Manausa RM. A review of telavancin in the treatment of complicated skin and skin structure infections (CSSSI). Ther Clin Risk Manag, 2008; 4(1):235–44. doi: https://doi.org/10.2147/tcrm.s1843
Eshwara VK, Munim F, Tellapragada C, Kamath A, Varma M, Lewis LE, Mukhopadhyay C. Staphylococcus aureus bacteremia in an Indian tertiary care hospital: observational study on clinical epidemiology, resistance characteristics, and carriage of the panton–valentine leukocidin gene. Int J Infect Dis, 2013; 17(11):e1051–55. doi: https://doi.org/10.1016/j.ijid.2013.06.002
Fu J, Ye X, Chen C, Chen S. The efficacy and safety of linezolid and glycopeptides in the treatment of Staphylococcus aureus infections. PLos One, 2013; 8(3):e58240. doi: https://doi.org/10.1371/journal.pone.0058240
George K, Abdulkader JK, Sugumar M, Rajagopal GK. Prevalence of MRSA nasal carriage in patients admitted to a tertiary care hospital in Southern India. J Clin Diagn Res JCDR, 2016; 10(2):DC011–13. doi: https://doi.org/10.7860/JCDR/2016/18259.7262
Ghosh S, Banerjee M. Methicillin resistance & inducible clindamycin resistance in Staphylococcus aureus. Indian J Med Res, 2016; 143(3):362–4. doi: https://doi.org/10.4103/0971-5916.182628
Goes ICRS, Romero LC, Turra AJ, Gotardi MA, de Oliveira Rodrigues TFS, de Oliveira Santos L, das Dores JC, do Nascimento MU, Cavalleri AC, Pinheiro-Hubinger L, Eller LKW, Pereira VC. Prevalence of nasal carriers of methicillin-resistant Staphylococcus aureus in primary health care units in Brazil. Rev Inst Med Trop S Paulo, 2021; 63:e14. doi: https://doi.org/10.1590/S1678-9946202163014
Gowrishankar S, Kamaladevi A, Balamurugan K, Pandian SK. In vitro and in vivo biofilm characterization of methicillin-resistant Staphylococcus aureus from patients associated with pharyngitis infection. BioMed Res Int, 2016; 2016:1289157. doi: https://doi.org/10.1155/2016/1289157
Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther, 2018; 12:1759–67. doi: https://doi.org/10.2147/DDDT.S164515
Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—a review of recent developments in MRSA management and treatment. Crit Care, 2017; 21(1):001–10. doi: https://doi.org/10.1186/s13054-017-1801-3
Hindy JR, Haddad SF, Kanj SS. New drugs for methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Curr Opin Infect Dis, 2022; 35(2):112–19. doi: https://doi.org/10.1097/QCO.0000000000000800
Holmes A, Ganner M, McGuane S, Pitt TL, Cookson BD, Kearns AM. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol, 2005; 43(5):2384–90. doi: https://doi.org/10.1128/JCM.43.5.2384-2390.2005
Hong J, Ensom M, Lau T. What is the evidence for co-trimoxazole, clindamycin, doxycycline, and minocycline in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia? Ann Pharmacother, 2019; 53(11):1153–61. doi: https://doi.org/10.1177/1060028019856721
Howden BP, Davies JK, Johnson PDR, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev, 2010; 23(1):99–139. doi: https://doi.org/10.1128/CMR.00042-09
Jang HC, Kim SH, Kim KH, Kim CJ, Lee S, Song KH, Jeon JH, Park WB, Kim HB, Park SW, Kim NJ, Kim EC, Oh M, Choe KW. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis, 2009; 49(3):395–401. doi: https://doi.org/10.1086/600295
Jorgensen SCJ, Zasowski EJ, Trinh TD, Lagnf AM, Bhatia S, Sabagha N, Abdul-Mutakabbir JC, Alosaimy S, Mynatt RP, Davis SL, Rybak MJ. Daptomycin plus β-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective, comparative cohort study. Clin Infect Dis, 2020; 71(1): 1–10. doi: https://doi.org/10.1093/cid/ciz746
Joshi S, Ray P, Manchanda V, Bajaj J, Chitnis DS, Gautam V, Goswami P, Gupta V, Harish BN, Kagal A, Kapil A, Rao R, Rodrigues C, Sardana R, Devi KS, Sharma A, Balaji V. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res, 2013; 137(2):363–69.
Junnila J, Hirvioja T, Rintala E, Auranen K, Rantakokko-Jalava K, Silvola J, Lindholm L, Gröndahl-Yli-Hannuksela K, Marttila H, Vuopio J. Changing epidemiology of methicillin-resistant Staphylococcus aureus in a low endemicity area—new challenges for MRSA control. Eur J Clin Microbiol Infect Dis, 2020; 39(12):2299–307. doi: https://doi.org/10.1007/s10096-020-03824-9
Kali A, Stephen S, Umadevi S. Laboratory evaluation of phenotypic detection methods of methicillin-resistant Staphylococcus aureus. Biomed J, 2014; 37(6):411–14. doi: https://doi.org/10.4103/2319-4170.132907
Kaur J, Kaur J, Dhama AS, Jindal S, Walia K, Singh H. Strengthening the surveillance of antimicrobial resistance in India using integrative technologies. Front Public Health, 2022; 10:861888. Available via https://www.frontiersin.org/article/10.3389/fpubh.2022.861888
Kaur H, Purwar S, Saini A, Kaur H, Karadesai SG, Kholkute SD, Roy S. Status of methicillin resistant Staphylococcus aureus infections and evaluation of PVL producing strains in Belgaum, South India. JKIMSU, 2012; 1(2):43–51.
Kaur DC, Sadhana SC. Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. J Global Infect Dis, 2015; 7(2):78–84. doi: https://doi.org/10.4103/0974-777X.157245
Kaur G, Singh K, Oberoi L, Sidhu SK. Prevalence and in vitro susceptibility pattern of MRSA, VISA and VRSA isolated from surgical site infection in tertiary care hospital. IJCMR, 2020; 7(9):16–9. doi: http://dx.doi.org/10.21276/ijcmr.2020.7.9.21
Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLos One, 2015; 10(11):e0143276. doi: https://doi.org/10.1371/journal.pone.0143276
Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis, 2019; 68(Suppl 3):S193–99. doi: https://doi.org/10.1093/cid/ciz002
Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr Opin Microbiol, 2020; 53:51–60. doi: https://doi.org/10.1016/j.mib.2020.02.005.
Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine, 2009; 88(5):263–67. doi: https://doi.org/10.1097/MD.0b013e3181b8fccb
Lee GC, Boyd NK, Lawson KA, Frei CR. Incidence and cost of skin and soft tissue infections in the United States. Value Health, 2015; 18(3):A245. doi: https://doi.org/10.1016/j.jval.2015.03.1424
Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Prim, 2018; 4(1):1–23. doi: https://doi.org/10.1038/nrdp.2018.33
Lepelletier D, Maillard JY, Pozzetto B, Simon A. Povidone iodine: properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization. Antimicrob Agents Chemother, 2020; 64(9):e00682–20. doi: https://doi.org/10.1128/AAC.00682-20
Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. Involvement of panton-valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis, 1999; 29(5):1128–32. doi: https://doi.org/10.1086/313461
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis, 2011; 52(3):e018–55. doi: https://doi.org/10.1093/cid/ciq146
Loewen K, Schreiber Y, Kirlew M, Bocking N, Kelly L. Community-associated methicillin-resistant Staphylococcus aureus infection. Can Fam Physician, 2017; 63(7):512–20.
Lohan K, Sangwan J, Mane P, Lathwal S. Prevalence pattern of MRSA from a rural medical college of North India: a cause of concern. J Fam Med Prim Care, 2021; 10(2):752–57. doi: https://doi.org/10.4103/jfmpc.jfmpc_1527_20
Mathur P. Hand hygiene: back to the basics of infection control. Indian J Med Res, 2011; 134(5):611–20. doi: https://doi.org/10.4103/0971-5916.90985
Mehta Y, Hegde A, Pande R, Zirpe KG, Gupta V, Ahdal J, Qamra A, Motlekar S, Jain R. Methicillin-resistant Staphylococcus aureus in intensive care unit setting of India: a review of clinical burden, patterns of prevalence, preventive measures, and future strategies. Indian J Crit Care Med, 2020; 24(1):55–62. doi: https://doi.org/10.5005/jp-journals-10071-23337
Mendem SK, Gangadhara TA, Shivannavar CT, Gaddad SM. Antibiotic resistance patterns of Staphylococcus aureus: a multi center study from India. Microb Pathog, 2016; 98:167–70. doi: https://doi.org/10.1016/j.micpath.2016.07.010
Mitiku A, Aklilu A, Biresaw G, Gize A. Prevalence and associated factors of methicillin resistance Staphylococcus aureus (MRSA) among urinary tract infection suspected patients attending at Arba Minch General Hospital, Southern Ethiopia. Infect Drug Resist, 2021; 14:2133–42. doi: https://doi.org/10.2147/IDR.S306648
Moosavian M, Shahin M, Navidifar T, Torabipour M. Typing of staphylococcal cassette chromosome mec encoding methicillin resistance in Staphylococcus aureus isolates in Ahvaz, Iran. New Microb New Infect, 2017; 21:090–4. doi: https://doi.org/10.1016/j.nmni.2017.11.006
Moussa I, Shibl AM. Molecular characterization of methicillin-resistant Staphylococcus aureus recovered from outpatient clinics in Riyadh, Saudi Arabia. Saudi Med J, 2009; 30(5):611–17.
National Nosocomial Infections Surveillance System. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control, 2004; 32(8):470–85. doi: https://doi.org/10.1016/S0196655304005425
NCDC. National guidelines for infection prevention and control in healthcare facilities. 2020. Available via https://www.mohfw.gov.in/pdf/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf (Accessed 3 January 2023)
Neetu TJ, Murugan S. Genotyping of methicillin resistant Staphylococcus aureus from tertiary care hospitals in Coimbatore, South India. J Global Infect Dis, 2016; 8:68–74. doi: https://doi.org/10.4103/0974-777X.182119
Pal S, Sayana A, Joshi A, Juyal D. Staphylococcus aureus: a predominant cause of surgical site infections in a rural healthcare setup of Uttarakhand. J Fam Med Prim Care, 2019; 8(11):3600–6. doi: 10.4103/jfmpc.jfmpc_521_19
Palavecino EL. Rapid methods for detection of MRSA in clinical specimens. Methods Mol Biol (Clifton, N.J.), 2014; 1085:71–83. doi: https://doi.org/10.1007/978-1-62703-664-1_3
Park B. Treatment options for methicillin-resistant Staphylococcus aureus pneumonia evaluated [Online]. 2019. Available via https://www.pulmonologyadvisor.com/home/topics/pneumonia/treatment-options-for-methicillin-resistant-staphylococcus-aureus-pneumonia-evaluated/ (Accessed 26 May 2023)
Patel M, Waites KB, Moser SA, Cloud GA, Hoesley CJ. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. J Clin Microbiol, 2006; 44(7):2481–84. doi: https://doi.org/10.1128/JCM.02582-05
Patil SS, Suresh KP, Shinduja R, Amachawadi RG, Chandrashekar S, Pradeep S, Kollur SP, Syed A, Sood R, Roy P, Shivamallu C. Prevalence of methicillin-resistant Staphylococcus aureus in India: a systematic review and meta-analysis. Oman Med J, 2022; 37(4):e440. doi: https://doi.org/10.5001/omj.2022.22
Peacock SJ, Paterson GK. Mechanisms of methicillin resistance in Staphylococcus aureus. Ann Rev Biochem, 2015; 84(1):577–601. doi: https://doi.org/10.1146/annurev-biochem-060614-034516
Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians, 2012; 4(2):83–8. doi: https://doi.org/10.4103/0974-2727.105587
Polyzos KA, Mavros MN, Vardakas KZ, Makris MC, Rafailidis PI, Falagas ME. Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS One, 2012; 7(8):e41870. doi: https://doi.org/10.1371/journal.pone.0041870
Pourmand MR, Hassanzadeh S, Mashhadi R, Askari E. Comparison of four diagnostic methods for detection of methicillin resistant Staphylococcus aureus. Iran J Microbiol, 2014; 6(5):341–44.
Pradhan P, Rajbhandari P, Nagaraja SB, Shrestha P, Grigoryan R, Satyanarayana S, Davtyan H. Prevalence of methicillin-resistant Staphylococcus aureus in a tertiary hospital in Nepal. Public Health Act, 2021; 11(Suppl 1):46–51. doi: https://doi.org/10.5588/pha.21.0042
Preeja PP, Kumar SH, Shetty V. Prevalence and characterization of methicillin-resistant Staphylococcus aureus from community- and hospital-associated infections: a tertiary care center study. Antibiotics (Basel, Switzerland), 2021; 10(2):197. doi: https://doi.org/10.3390/antibiotics10020197
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health, 2015; 109(7):309–18. doi: https://doi.org/10.1179/2047773215Y.0000000030
Price JR, Cole K, Bexley A, Kostiou V, Eyre DW, Golubchik T, Wilson DJ, Crook DW, Walker AS, Peto TEA, Llewelyn MJ, Paul J. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis, 2017; 17(2):207–14. doi: https://doi.org/10.1016/S1473-3099(16)30413-3
Punjabi CD, Madaline T, Gendlina I, Chen V, Nori P, Pirofski L. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in respiratory cultures and diagnostic performance of the MRSA nasal polymerase chain reaction (PCR) in patients hospitalized with coronavirus disease 2019 (COVID-19) pneumonia. Infect Control Hosp Epidemiol, 2020; 42(9):1156–58. doi: https://doi.org/10.1017/ice.2020.440
Radhakrishna M, D’Souza M, Kotigadde S, Vishwas Saralaya K, Shashidar Kotian M. Prevalence of methicillin resistant Staphylococcus aureus carriage amongst health care workers of critical care units in Kasturba Medical College Hospital, Mangalore, India. J Clin Diagnostic Res JCDR, 2013; 7(12):2697–700. doi: https://doi.org/10.7860/JCDR/2013/5160.3735
Reveles KR, Mortensen EM, Attridge RT, Frei CR. Comparative-effectiveness of vancomycin and linezolid as part of guideline-recommended empiric therapy for healthcare-associated pneumonia. BMC Res Notes, 2015; 8(1):1–8. doi: https://doi.org/10.1186/s13104-015-1396-1
Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis, 2014; 58(Suppl 1):S020–27. doi: https://doi.org/10.1093/cid/cit614
Rubinstein E, Keynan Y. Vancomycin revisited–60 years later. Front Public Health, 2014; 2:217. doi: https://www.frontiersin.org/article/10.3389/fpubh.2014.00217
Sasirekha B, Usha MS, Amruta JA, Ankit S, Brinda N, Divya R. Incidence of constitutive and inducible clindamycin resistance among hospital-associated Staphylococcus aureus. 3 Biotech, 2014; 4(1):85–9. doi: https://doi.org/10.1007/s13205-013-0133-5
Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep, 2020; 10(1):1–16. doi: https://doi.org/10.1038/s41598-020-69058-z
Siddiqui AH, Koirala J. Methicillin resistant Staphylococcus aureus. StatPearls Publishing, Treasure Island, FL, 2023. Available via http://www.ncbi.nlm.nih.gov/books/NBK482221/
Soo Yean CY, Raju KS, Xavier R, Subramaniam S, Gopinath SCB, Chinni SV. Molecular detection of methicillin-resistant Staphylococcus aureus by non-protein coding RNA-mediated monoplex polymerase chain reaction. PLoS One, 2016; 11(7):e0158736. doi: https://doi.org/10.1371/journal.pone.0158736
Spagnolo AM, Orlando P, Panatto D, Amicizia D, Perdelli F, Cristina ML. Staphylococcus aureus with reduced dusceptibility to vancomycin in healthcare settings. J Prev Med Hyg, 2014; 55(4):137–44.
Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog, 2002; 85(Pt 1):57–72. doi: https://doi.org/10.3184/003685002783238870
Stryjewski ME, Lentnek A, O’Riordan W, Pullman J, Tambyah PA, Miró JM, Fowler Jr VG, Barriere SL, Kitt MM, Corey GR. A randomized phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: the ASSURE study. BMC Infect Dis, 2014; 14(1):1–10. doi: https://doi.org/10.1186/1471-2334-14-289
Sunagar R, Hegde NR, Archana GJ, Sinha AY, Nagamani K, Isloor S. Prevalence and genotype distribution of methicillin-resistant Staphylococcus aureus (MRSA) in India. J Glob Antimicrob Resist, 2016; 7:46–52. doi: https://doi.org/10.1016/j.jgar.2016.07.008
Sutton JP, Steiner CA. Hospital-, Health Care-, and community-acquired MRSA: estimates from California hospitals, 2013. Healthcare cost and utilization project (HCUP) statistical briefs. Agency for Healthcare Research and Quality (US)[Online] Available via https://www.ncbi.nlm.nih.gov/books/NBK396238/ (Accessed 8 May 2023)
Szymanek-Majchrzak K, Mlynarczyk A, Mlynarczyk G. Characteristics of glycopeptide-resistant Staphylococcus aureus strains isolated from inpatients of three teaching hospitals in Warsaw, Poland. Antimicrob Resist Infect Control, 2018; 7(1):1–6. doi: https://doi.org/10.1186/s13756-018-0397-y.
Taneja N, Sharma M. Antimicrobial resistance in the environment: the Indian scenario. Indian J Med Res, 2019; 149(2):119–28. doi: https://doi.org/10.4103/ijmr.IJMR_331_18
Thapa D, Pyakurel S, Thapa S, Lamsal S, Chaudhari M, Adhikari N, Shrestha D. Staphylococcus aureus with inducible clindamycin resistance and methicillin resistance in a tertiary hospital in Nepal. Trop Med Health, 2021; 49(1):1–7. doi: https://doi.org/10.1186/s41182-021-00392-2
Thimmappa L, Bhat A, Hande M, Mukhopadhyay C, Devi E, Nayak B, George A. Risk factors for wound infection caused by methicillin resistant Staphylococcus aureus among hospitalized patients: a case control study from a tertiary care hospital in India. Afr Health Sci, 2021; 21(1):286–94. doi: https://doi.org/10.4314/ahs.v21i1.37
Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis, 2006; 6 (1):1–6. doi: https://doi.org/10.1186/1471-2334-6-156
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev, 2015; 28(3):603–61. doi: https://doi.org/10.1128/CMR.00134-14
Udo EE. Community-acquired methicillin-resistant Staphylococcus aureus: the new face of an old foe? Med Princ Pract, 2013; 22(Suppl. 1):20–9. doi: https://doi.org/10.1159/000354201
Urushibara N, Aung MS, Kawaguchiya M, Kobayashi N. Novel staphylococcal cassette chromosome mec (SCCmec) type XIV (5A) and a truncated SCCmec element in SCC composite Islands carrying SpeG in ST5 MRSA in Japan. J Antimicrob Chemother, 2020; 75(1):46–50. doi: https://doi.org/10.1093/jac/dkz406
van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev, 2012; 25(2):362–86. doi: https://doi.org/10.1128/CMR.05022-11
Walia K, Madhumathi J, Veeraraghavan B, Chakrabarti A, Kapil A, Ray P, Singh H, Sistla S, Ohri VC. Establishing antimicrobial resistance surveillance & research network in India: journey so far. Indian J Med Res, 2019; 149(2):164–79. doi: https://doi.org/10.4103/ijmr.IJMR_226_18
Wangai FK, Masika MM, Maritim MC, Seaton RA. Methicillin-resistant Staphylococcus aureus (MRSA) in East Africa: red alert or red herring? BMC Infect Dis, 2019; 19(1):103–66. doi: https://doi.org/10.1186/s12879-019-4245-3
WHO. Infection prevention and control recommendations. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. World Health Organization, 2014. Available via https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care (Access 2 February 2023)
Wiseman JT, Fernandes-Taylor S, Barnes ML, Saunders RS, Saha S, Havlena J, Rathouz PJ, Kent KC. Predictors of surgical site infection after hospital discharge in patients undergoing major vascular surgery. J Vasc Surg, 2015; 62(4):1023–31.e5. doi: https://doi.org/10.1016/j.jvs.2015.04.453
Wong J, Ip M, Tang A, Wei VW, Wong SY, Riley S, Read JM, Kwok KO. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: a systematic review and meta-analysis. Clin Epidemiol, 2018; 10:1489–501. doi: https://doi.org/10.2147/CLEP.S160595
Woods CR. Macrolide-inducible resistance to clindamycin and the D-test. Pediatr Infect Dis J, 2009; 28(12):1115–8. doi: 10.1097/INF.0b013e3181c35cc5
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis, 2012; 54(5):621–29. doi: https://doi.org/10.1093/cid/cir895
Yue J, Dong BR, Yang M, Chen X, Wu T, Liu GJ. Linezolid versus vancomycin for skin and soft tissue infections. Evid Based Child Health A Cochrane Rev J, 2014; 9(1):103–66. doi: https://doi.org/10.1002/ebch.1961
Zalavras CG. Prevention of infection in open fractures. Infect Dis Clin North Am, 2017; 31(2):339–52. doi: https://doi.org/10.1016/j.idc.2017.01.005
Zhen X, Lundborg CS, Zhang M, Sun X, Li Y, Hu X, Gu S, Gu Y, Wei J, Dong H. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: a multicentre study in China. Sci Rep, 2020; 10(1):3900. doi: https://doi.org/10.1038/s41598-020-60825-6