INTRODUCTION
Southern Africa is recognized for its rich and remarkable floral diversity, with nearly 22,755 plant species. Besides, approximately 3,000 plant species are used to treat numerous diseases (Neffati et al., 2017; Twilley et al., 2020; Van Wyk and Prinsloo, 2018). Since the beginning of time, humankind has been utilizing plants for their basic requirements, such as clothing, food, shelter, flavors, fertilizers, and fragrances (Jain et al., 2019). Furthermore, traditionally the indigenous people of South Africa have consumed medicinal plants to cure infectious diseases and treat ailments throughout history (Oguntibeju, 2018). Street et al. (2008) have estimated that almost 52% of the South African population, which accounts for nearly 27 million, relies on native medicinal plants for their essential healthcare treatments.
In light of the above, African traditional medicine (ATM) has been part and parcel of the legislative framework of South Africa (Gqaleni et al., 2007). This is because traditional medicine and practices have played an integral role in the nation’s history and plant knowledge has been passed down from generation to generation. Furthermore, the use of traditional medicine has exploded because of its easy accessibility, affordability, and considerable tribal knowledge (Semenya et al., 2012). Various scientific reports have supported the claims made by traditional healers about the countless biological properties of plants, including their antiviral, antibacterial, antioxidant and anti-inflammatory, immunomodulatory, and anticancer activities (Kshirsagar and Rao, 2021; Reddy et al., 2020). Tuyiringire et al. (2020) have shown increasing evidence of medicinal plants as a natural source of anti-tuberculosis (TB) agents. Their study further highlighted that the selected candidates could combat multidrug-resistant (MDR) TB (Tuyiringire et al., 2020).
South Africa is among the eight countries with the highest TB burden worldwide. TB is now the second most infectious disease due to the high number of fatalities worldwide, surpassing the human immunodeficiency virus (HIV). TB is caused by infectious Mycobacterium tuberculosis that usually affects the lungs (Berkowitz et al., 2018). In 2018, approximately 1.5 million individuals died from TB, 10.0 million patients became sick from TB, and nearly 1.7 billion people suffered from M. tuberculosis infection worldwide (Padmapriyadarsini et al., 2021). Moreover, persistent TB infection in patients with HIV is responsible for countless fatalities and has become a major driver of antimicrobial resistance. The current TB treatment has displayed numerous challenges, including expensive drug therapy, its ineffectiveness since the development of drug resistance, and the counteractions of HIV infection.
Moreover, combination therapy causes serious symptoms and high toxicity levels (Anochie et al., 2018). Regarding the current antibiotic therapy, failure is inevitable. Well-documented studies are available on the various biological activities and numerous secondary biocompounds (e.g., polyphenols, flavonoids, and alkaloids) of traditional medicinal plants (Chiocchio et al., 2021; Oguntibeju, 2018; Street et al., 2008). On the other hand, South Africa has an abundant wealth of traditional medicinal plants with tremendous anti-TB properties (Anochie et al., 2018; Madikizela and McGaw, 2017). Hence, untapped alternatives in the drug discovery and development process must be explored to uncover new cost-effective, accessible, more potent, and less toxic natural plant-based drugs. In conclusion, these medicinal plants might be explored for therapeutic prevention, control, management, and treatment of TB.
METHODOLOGY FRAMEWORK
In the present review, we searched online databases such as Google Scholar, ScienceDirect, Scopus, and Web of Science to screen and select research papers. Terminologies including biocompounds, TB, medicinal plants, and South Africa alone or in combination were used to search the electronic databases for research papers.
South African medicinal plants as an alternative to combating infectious TB
Artemisia afra Jacq. Ex Willd. (African wormwood)
Tradition uses of the African wormwood plant
Artemisia afra Jacq. Ex Willd. is a native aromatic plant distributed in the southern locations of Africa, including South Africa, Zimbabwe, and Namibia; thus, it flourishes in the Gauteng and Limpopo areas of South Africa. The Zulu people named the plant “Mhlonyane,” Xhosa “Umhlonyane,” Sotho “Lanyana,” Afrikaans “Wildeals,” and Tswana “Lengana,” and English speakers refer to the plant as “African wormwood” (Fig. 1) (Liu et al., 2009). Moreover, traditional use of the plant involves the treatment of coughs, fever, headaches, chills, indigestion, loss of appetite, gastric disorders, colic, croup, gout, whooping cough, asthma, diabetes, malaria, bladder, influenza, kidney disorders, heart inflammation, purgative, convulsions, and rheumatism. Various scientific studies have supported the traditional claims that African wormwood showed promising antiviral, anti-inflammatory, and antibacterial activities. In rural areas in the Eastern Cape Province of South Africa, people treat diabetes by infusing the leaves or roots of African wormwood “Umhlonyane” (Kshirsagar and Rao, 2021). Others boil the leaves to cure respiratory infections and inhale the vapor to cure menstrual chill. A tea preparation of African wormwood sweetened with honey is also used for therapeutic purposes. For this, an increasing number of people are becoming more interested in African wormwood due to its infinite possibilities. Artemisia afra “African wormwood” is currently one of the most talked-about medicinal plants in South Africa (Du Toit and Van der Kooy, 2019).
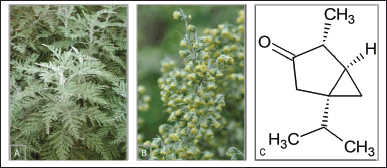 | Figure 1. Artemisia afra (African wormwood): (A) Artemisia afra plant, (B) creamy yellow flowers, and (c) monoterpenes α-thujone. [Click here to view] |
Phytochemical compounds isolated from the African wormwood plant
Several studies have extracted volatile secondary metabolites from African wormwood. Mbokane and Moyo (2018) reported that numerous biocompounds were detected in A. afra extract in high levels, such as polyphenols, phenols, flavonoids, and alkaloids (Mbokane and Moyo, 2018). In 2009, Liu et al. (2009) estimated that 131 biocompounds have been described from the essential oil of A. afra. Moreover, they provided a detailed report on the secondary metabolites isolated from the African wormwood. Several monoterpenoids were also highlighted, such as artemisia alcohol, artemisia ketone, artemisyl acetate, camphene, camphor, cis-carveol, caryophylla-2(12),6(13)-dien-5-one, cis-chrysanthenol, chrysanthenone, cis-chrysanthenyl, acetate, 1,8-cineole, cumin alcohol, dihydrocarvyl acetate, limonene, linalool, and linalool acetate. Other diverse chemicals were extracted from the African wormwood plants, such as sesquiterpenes, artemisal, berbenome, cuminaldehyde, p-cymen-8-ol, and p-cymene. Furthermore, they categorized them under monoterpenes and sesquiterpenes. Another study identified the flavone acacetin and the sesquiterpene lactone lα,4α-dihydroxyguaia-2,10(14),11(13)-trien-12,6α-olide in the A. afra extract. The essential oils of African wormwood showed significant levels of α-and β-thujone (Fig. 1C), ketone, alcohol, camphor, and 1,8-cineole (Alhassan, 2017).
Anti-TB properties of the African wormwood plant
Various studies have reported African wormwood as a potent medicinal plant with superior bioactivities (Kshirsagar and Rao, 2021). A recent study determined that the minimum inhibitory concentrations (MICs) of Artemisia annua and A. afra extracts against Mycobacteroides abscessus and M. tuberculosis showed excellent bactericidal activity against M. tuberculosis. The results further showed that African wormwood exhibited potent anti-TB effects. These strains have been reported to be virulent Erdman strains and might be regarded as resistant strains, hence acting as chemotherapeutic agents against infectious M. tuberculosis (Martini et al., 2020). According to Ntutela et al. (2009), the dichloromethane extract of African wormwood inhibited the bacterial replication of Mycolicibacterium aurum. The actions ensured that the drug copying was entirely stopped. This was due to the invasion of the heme molecule that affects the reduced oxygen for the survival of the pathogen (Ntutela et al., 2009). Several studies have established that African wormwood might be a suitable candidate as an anti-TB agent (Gemechu et al., 2013; More et al., 2012). Mativandlela et al. (2008) determined antimycobacterial activity using the MIC of A. afra extracts against Mycobacterium smegmatis and M. tuberculosis. The results showed that A. afra exhibited some anti-TB activity and could be a good candidate for treating TB and TB-related symptoms (Mativandlela et al., 2008).
Three local medicinal plants commonly used for the treatment of TB in the Eastern Cape Province, South Africa, were screened using a twofold microdilution bioassay. The A. afra dichloromethane and ethanol extracts showed good anti-TB activity against M. aurum A+. Further analysis also indicated that A. afra extracts inhibited the growth of Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, and Bacillus cereus (Buwa and Afolayan, 2009). Interestingly, a survey study was conducted on 52 Bapedi traditional healers in the districts of Limpopo, South Africa. The results indicated that A. afra was the most widely used medicinal plant in treating and managing TB in the Limpopo province of South Africa (Semenya and Maroyi, 2013). Despite the extensive biological activities of A. afra, several studies have demonstrated that A. afra extracts and their volatile oils possess some toxicological effects (Kane et al., 2019; Mongalo et al., 2020; Mukinda and Syce, 2007; Mungho et al., 2018; Sunmonu and Afolayan, 2013).
Ziziphus mucronate Willd. (the buffalo thorn)
Tradition uses of the buffalo thorn plant
Ziziphus mucronata Willd. is a deciduous native sub-Saharan Africa plant, growing from South Africa northwards to Eritrea and Ethiopia. Historians believe that Christ’s crown was made from the Ziziphus spina-christi Willd. plant, which closely resembles Z. mucronata. Ziziphus mucronata is commonly known as buffalo thorn due to its pointed leaves. Moreover, in Latin, “ziziphus” means thorn, and “mucronata” refers to pointed leaves. Various plant parts of Z. mucronata are used traditionally to treat several ailments, such as gastrointestinal conditions, rheumatism, and snake bites. The bark infusions help relieve cough, body pains, respiratory conditions, and chest issues. For the treatment of gonorrhea, diarrhea, and dysentery, indigenous people use Z. mucronata root drinks (Mekonen et al., 2020; Fig. 2).
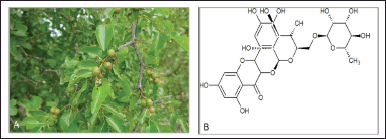 | Figure 2. (A) Ziziphus mucronate with distinct thrones and (B) chemical compound rutin isolated from Z. mucronate. [Click here to view] |
Phytochemical compounds isolated from the buffalo thorn plant
Olajuyigbe and Afolayan (2011) reported that in the Z. mucronata extracts, phenolics were the highest, followed by flavonoids and proanthocyanidin contents. A comprehensive study established that Z. mucronata extracts produced various biocompounds, including quinones, terpenoids, alkaloids, flavonoids, phenolics, and quercetin (Auvin et al., 1996; Olajuyigbe and Afolayan, 2011). Mucronine J, a novel cyclopeptide alkaloid biocompound, was isolated from the root bark of Z. mucronata (Rhamnaceae), which was the structure elucidated by mass spectrometry and 1D and 2D NMR (Mukinda and Syce, 2007). Another study concluded that five cyclopeptide alkaloids (sanjoinines A, B, F, G1, and G2) were extrapolated from Z. mucronata leaf extract (Foyet et al., 2019). According to Koà et al. (2017), 11 phenolics compounds were extracted from an aqueous extract of Z. mucronate, including rutin (quercetin-3–O–rutinoside), isoquercetin (hyperoside), catechin, quercitrin (quercetin-3,7–O–L- dirham pyranoside), and delphinidin-3-glucoside.
Anti-TB properties of the buffalo thorn plant
An in-depth study was conducted on the ATM system for treating TB using medicinal plants. The study highlighted various herbal plants producing secondary biocompounds as potential anti-TB agents and Z. mucronata plant was listed (Anochie et al., 2018). Indigenous people of the South African tribes used medicinal plants to combat TB (Dzoyem et al., 2016; Green et al., 2010; Sharifi-Rad et al., 2020). Moreover, Sigidi et al. (2016) reported that Z. mucronata extracts exhibited some anti-TB activity at concentrations >500 μg/ml against the M. tuberculosis H37 strain. Another research study demonstrated that Z. mucronata extracts produced cyclopeptide alkaloids, which were well-known to exhibit significant anti-TB effects against M. tuberculosis (Lomchoey, 2014). The Mycobacterium species have structural cell wall functions to withstand the habitat and anti-TB agents and survive adverse environmental conditions for countless years. This has led to the ever-increasing MDR and extensively drug-resistant genes, which make the treatment of TB challenging (Mongalo et al., 2020).
Knowltonia vesicatoria (brandblaar)
Tradition uses of the brandblaar plant
Knowltonia vesicatoria is a slow-growing perennial fynbos indigenous South African plant that is widely distributed in the Cape Peninsula stretching to Grahamstown. Furthermore, K. vesicatoria is referred to as “brandblaar” by the Afrikaans people of South Africa. It has been popular in South Africa since European colonization due to its wide range of beneficial uses. The native tribes used K. vesicatoria leaves to soothe aching backs and joints. Moreover, it was used to treat colds, arthritis, rheumatism, flu, and, sometimes, toothache (van Wyk and Gericke, 2000; Fig. 3).
Phytochemical compounds isolated from brandblaar plant
A study was conducted on a crude ethanol extract of K. vesicatoria as a promising candidate and a new natural alternative to antimycobacterial drugs. This study was the first to use NMR and silica column chromatography to extract stigmasta-5,23-dien–3–ol and 5–(hydroxymethyl)furan-2(5H)–one from K. vesicatoria using. Other biocompounds extracted included isomer mixture [5-(hydroxymethyl)furan-2(5H)-one, 5-(hydroxymethyl)dihydrofuran-2(3H)-one] and 5-(hydroxymethyl)furan-2(5H)-one. Moreover, stigmasta-5,23-dien-3-ol displayed the most potency against drug-sensitive M. tuberculosis with inhibition of 50.00 μg/ml MIC (Labuschagné et al., 2012). An in-depth study on the phytochemicals and bioactivity of southern African flora established that K. vesicatoria produced flavonoids, quinones, and other beneficial biocompounds (Babiaka et al., 2015). Despite the evidence, there is limited research on the phytochemical compounds produced by K. vesicatoria.
Anti-TB properties of brandblaar plant
In line with the above, another study revealed that K. vesicatoria (aerial parts) extracts demonstrated the best bioactive activities against M. tuberculosis with inhibition of MIC of 39.06 μg/ml. In contrast, the extracts showed weak bioactivity against nonpathogenic M. smegmatis. Moreover, Labuschagné et al. (2012) showed that K. vesicatoria extract possessed strong anti-TB properties against drug-resistant M. tuberculosis isolates and exhibited a MIC value of 50.00 μg/ml. The combination effect of K. vesicatoria extract with other compounds reduces the MIC value (Labuschagné et al., 2012). Regardless of the limited data, various researchers have advocated for using K. vesicatoria as a substitute for commercialized anti-TB agents (Anochie et al., 2018; Babiaka et al., 2015; Martolia et al., 2020; Nielsen et al., 2012). Despite the limited studies, several studies supported the anti-TB effects of K. vesicatoria based on in vitro laboratory-based reports (Krige et al., 2009; Kuete et al., 2021; Labuschagné et al., 2008; Nielsen et al., 2012; Rawat et al., 2021).
 | Figure 3. (A) Knowltonia vesicatoria plant; (B) Blisterleaf; and (C) chemical compound stigmasta-5,23-dien-3-ol. [Click here to view] |
Lippia javanica (the fever tea)
Traditional uses of the fever tea plant
Lippia javanica is an erect woody perennial shrub or herb of 4.5 m tall, with a brownish stem and strong aromatic leaves that emit a lemon-like fragrance when crushed (Maroyi, 2017; van Wyk and Gericke, 2000). It belongs to the verbena (Verbenaceae) family and can be naturally found throughout South Africa (except in Western Cape), extending from Eastern Cape to other tropical African countries (Kipkore et al., 2014; Shikanga et al., 2010). It is referred to as fever tea or lemon bush (English); “lemoenbossie,” “beukesbossie,” or “koorsbossie” (Afrikaans); “umsutane” or “mutswane” (Swati); “inzinziniba” (Xhosa); “umsuzane” or “umswazi” (Zulu); “musukudu” or “bokhukhwane” (Tswana). Generally, L. javanica is traditionally consumed as an herbal tea for either recreational or medicinal purposes. When applied for ethnomedicinal purposes, it is consumed in limited amounts for a few days (Krige et al., 2009). Lippia javanica is medically used to treat cough, cold, bronchitis, asthma, chest pains, and other diseases (Chigora et al., 2007; Davids et al., 2014; Njoroge and Bussmann, 2006; Semenya et al., 2013; Fig. 4).
Phytochemical compounds isolated from the fever tea plant
Lippia javanica is rich in biocompounds and essential oils, such as pipertenone, tagetenone, ipsdienone, p-cymene, ipsenone, myrcene, linalool, carvone, sabinene, ocimenone, limonene, caryophyllene, and myrcenone (Shikanga et al., 2010). It has been revealed that piperitenone extracted from L. javanica could inhibit the growth of E. coli and Bacillus subtilis at a dilution rate of 1% (Manenzhe et al., 2004). It can also inhibit the growth of Acinetobacter calcoaceticus, Salmonella typhi, S. aureus, and Micrococcus kristinae with a MIC range of 12– 50 μg/ml, using kanamycin and dimethyl sulphoxide as control (Samie et al., 2009). The same was observed in the leaf extract (acetone, methanol, and hexane extract) of L. javanica against 15 bacterial species with a MIC range of 1.5 to >12 mg/ml (Nyahangare et al., 2012). Biocompounds such as lippialactone derived from ethyl acetate extract of the aerial parts of L. javanica, according to Ludere et al. (2013), prevent the growth of E. coli and S. aureus at 10 mg/ml. Apigenin, a phenolic compound, which is a well-known antibacterial agent, is also extracted from L. javanica and has an antibacterial effect on Vibrio cholera, Enterococcus faecalis, S. typhi, Proteus mirabilis, and Pseudomonas aeruginosa (Cushnie and Lamb, 2005; Martini et al., 2004; Shikanga et al., 2010). Isoverbascoside and verbascoside were extracted from the leaf of L. javanica and can exhibit antifungal activities against mycelia at above 0.6 mg/ml−1 (Lima et al., 2003).
Anti-TB properties of the fever tea plant
Mujovo et al. (2008) reported that among all the compounds isolated from L. javanica, only euscaphic acid, which is a triterpenoid carboxylic acid, showed antimycobacterial activity when tested against a drug-sensitive strain of M. tuberculosis with a MIC of 50 μg/ml. Acetone leaf extract of L. javanica showed antimycobacterial activity against M. smegmatis with the lowest MIC value of 0.47 mg/ml (Masoko and Nxumalo, 2013). These compounds with antimycobacterial activities extracted from L. javanica have passed through preliminary evaluations and have turned out to be very effective. Thus, they can be used to produce new and more effective drugs for the treatment of TB (Maroyi, 2017; Masoko and Nxumalo, 2013; Mujovo et al., 2008). Lippia javanica is regarded as one of the most commonly used medicinal plants by Bapedi traditional healers to cure TB infection in the Limpopo province of South Africa (Semenya and Maroyi, 2013).
 | Figure 4. (A) Lippia javanica plant with white flower and (B) chemical compound pipertenone. [Click here to view] |
Euclea natalensis A. DC. (Natal guarri)
Traditional uses of the Natal guarri plant
Euclea natalensis is a shrub with a dense spreading crown, straight trunk, and branchlets covered in fine rusty hair. It is indigenous in the southeastern part of Africa. It is called “Natal ebony,” “Natal guarri,” and “large-leaved guarri” in English; “Natalghwarrie,” “berggwarrie,” and “swartbasboom” in Afrikaans; “umTshekisani” and “umKhasa” in Xhosa; “iDungamuzi,” “iChitamuzi,” “umZimane,” “umTshikisnae,” “inKunzane,” “inKunzi-emnyama,” umHlalanyamazane,” and “umAnyathi” in Zulu; “umHlangula” in Tsonga. Moreover, its parts are used for different purposes in different communities. The wood, roots, and twigs from E. natalensis are used as building materials and for the production of dark red dye and toothbrushes, respectively (Oosthuizen et al., 2020). The roots and twigs of E. natalensis are generally used to treat chest ailments, toothache, bronchitis, asthma, snake bite, diarrhea, malaria, stomach problems, diabetes, venereal diseases, and wounds (Lall et al., 2005; Maroyi, 2017; Fig. 5).
Phytochemical compounds isolated from the Natal guarri plant
Several compounds from the class of naphthoquinone and pentacyclic terpenoids were isolated from E. natalensis by different researchers (van der Kooy, 2004). Octahydroeuclein, 20(29)-lupene-3β-isoferulate, shinanolone, lupeol, and betulin were isolated from the ethanol extract of E. natalensis’ root bark, and they all exhibited inhibitory characteristics against Gram-positive bacteria species (Lall et al., 2006). According to Lall et al. (2001), 50% inhibition in the replication of herpes simplex virus cells was achieved with 0.1 and 0.2 mg/ml of acetone and water extract from the root bark of E. natalensis. Other compounds isolated from the root bark of E. natalensis include isodiospyrin, mamegakione, natalenone, 8’-hydroxydiospyrin, euclanone, galpinone, methylnaphthazarin, neodiospyrin, diospyrin, 7-methyljuglone, β-sitosterol, 5-hydroxy-4-methoxy-2-metaldehyde (Lall et al., 2005; Lal et al., 2016; Maroyi, 2017; van der Kooy, 2004).
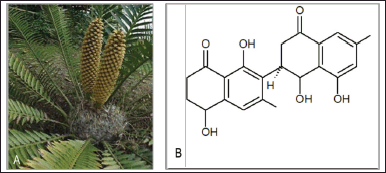 | Figure 5. (A) Euclea natalensis plant and (B) chemical compound octahydroeuclein. [Click here to view] |
Anti-TB properties of the Natal guarri plant
Most of the compounds isolated from E. natalensis exhibited inhibitory activities against Mycobacterium species. Using a radiometric respiratory BACTEC assay, Lall and Meyer (2001) observed a MIC value of 100 μg/ml while evaluating the antimycobacterial activities of both drug-sensitive and drug-resistant strains of M. tuberculosis. Evaluating the antimycobacterial activities of crude extract, diospyrin, and 7-methyljuglone, isolated from the root of E. natalensis, against M. tuberculosis produced MIC values of 8.0, 8.0, and 0.5 μm/ml, respectively (Lall and Meyer, 2005). Furthermore, 7-methyljuglone and diospyrin are the most bioactive and antimycobacterial compounds isolated from E. natalensis based on all evaluations and previous studies (Mahapatra et al., 2007; Maroyi, 2017; Lall; 2001; van der Kooy, 2004; van der Kooy, 2007).
A previous study by Bapela et al. (2008) revealed that naphthoquinone, 7-methyljuglone was the lead bioactive compound in E. natalensis extracts. The data showed that E. natalensis extracts were potent against M. tuberculosis and M. smegmatis. In another study, naphthoquinone, 7-methyljuglone was also isolated from the acetone extract of E. natalensis and showed a good inhibitory effect against Mycobacterium bovis (MIC?=?26?μg/ml), compared to M. smegmatis and Mycobacterium fortuitum. Even though the strains were nonpathogenic, they were used as prototypes to identify the potency of plant-based extracts (McGaw et al., 2008). Furthermore, E. natalensis has been shown to be a potent antimycobacterial candidate in the literature; however, more research is warranted as the studies in this area are limited.
Bridelia micrantha (Hochst.) Baill. (coastal golden-leaf)
Traditional uses of the coastal golden-leaf plant
Bridelia micrantha (Hochst.) Baill. is a small- to medium-sized tree with a spreading crown, from the family of Phyllanthaceae (formally Euphorbiaceae). It is commonly known as “mitzeerie” or “bruin stinkhout” (Afrikaans), “motsere” (Sotho), “ndzerhe” (Tswana), and coastal golden-leaf (English). It is widely distributed throughout tropical Africa to the Eastern Cape, South Africa. The fruits are widely eaten and can be used for jam and juice, while the root, bark, leaf sap, and leaves are used for medicinal purposes in African countries such as South Africa, Cameroon, Malawi, and Nigeria (Ingram and Shure, 2010; Maroyi, 2017; Meke et al., 2017). The wood from B. micrantha is used for construction, furniture, tool handles, and kitchen utensils in tropical Africa, especially Ethiopia and Kenya (Bosch, 2012; Chekole et al., 2015). B. micrantha is used to treat burns, wounds, stomach ache, dysentery, constipation, malaria, tapeworms, sexually transmitted infections, diarrhea, conjunctivitis, and painful eyes (Maroyi, 2017; Fig. 6).
Phytochemical constituents isolated from the coastal golden-leaf plant
Bridelia micrantha is rich in phytochemicals such as alkaloids, flavonoids, essential oil, phenolic compounds, sterols, tannins, terpenoids, and oxalate. Compounds isolated from fruit, leaves, and stem of B. micrantha include the following: caffeic acid, cycloartenol acetate, ergosterol, stigmast-8(14)-en-3-ol, 5β, 6β-epoxy-7-bromocholoratan-3-one, acacic acid lactone, quercetin, quercetin-3-O-glucoside, delphinidin, ellagic acid, gallic acid, friedelin, taraxerol, taraxerone, trans-triacontyl-4-hydroxy-3- methoxycinnamate, betulinic acid, catechin, and oleanolic acid (Akinyeye and Olatunya, 2014; Chekole et al., 2015; Munayi, 2016; Shelembe et al., 2016). Adefuye et al. (2011)revealed that ethyl acetate extract of the stem bark of B. micrantha was more active in inhibiting the growth of S. aureus with a MIC50 value of 0.078 mg/ml than other extracts.
Anti-TB properties of the coastal golden-leaf plant
Using tetrazolium microplate assay, Green et al. (2010) observed that acetone leaf extract of B. micrantha was more active against M. tuberculosis with a MIC value of 25 μg/ml than other extracts (methanol, hexane, and ethanol). Moreover, Green et al. (2011) used a resazurin microplate assay to evaluate the antimycobacterial activity of n-hexane fraction, a subfraction of ethyl acetate fraction of the acetone stem extract of B. micrantha against M. tuberculosis. They observed 20% and 35% inhibition in the growth of M. tuberculosis H37Ra and M. tuberculosis that is resistant to all first-line drugs at 10 μg/ml. The primary ethyl acetate fraction inhibited the growth of M. tuberculosis resistant to streptomycin, rifampicin, isoniazid, and ethambutol at 50 μg/ml. The evaluations from researchers (Akinyeye and Olatunya, 2014; Green et al., 2010; van der Kooy, 2007) can serve as scientific validations for producing more effective and efficient drugs for the treatment of TB. Numerous biocompounds with abundant biological activities have been isolated from B. micrantha, including alkaloids, flavonoids, essential oils, phenolic compounds, sterols, terpenoids, and tannins, saponins (Akinyeye and Olatunya, 2011; Anywar et al., 2021; Maluleke, 2019; Ngane, 2019; Okeleye et al., 2011).
 | Figure 6. (A) Bridelia micrantha (Hochst.) Baill. plant and (B) basic structure of monoterpenes. [Click here to view] |
Immune adjuvant effect of medicinal plants against infectious TB
Biological therapy, often known as immunotherapy, is a type of treatment that uses the body’s natural immune system to protect against infection, cancer, and other disorders and mitigate the effects of other treatments. Biological response modifiers (BRMs), which include vaccinations, monoclonal antibodies, cytokines, and adjuvants, are used in biotherapy. BRMs can be utilized individually or in combination (Kuroki et al., 2012). Immunomodulatory activities have been discovered in medicinal plants typically used as herbal medications. Both nonspecific and specific immunity may be stimulated by these substances. These plants may promote both humoral and cell-mediated immunity, or simply one of them, while reducing the immune system’s cell-mediated component (Rao et al., 1994).
Immunomodulatory therapy may be a viable alternative to traditional chemotherapy for a variety of diseases, particularly when the host’s defense mechanisms must be activated in the face of impaired immune responsiveness or when selective immunosuppression is required in situations such as inflammatory diseases, autoimmunity, and organ/bone marrow transplantation (John et al., 2022). Numerous polysaccharides derived from TCM plants have recently been discovered to be biocompatible, biodegradable, and immunomodulatory, indicating that they could be used as vaccine adjuvant options (Khademi et al., 2018; Wan et al., 2022). Due to the lack of innate immune stimulation, conventional vaccinations have been proven to be insufficiently immunogenic. As a result, adjuvants for new-generation vaccines must ensure that the vaccine closely resembles illness in order to elicit a strong immune response. While adjuvants are powerful immunostimulators, most of them are not approved for use in the clinic due to unfavorable side effects. As a result, a substantial study focused on identifying the active components of adjuvants and then modifying them to reduce adverse effects (Song and Hu, 2009).
In a 2020 study, Roy et al. (2020) discussed the possible use of phytochemical-derived efflux pump inhibitors and immunotherapy to combat TB and provided a brief look at the molecular mechanism and structure of drug-efflux pumps seen in MTB. A recent article on monoclonal antibodies against antigens of Mycobacterium TB has produced mixed results. It showed that phagocytic cells enhanced antibody-mediated phagocytosis of mycobacteria by phagocytic cells using sera from patients vaccinated with bacillus Calmette-Guérin. Furthermore, cytokines and inhibitors as immunomodulators were examined in a clinical trial involving 50 patients with MDR-TB, where adjunct supplementation with recombinant human IL-2 for 7 months resulted in increased sputum smear conversion and improved immune status (Gupta et al., 2016).
Immunoxel (Dzherelo) is a water–alcohol extract of medicinal plants used in Ukraine as a TB and HIV immunotherapy adjuvant. Patients exhibited faster defervescence, weight increase, higher hemoglobin content, and less inflammation, as shown by the lower leukocyte counts and erythrocyte sedimentation rate. Only 19% of the individuals in the placebo group converted. These data suggest that solid Immunoxel delivered through the mucosa is equal to the original liquid Immunoxel given twice daily for 2–4 months (Efremenko et al., 2012). In a recent study, patients were divided into two groups: the first (n = 137) received a sublingual honey lozenge formulated with the botanical immunomodulator Immunoxel once daily; the second (n = 132) was given placebo lozenges in addition to conventional ATT. Immunoxel’s sputum clearing was effective in all types of TB. Immunoxel reduced TB-related inflammation, as shown by the decreased defervescence and normalization of high leukocyte counts and erythrocyte sedimentation rate. There were no adverse side effects observed at any period. Immunoxel is a commercially accessible immunotherapeutic intervention that is cost-efficient, safe, and successful in treating TB (Batbold et al., 2017).
In the in vitro and aerosol mouse models of MTB infection, researchers looked at the immunomodulatory effects of G1-4A, a polysaccharide extracted from the Indian medicinal plant Tinospora cordifolia. The findings showed that modulating host immune responses with G1-4A could increase the therapeutic efficacy of existing anti-tubercular medicines and could be a promising technique for developing new TB treatments (Gupta et al., 2016). Isoniazid as a potential host-directed therapy when used with luteolin, a plant-derived hepatoprotective immunomodulator, enhances anti-TB immunity, decreases the period of TB treatment, and avoids disease relapse. Luteolin also promotes central memory T-cell responses, which improves long-term anti-TB immunity. We also discovered that luteolin boosts the activity of natural killer and natural killer T-cells, both of which have antitubercular properties (Singh et al., 2021).
CONCLUDING REMARKS
Indigenous people of South Africa have relied on medicinal plants for their anti-TB healing properties. In the current review, it can be further deduced that plant-based biocompounds exhibit excellent therapeutic properties and are thus suitable candidates for treating TB, especially multiresistant M. tuberculosis strains. These findings should encourage researchers to pursue such TB management treatments in order to discover novel, cost-effective, efficacious, and sustainable drugs that can inhibit the growth and proliferation of M. tuberculosis and reduce the fatality rate of TB, even in patients with HIV in South Africa and beyond. They also shed light on the importance of South African medicinal plants and the need for more in-depth studies, including clinical studies in TB management.
AUTHOR CONTRIBUTIONS
All authors (MCM, COO, DOM, CAN) have collected the research data and wrote, edited, reviewed, and agreed on the final draft of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
All authors declare no financial or any other conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adefuye AO, Samie A, Ndip RN. In-vitro evaluation of the antimicrobial activity of extracts of Bridelia micrantha on selected bacterial pathogens. J Med Plants Res, 2011; 5(20):5116–22.
Akinyeye RO, Olatunya AM Phytochemical screening and mineral composition of the bark of some medicinal trees in Ondo State, Nigeria. Med Aromat Plant Res J, 2014; 2:44–9.
Alhassan M. South African plants as a source of herbicides: identification of a compound with phytotoxic activity from Artemisia afra Jacq. ex Willd. Doctoral dissertation, University of KwaZulu-Natal, Pietermaritzburg, South Africa,
Anochie PI, Ndingkokhar B, Bueno J, Anyiam FE, Ossai-Chidi LN, Onyeneke EC, Onyeozirila AC. African medicinal plants that can control or cure tuberculosis. Int J Pharm Sci Dev Res, 2018; 4(1):001–8.
Anywar G, Kakudidi E, Byamukama R, Mukonzo J, Schubert A, Oryem-Origa H, Jassoy C. A review of the toxicity and phytochemistry of medicinal plant species used by herbalists in treating people living with HIV/AIDS in Uganda. Front Pharmacol, 2021; 12:615147.
Auvin C, Lezenven F, Blond A, Augeven-Bour I, Pousset JL, Bodo B, Camara J. Mucronine J, a 14-membered cyclopeptide alkaloid from Zizyphus mucronata. J Nat Prod, 1996; 59(7):676–8.
Babiaka SB, Ntie-Kang F, Ndingkokhar B, Mbah JA, Sippl W, Yong JN. The chemistry and bioactivity of Southern African flora II: flavonoids, quinones and minor compound classes. RSC Adv, 2015; 5(71):57704–20.
Bapela JM, Kuete V, Du Toit E, Meyer MJ, Lall N. Fertilization-induced changes in growth parameters and antimycobacterial activity of Euclea natalensis (Ebenaceae). S Afri J Bot, 2008; 74(2):244–50.
Batbold U, Butov DO, Kutsyna GA, Damdinpurev N, Grinishina EA, Mijiddorj O, Kovolev ME, Baasanjav K, Butova TS, Sandagdorj M, Batbold O. Double-blind, placebo-controlled, 1: 1 randomized Phase III clinical trial of Immunoxel honey lozenges as an adjunct immunotherapy in 269 patients with pulmonary tuberculosis. Immunotherapy, 2017; 9(1):13–24.
Berkowitz N, Okorie A, Goliath R, Levitt N, Wilkinson RJ, Oni T. The prevalence and determinants of active tuberculosis among diabetes patients in cape town, South Africa, a high HIV/TB burden setting. Diabetes Res Clin Pract, 2018; 138:16–25.
Bosch CH. Bridelia micrantha (Hochst.) Baill. In: Lemmens RHMJ, Louppe D, Oteng-Amoako AA (Eds.). Plant resources of tropical Africa 7(2). Timbers 2, PROTA Foundation, Wageningen, The Netherlands, pp 169–71, 2012.
Buwa LV, Afolayan AJ. Antimicrobial activity of some medicinal plants used for the treatment of tuberculosis in the Eastern Cape province, South Africa. Afr J Biotech, 2009; 8(23):2009.
Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia J Ethnobiol Ethnomed, 2015; 11:4.
Chigora P, Masocha R, Mutenheri F. The role of indigenous medicinal knowledge (IMK) in the treatment of ailments in rural Zimbabwe: the case of Mutirikwi communal lands. J Sustain Dev Afr, 2007; 9(2):26–43.
Chiocchio I, Mandrone M, Tomasi P, Marincich L, Poli F. Plant secondary metabolites: an opportunity for circular economy. Molecules, 2021; 26(2):495.
Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Inter J Antimicrob Agents, 2005; 26(5):343–56.
Davids D, Blouws T, Aboyade O, Gibson D, De Jong JT, Van’t Klooster C, Hughes G. Traditional health practitioners’ perceptions, herbal treatment and management of HIV and related opportunistic infections. J Ethnobiol Ethnomed, 2014; 10(1):1–4.
Du Toit A, Van der Kooy F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J Ethnopharmacol, 2019; 244:112127.
Dzoyem JP, Aro AO, McGaw LJ, Eloff JN. Antimycobacterial activity against different pathogens and selectivity index of fourteen medicinal plants used in southern Africa to treat tuberculosis and respiratory ailments. S Afr J Bot, 2016; 102:70–4.
Efremenko YV, Arjanova OV, Prihoda ND, Yurchenko LV, Sokolenko NI, Mospan IV, Pylypchuk VS, Rowe J, Jirathitikal V, Bourinbaiar AS, Kutsyna GA. Clinical validation of sublingual formulations of Immunoxel (Dzherelo) as an adjuvant immunotherapy in treatment of TB patients. Immunotherapy, 2012; 4(3):273–82.
Foyet HS, Keugong Wado E, Ngatanko Abaissou HH, Assongalem EA, Eyong OK. Anticholinesterase and antioxidant potential of hydromethanolic extract of Ziziphus mucronata (rhamnaceae) leaves on scopolamine-induced memory and cognitive dysfunctions in mice. Evid Based Complement Alternat Med, 2019; 24:4568401.
Gemechu A, Giday M, Worku A, Ameni G. In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med, 2013; 13(1):1–6.
Gqaleni N, Moodley I, Kruger H, Ntuli A, McLeod H. Traditional and complementary medicine: health care delivery. S Afr Health Rev, 2007; 1:175–88.
Green E, Obi LC, Samie A, Bessong PO, Ndip RN. Characterization of n-hexane subfraction of Bridelia micrantha (Berth) and its antimycobacterium activity. BMC Complement Altern Med, 2011; 11:28.
Green E, Samie, A Obi, CL Bessong, PO Ndip, RN. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J Ethnopharmacol, 2010; 130(1):151–7.
Gupta PK, Chakraborty P, Kumar S, Singh PK, Rajan MG, Sainis KB, Kulkarni S. G1-4A, a polysaccharide from Tinospora cordifolia inhibits the survival of Mycobacterium tuberculosis by modulating host immune responses in TLR4 dependent manner. PLoS One, 2016; 11(5):e0154725.
Ingram V, Schure J. Review of non timber forest products (NTFPs) in central Africa: Cameroon. Center for International Forestry Research (CIFOR), Yaounde, Cameroon, p 167, 2010.
Jain C, Khatana S, Vijayvergia R. Bioactivity of secondary metabolites of various plants: a review. Int J Pharm Sci Res, 2019; 10(2):494–8.
John CL, Chelliah LP, Chelliah GP. Kayakalpam (Rejuvenative) herbs: an immunomodulators in Siddha system of medicine: a scientific review. World J Adv Res Rev, 2022; 13(02):505–10.
Kane NF, Kyama MC, Nganga JK Hassanali A, Diallo M, Kimani FT. Acute toxicity effect of Artemisia afra plant extracts on the liver, kidney, spleen and in vivo antimalarial assay on Swiss albino mice. Adv Biosci Bioengin, 2019; 7(4):64.
Khademi F, Taheri RA, Avarvand AY, Vaez H, Momtazi-Borojeni AA, Soleimanpour S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb Pathogen, 2018; 121:218–23.
Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed, 2014; 10(1):1–22.
Koà K, Neya FB, Opoku N, Baissac Y, Campa C and Sankara P. Phytochemical analysis of Ziziphus mucronata Willd. extract and screening for antifungal activity against peanut pathogens. Afr J Plant Sci, 2017; 11(11):394–02.
Krige J, Dreyer LL and Mucina L. Floristic links between the west Coast and south Coast (South Africa)-is the Breede river valley a migration route? S Afr J Bot, 2009; 75(2):408.
Kshirsagar SG, Rao RV. Antiviral and immunomodulation effects of Artemisia. Medicina, 2021; 57(3):217.
Kuete V, Lall N, Efferth T. Anti-infective and antiproliferative potential of African medicinal plants. Evid Based Complement Alternat Med, 2012; 2012:53219.
Kuroki M, Miyamoto S, Morisaki T, Yotsumoto F, Shirasu N, Taniguchi Y, Soma GI. Biological response modifiers used in cancer biotherapy. Anticancer Res, 2012; 32(6):2229–33.
Labuschagné A, Hussein AA, Rodríguez B, Lall N. Synergistic antimycobacterial actions of Knowltonia vesicatoria (Lf) Sims. Evid Based Complement Alternat Med, 2012; 2012:808979.
Labuschagné A, Lall N, Mativandlela SPN, McGaw LJ, Hamilton C. Antimycobacterial activity of seven herbaceous plants. Unpublished Honour’s dissertation, University of Pretoria, Pretoria, South Africa, 2008.
Lall N, Weiganand O, Hussein AA, Meyer JJM. Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. S Afr J Bot, 2006; 72:579–83.
Lall N, Meyer JJM, Taylor MB, van Staden J. Anti-HSV-1 activity of Euclea natalensis. S Afr J Bot, 2005; 71(3):444–6.
Lall N, Meyer JJM, Wang Y, Bapela NB, van Rensburg CEJ, Fourie B, Franzblau SG. Characterization of intracellular activity of antitubercular constituents from the roots of Euclea natalensis. Pharm Biol, 2005; 43:353–57.
Lall N, Meyer JJM. Inhibition of drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis by diospyrin, isolated from Euclea natalensis. J. Ethnopharmacol, 2001; 78:213–6.
Lall N. Isolation and Identification of Naphthoquinones from Euclea Natalensis with activity against Mycobacterium tuberculosis, other pathogenic bacteria and herpes simplex virus. PhD Thesis, University of Pretoria, Pretoria, South Africa, .
Lima CSA, de Amorim ELC, de Sena KX, Chiappeta AD, Nunes XP, Agra MD, da-Cunha EV, Silva MS, Barbosa-Filho JM. Antimicrobial activity of a mixture of two isomeric phenylpropanoid glycosides from Arrabidaea harleyi A. H. Gentry (Bignoniaceae). Braz J Pharm Sci, 2003; 39(1):77–81.
Liu NQ, Van der Kooy F, Verpoorte. R. Artemisia afra: a potential flagship for African medicinal plants? S Afri J Bot, 2009; 75(2):185–95.
Lomchoey N. Cyclopeptide alkaloids from some Ziziphus plants. Doctoral dissertation, Srinakharinwirot University, Bangkok, Thailand,
Ludere MT, Van Ree T, Vleggaar RIsolation and relative stereochemistry of lippialactone, a new antimalarial compound from Lippia javanica. Fitoterapia, 2013; 86(3):188–92.
Madikizela B, McGaw. L Anti-mycobacterial, cytotoxicity and genotoxicity effects of five traditionally used anti-tuberculosis plants in South Africa. Planta Med Inter Open, 2017; 4(S 01):We–SL.
Mahapatra A, Mativandlela SPN, Binneman B, Fourie BP, Hamilton CJ, Meyer JJM, van der Kooy F, Houghon P, Lall N. Activity of 7-methyljuglone derivatives against Mycobacterium tuberculosis and as subversive substrate for mycothiol disulfide reductase. Bioorg Med Chem, 2007; 15:7638–46.
Maluleke KA. Isolation and characterization of antidiabetic constituents of Bridelia micrantha. Doctoral dissertation, University of Venda, Limpopo, South Africa,
Manenzhe NJ, Potgieter N, Van Ree T. Composition and antimicrobial activities of volatile components of Lippia javanica. Phytochemistry, 2004; 65(16):2333–6.
Maroyi A. Ethnopharmacology and therapeutic value of Bridelia micrantha (Hochst.) Baill. in tropical Africa: a comprehensive review. Molecules, 2017; 22:1493.
Maroyi A. Lippia javanica (Burm. F.) Spreng: traditional and commercial uses and phytochemical and pharmacological significance in the African and Indian subcontinent. Evid Based Complement Alternat Med, 2017; 34:6746071
Maroyi A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of Euclea natalensis A.DC. Molecules, 2017; 22:2128.
Martini MC Zhang T, Williams JT, Abramovitch RB, Weathers PJ, Shell SS. Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J Ethnopharmacol, 2020; 262:113191.
Martini ND, Katerere DRP, Eloff JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol, 2004; 93(2):207–12.
Martolia J, Soni H, Tandel F. Natural perspective for management of drug resistant tuberculosis: a Review. Americas, 2020; 3:1.
Masoko P, Nxumalo KM. Validation of antimycobacterial plants used by traditional healers in three districts of the Limpopo Province (South Africa)” Evid Based Complement Alternat Med, 2013; 2013:586247.
Mativandlela SPN, Meyer JJM, Hussein AA, Houghton PJ, Hamilton CJ, Lall N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South African medicinal plants. Phytother Res, 2008; 22(6):841–5.
Mbokane EM, Moyo NAG. A preliminary investigation into the potential effect of Artemisia afra on growth and disease resistance in sub-adults of Oreochromis mossambicus. Aquaculture, 2018; 482:197–202.
McGaw LJ, Lall N, Hlokwe TM, Michel AL, Meyer JJM, Eloff JN. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol Pharm Bull, 2008; 31(7):1429–33.
Meke GS, Mumba RFE, Bwanali RJ, Williams VL. The trade and marketing of traditional medicines in southern and central Malawi. Int J Sustain Dev World Ecol, 2017; 24:73–87.
Mekonen K, Afework M, Makonnen E, Debela A, Ergete W, Tolessa, T. Evaluation of acute and sub-acute toxicity of aqueous extracts of Artemisia afra leaves on brain heart and suprarenal glands in swiss albino mice. Ethiopian J Health Sci, 2020; 30:6.
Mongalo NI, Mashele SS, Makhafola TJ. Ziziphus mucronata Willd. (Rhamnaceae): it’s botany, toxicity, phytochemistry and pharmacological activities. Heliyon, 2020; 6(4):e03708.
More G, Lall N, Hussein A, Tshikalange TE. Antimicrobial constituents of Artemisia afra Jacq. ex Willd. against periodontal pathogens. Evid Based Complement Alternat Med, 2012; 2012:252758.
Mujovo SF, Hussein AA, Meyer JJM, Fourie B, Muthivhi T, Lall T. Bioactive compounds from Lippia javanica and Hoslundia opposite. Nat Prod Res, 2008; 22(12):1047–54.
Mukinda JT, Syce JA Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J ethnopharmacol, 2007; 112(1):138–44.
Munayi RR. Phytochemical investigation of Bridelia micrantha and Tabernaemontana ventricosa for cytotoxic principles against drug sensitive leukemia cell lines. Master’s Thesis, University of Nairobi, Nairobi, Kenya,
Mungho TC, Tobela GE, Olasunkanmi AO, Constance SR. Acute toxicity and antihypertensive effects of Artemisia afra and Leonotis leonurus in spontaneously hypertensive rats. Res J Biotechnol, 2018; 13:1.
Neffati M, Najjaa H, Máthé Á. (Eds.). Medicinal and aromatic plants of the World-Africa. Springer, p 3, 2017.
Ngane RAN. Antibacterial activity of methanol extract and fractions from stem Bark of Bridelia micrantha (Hochst.) Baill. (Phyllanthaceae). EC Pharmacol Toxicol, 2019; 7:609–16.
Nielsen TR, Kuete V, Jäger AK, Meyer JJM, Lall N. Antimicrobial activity of selected South African medicinal plants. BMC Complement Alternat Med, 2012; 12(1):1–6.
Njoroge NJ, Bussmann RW. Traditional management of ear, nose and throat (ENT) diseases in Central Kenya. J Ethnobiol Ethnomed, 2006; 2(54):1–9.
Ntutela S, Smith P, Matika L, Mukinda J, Arendse H, Allie N. Efficacy of Artemisia afra phytotherapy in experimental tuberculosis. Tuberculosis, 2009; 89:S33–40.
Nyahangare ET, Mvumi BM, Stevenson PC. Tick control measures from nature. Afgriland, 2012; 56(3):76–7.
Oguntibeju OO. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J Inflamm Res, 2018; 11:307.
Okeleye BI, Bessong PO, Ndip RN. Preliminary phytochemical screening and in vitro anti-helicobacter pylori activity of extracts of the stem bark of Bridelia micrantha (Hochst., Baill., Euphorbiaceae). Molecules, 2011; 16(8):6193–05.
Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement Altern Med, 2011; 11(1):1–8.
Oosthuizen CB, Lall N. Euclea natalensis, Underexplored medicinal plants from Sub-Saharan Africa plants with therapeutic potential for Human Health. Academic Press, pp 111–6, 2020.
Padmapriyadarsini C, Sachdeva KS, Nair D, Ramachandran R. The paradigm shift in the approach to management of latent tuberculosis infection in high tuberculosis burden countries. Expert Rev Respir Med, 2021; 15(7):899–10.
Rao CS, Raju C, Gopumadhavan S, Chauhan BL, Kulkarni RD, Mitra SK. Immunotherapeutic modification by an ayurvedic formulation Septilin, Indian J Experiment Biol, 1994, 32:553–58.
Rawat S, Raizaday A, Pathak S, Singh H, Mishra A, Singh SK, Dua K, Gupta G. Medicinal plants in the treatment of tuberculosis III. Medicinal plants for lung diseases. Springer, Singapore, pp 217–27, 2021.
Reddy MN, Adnan M, Alreshidi MM, Saeed M, Patel M. Evaluation of anticancer, antibacterial and antioxidant properties of a medicinally treasured fern Tectaria coadunata with its phytoconstituents analysis by HR-LCMS. AntiCancer Agents Med Chem, 2020; 20(15):1845–56.
Roy A, Hussain A, Sinha K, Praharaju M, Khatun S. EPIs and immunotherapy–an answer to drug-resistant tuberculosis. Int J Med Health Sci, 2020; 10:1306−19.
Samie A Housein A, Lall N, Meyer JJM. Crude extracts of, and purified compounds from, Pterocarpus angolensis, and the essential oil of Lippia javanica: their in-vitro cytotoxicitiesand activities against selected bacteria and Entamoeba histolytica. Ann Trop Med Parasitol, 2009; 103(5):427–39.
Semenya S, Potgieter M, Erasmus L. Ethnobotanical survey of medicinal plants by Bapedi healers to treat diabetes mellitus in the Limpopo province, South Africa. J Ethnopharmacol, 2012; 141(1):440–5.
Semenya SS, Maroyi A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo province, South Africa. Afr J Tradit Complement Altern Med, 2013; 10(2):316–23.
Semenya SS, Potgieter MJ, Tshisikhawe MP. Use, conservation and present availability status of ethnomedicinal plants of Matebele-village in the Limpopo province, South Africa. Afr J Biotechnol, 2013; 12(18):2392–05.
Sharifi-Rad J, Salehi B, Stojanovi?-Radi? ZZ, Fokou PVT, Sharifi-Rad M, Mahady GB. Medicinal plants used in the treatment of tuberculosis-ethnobotanical and ethnopharmacological approaches. Biotechnol Adv, 2020; 107629.
Shelembe BG, Moodley R, Jonnalagadda SB. Secondary metabolites isolated from two medicinal plant species, Bridelia micrantha and Sideroxylon inerme and their antioxidant activities. Acta Pol Pharm Drug Res, 2016; 73:1249–57.
Shikanga EA, Combrinck S, Regnier T. South African Lippia herbal infusions: total phenolic content, antioxidant and antibacterial activities. S Afr J Bot, 2010; 76(3):567–71.
Sigidi MT, Anokwuru CP, Zininga T, Tshisikhawe MP, Shonhai A, Ramaite IDI. Comparative in vitro cytotoxic, anti-inflammatory and anti-microbiological activities of two indigenous Venda medicinal plants. Transl Med Commun, 2016; 1(1):1–7.
Singh DK, Tousif S, Bhaskar A, Devi A, Negi K, Moitra B, Ranganathan A, Dwivedi VP, Das G. Luteolin as a potential host-directed immunotherapy adjunct to isoniazid treatment of tuberculosis. PLoS Pathogens, 2021; 17(8):e1009805.
Song X, Hu S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine, 2009; 27:4883–90.
Street RA Stirk WA, Van Staden J. South African traditional medicinal plant trade—challenges in regulating quality, safety and efficacy. J Ethnopharmacol, 2008; 119(3):705–10.
Sunmonu TO, Afolayan AJ. Evaluation of antidiabetic activity and associated toxicity of Artemisia afra aqueous extract in Wistar rats. Evid Based Complement Alternat, 2013; 2013:929074.
Tuyiringire N, Deyno S, Weisheit A, Tolo CU, Tusubira D, Munyampundu JP. Three promising antimycobacterial medicinal plants reviewed as potential sources of drug hit candidates against multidrug-resistant tuberculosis. Tuberculosis, 2020; 124:101987.
Twilley D, Rademan S, Lall, N. A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J Ethnopharmacol, 2020; 261:113101.
Van Der Kooy F. Characterisation, synthesis and antimycobacterial activity of naphthoquinones isolated from Euclea natalensis. MSc Dissertation, University of Pretoria, Pretoria, South Africa.
Van Der Kooy F. The medicinal and chemical aspects of naphthoquinones isolated from Euclea natalensis A. DC. on Mycobacterium tuberculosis. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa.
Van Wyk AS, Prinsloo G. Medicinal plant harvesting, sustainability and cultivation in South Africa Biol Conserv, 2018; 227:335–42.
Van Wyk B-E, Gericke N. People’s plants: a guide to useful plants of southern Africa. Briza. Publications, Pretoria, South Africa.
Wan X, Yin Y, Zhou C, Hou L, Cui Q, Zhang X, Cai X, Wang Y, Wang L, Tian J. Polysaccharides derived from Chinese medicinal herbs: a promising choice of vaccine adjuvants. Carbohydr Poly, 2022; 276:118739.