INTRODUCTION
Background
Under current conditions of the information revolution and scientific and technological progress (Gruzina, 2020; The Eurasian Economic Union, 2022; United Nations Industrial Development Organization, 2021), pharmaceutical companies around the world are actively investing in research and development (R&D) of medicines (Armstrong, 2022; Assis, 2022; Atradius, 2022; Buntz, 2022; Citeline, 2021; Congressional Budget Office, 2021) [a compound annual growth rate (CAGR) of new drugs in pipeline, calculated according to Pharmaprojects data (2001–2021), is 5.53% (Citeline, 2021)]. Consequently, in the pharmaceutical market, the number of drug products (DPs) is rapidly increasing [a CAGR of first approved DPs by the Food and Drug Administration, calculated according to the Center for Drug Evaluation and Research (2008–2021), is 5.38%] (Mikulic, 2022; Center for Drug Evaluation and Research FDA, 2022). No doubt, this trend is favorable for pharmacotherapeutic medical care (PhTMC), but it is obvious that in the pharmaceutical market, quantity does not always result in quality.
Since the rise of DPs leads to the growth of its information, it will be more difficult for the workers of the pharmaceutical product lifecycle (PhPL) to process this amount of data and make the right decisions in their work. Without convenient IT-solutions (ITSs), there is a high probability of reduced quality of PhTMC.
Purpose and objectives
Our aim is to systematize innovative domestic IT-solutions (dITSs) for the optimization of remote control (RC) of drug information (DI) that is generated through the PhPL. To achieve the goal, we set the following tasks: determine the key terms, define a literature search strategy, find the dITSs that allow to optimize DI on the PhPL stages and describe the results in a tabular form.
MATERIALS AND METHODS
This review tends to be systematized due to the presence of a search strategy, a narrative synthesis with tabular accompaniment, and an analysis of what is known and what is uncertain [Table 1 where the criteria of compliance were checked according to SALSA framework utilized by Grant and Booth (2009)].
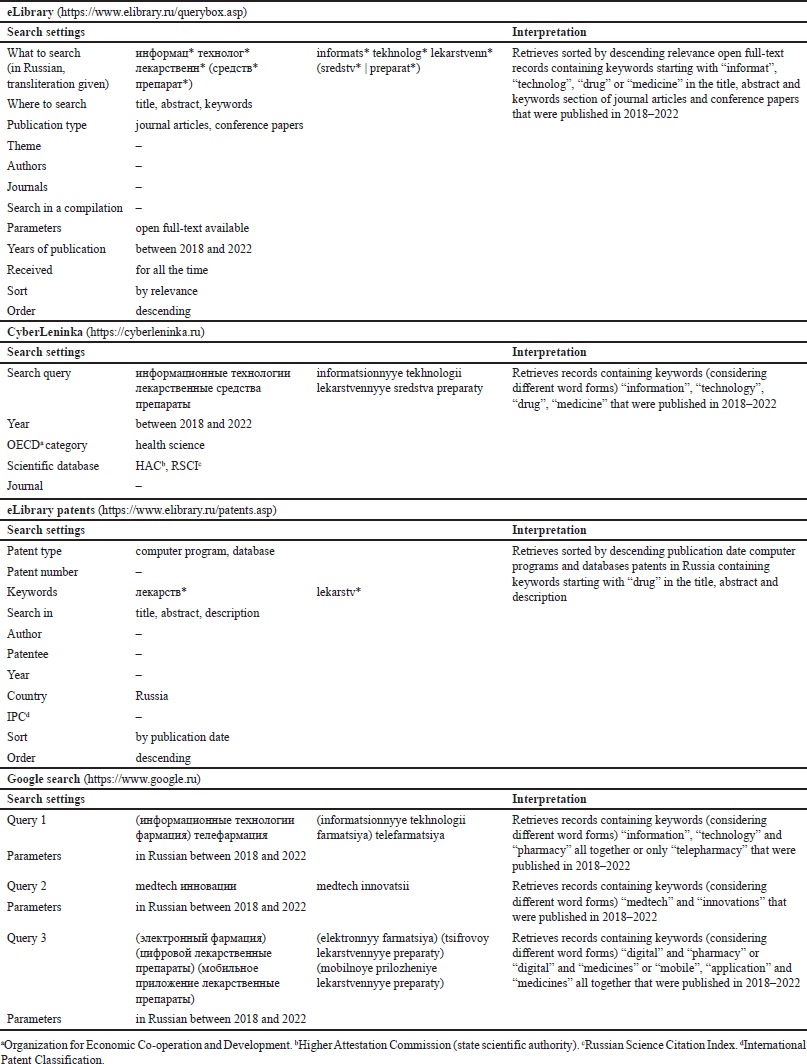
The search for dITSs consisted of three parts and was conducted on November 26, 2022, in Russian and in accordance with the search settings specified for each search engine (Table 2).
Initially, we carried out a literature search via eLibrary and CyberLeninka. The exclusion criteria at the screening step were: publications earlier than 2018 (5-year relevance), limited access (paid, on request), a foreign language, and a mismatch of titles and/or abstracts to the purpose of the review. The exclusion criterion at the eligibility assessment step was an absence of reference to the specific dITSs for the DI optimization within the PhPL.
The second part of the search was done through the Russian patent database provided by eLibrary. The exclusion criteria at the screening step were next: records earlier than 2018 and a mismatch of titles to the purpose of the review. The exclusion criterion at the eligibility assessment step was an absence of the patent’s description.
 | Table 1. Checking the review for the systematized review criteria of compliance (according to SALSA framework utilized by Grant and Booth (2009) . [Click here to view] |
 | Table 2. Search settings by the search engine. [Click here to view] |
The third part was a Google search. We did not include web pages further than the 10th search result [onwards the value of the results significantly drops, which is associated with Google search algorithms (Chitika, 2013; Google, n.d.; Google Help, n.d.)]. The exclusion criteria at the screening step were the following: publications earlier than 2018, a limited access, a foreign language, and a mismatch of titles and/or snippets to the purpose of the review. The exclusion criterion at the eligibility assessment step was a mismatch between the web page’s full text and the purpose of the review.
As the search was over, we formed a PRISMA flowchart (Fig. 1). All the included records of the search are summarized in Table 3. The key terms are introduced in the authors’ interpretation. The synthesis was done narratively with a tabular appendix (Table 4).
RESULTS AND DISCUSSION
Key terms
In the study, we have identified and defined the following terms. DI: any information about a drug, pharmaceutical information: any information related to pharmaceutical and medical activities within the circulation of medicines, ITSs for the optimization of information RC: automated information systems (IS) to save consumer resources for information search and processing, automation: the act of changing processes in order to reduce the human participation (both the developer’s and the user’s), optimization: the act of modifying a process to achieve its better efficiency and innovation: a new product (idea, method, item, device, etc.) or the act of innovating (i.e., creating an innovation).
Output of the systematic search and innovation vector
Out of 312 search results, 18 (5.77%) were included in the review. 23 dITSs were extracted from the studied resources. Some of them are multifunctional and solve several problems, use a combination of ITs, are used in several fields, and are developed jointly by several teams from different geographical centers.
The vector of dITSs is aimed at optimizing DI in medical organizations (16 out of 23, 69.6%; the PhPL has nothing to do with it) and in retail (6 out of 23, 26.1%). We have identified 37 directions in total, while the predominant directions are still the same (Fig. 2). The remaining directions are distributed approximately evenly across the PhPL except for the stage of disposal and waste management. Our review found no innovation in this sector. To identify the stages of PhPL, we have turned to the textbook “Pharmaceutical Informing” (under the editorship of Svistunov and Tarasov, 2020) and looked through the paper of Pyatigorskaya et al. (2020).
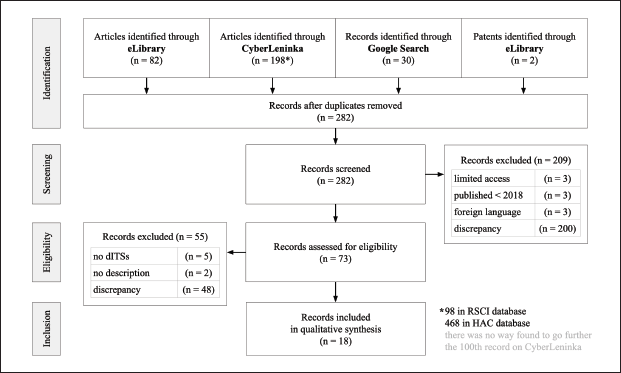 | Figure 1. PRISMA flowchart of the search. [Click here to view] |
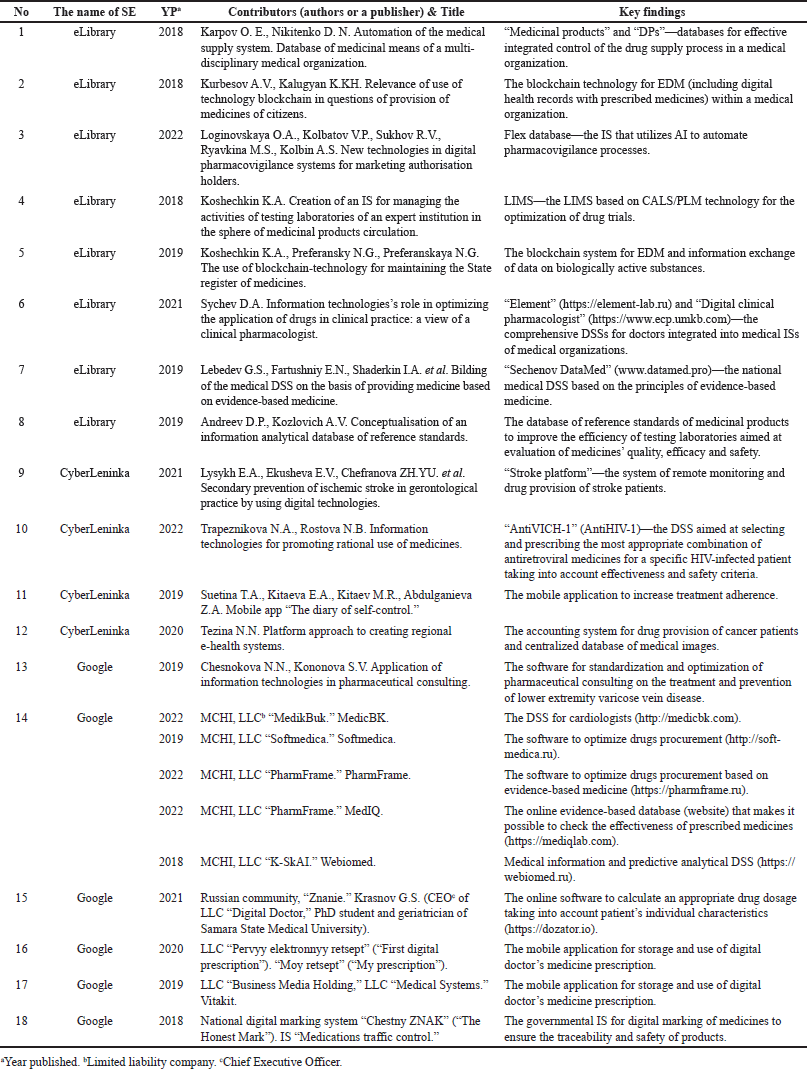 | Table 3. Included recordings on the dITSs topic. [Click here to view] |
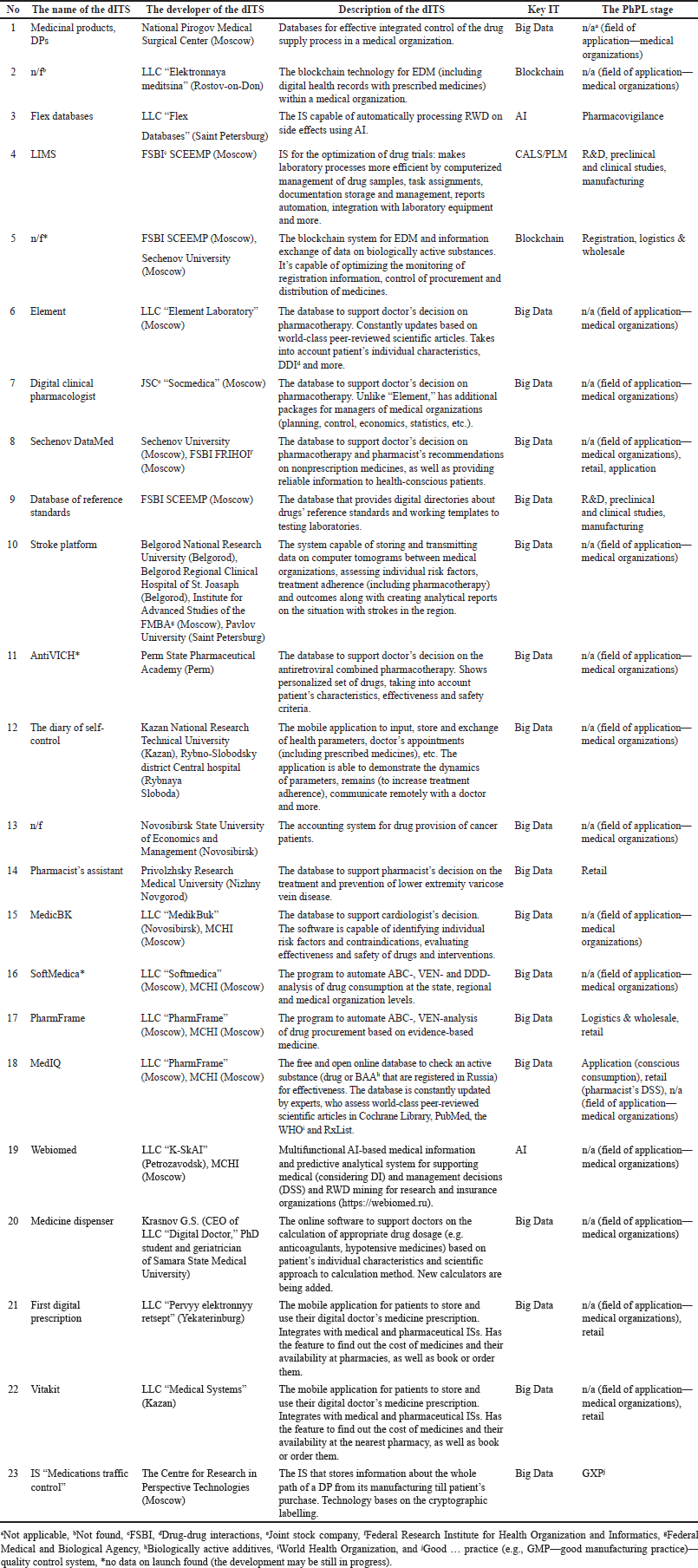 | Table 4. The dITSs for the optimization of DI RC and their supposed application on the stages of the PhPL. [Click here to view] |
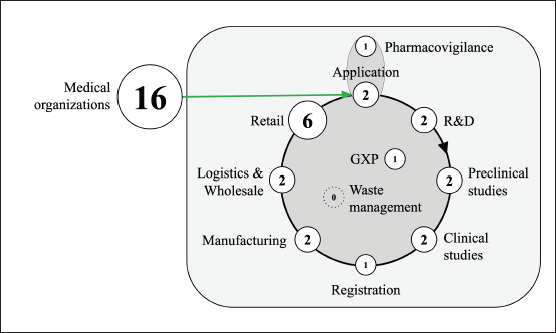 | Figure 2. The number of innovative dITSs applied to the PhPL (2018–2022). [Click here to view] |
Utilized technologies and their practical applications
18 out of 23 (78.3%) dITSs used big data as the main technology, 2 (8.7%) blockchain, 2 (8.7%) artificial intelligence (AI), and 1 (4.3%) continuous acquisition and lifecycle support/product lifecycle management (CALS/PLM) (Fig. 3). When selecting and describing ITs, we relied on the works of Vardomatskaya and Lilyukhin (2020), Salimjanova and Dyachuk (2021). However, as the complex CALS/PLM technology was difficult to define in one of the groups given in the works above, so we have singled it out.
It is important to understand what these technologies are. Big data can be defined as a technology for processing (collecting, analyzing, and visualizing) large and complex datasets. In pharmacy, this technology is particularly useful for extracting data from arrays of electronic medical records of patients and clinical trial participants. The technology helps to accelerate the development of new drugs and make data-driven decisions for healthcare practitioners.
Blockchain is an encrypted storage technology. It stands up for a decentralized database consisting of algorithmically linked blocks of information that are simultaneously stored on multiple computer devices connected via a mutual network. The key advantage of the technology is the transparent tracking of data that cannot be altered without leaving a trace. This is particularly important to stand against counterfeit pharmaceutical products and for monitoring healthcare practitioners’ prescriptions.
AI is a technology that imitates human intelligence to solve human mind-dependent (“sapiodependent”—authors’ expression”) tasks based on preset self-improving algorithms. AI can accelerate diagnosis and prescription of personalized pharmacotherapy (including genetic features). AI can be one of the methods of big data technology (we assume to call it “technology within technology”).
CALS/PLM according to Koshechkin (2018) is a combination of CALS technologies, which include technologies for continuous information support of supply and product lifecycle, and PLM, which is an organizational and technical system for managing product-related information throughout its lifecycle (from R&D to market exit).
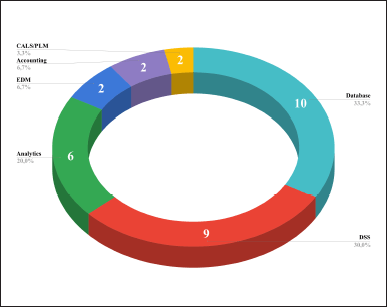
Our research showed the following forms in which these technologies are utilized: databases (10 out of 23, 43.5%), decision support systems (DSS; 9 out of 23, 39.1%), automated analytics (6 out of 23, 26.1%), electronic document (EDM; 2 out of 23, 8.7%), and DI management systems during the lifecycle (1 out of 23, 4.3%), as well as improving accounting systems (1 out of 23, 4.3%).
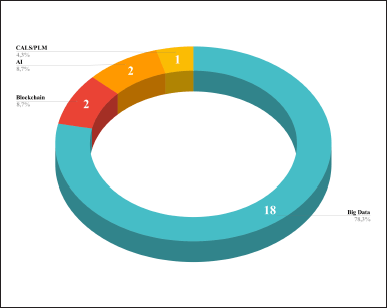 | Figure 3. The main utilized ITs. [Click here to view] |
Based on the articles included in the review, we can synthesize the definitions of each extracted form. Database refers to a structured dataset stored electronically. DSS acts as a computer program that processes data to provide useful information for decision-making. Automated analytics may be defined as interactive data visualization software or cloud-based services (graphs, charts, maps, schemes, etc.). EDM (as well as DI management) system is a software or cloud system aimed at storing and organizing digital documents. Finally, accounting systems are computer programs to track and manage processes (tasks, medical interventions, financial data, etc.).
A total of 30 points of practical application were found, grouped, and applied to PhPL (Fig. 4). At the stages of R&D, preclinical and clinical trials, it is possible to use the laboratory information management system (LIMS) and the database of reference standards by Federal State Budgetary Institution “Scientific Centre for Expert Evaluation of Medicinal Products (FSBI SCEEMP). In the next step of registration, another IT by FSBI SCEEMP may be applied—an EDM system based on blockchain technology. The manufacturing stage also involves the application of the LIMS and the database of reference standards. Next, logistics and wholesale are points for the application of blockchain EDM system (FSBI SCEEMP) and “PharmFrame” software to optimize drug procurement. The segment of medicine retail may benefit from “Sechenov DataMed” (DSS for healthcare practitioners), “Pharmacist’s assistant” (DSS for a pharmacist), “PharmFrame” and “MedIQ” (both contain evidence-based information on medicines), and “First digital prescription” and “Vitakit” (both are mobile applications to store doctor’s prescriptions). After all, at the stage of applying a medication, health-conscious patients can obtain reliable information from “MedIQ” and “Sechenov DataMed” as these services are open-access.
 | Figure 4. Practical application of the dITSs. [Click here to view] |
Pharmacovigilance and GXP are at the level of monitoring and control, so they are not depicted as a link in the lifecycle. Nevertheless, they have a direct impact on it. “Flex Database” automatically processes real-world data (RWD) on side effects using AI and therefore applies at the pharmacovigilance stage. The medication traffic control system by the Centre for Research in Perspective Technologies cryptographically stores information about the whole path of a DP and that is the reason for its application in GXP.
Finally, the predominant point of application of the discovered technologies in medical organizations. They are not part of the PhPL, but they are related to one of its stages—the stage of applying medicine. Different technologies are used here in various forms and for various purposes: databases (“Medicinal products” and “DPs,” “Stroke platform,” “The diary of self-control,” “MedIQ,” “First digital prescription” and “Vitakit”), EDM systems (LLC “Elektronnaya meditsina”), DSS (“Element,” “Digital clinical pharmacologist,” “Sechenov DataMed,” “AntiVICH,” “MedicBK,” “Medicine dispenser”), accounting system for drug provision (by Novosibirsk State University of Economics and Management), automated analytics (“SoftMedica”), and multifunctional AI-based system (“Webiomed”). Detailed information is provided in Table 4.
 | Figure 5. The main developer groups. [Click here to view] |
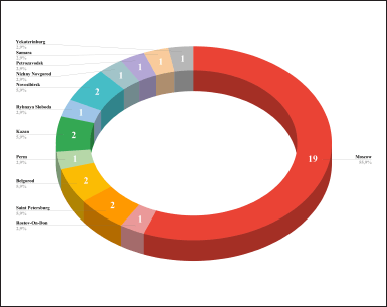 | Figure 6. The major geographical centers of the dITSs in Russia. [Click here to view] |
Developers and geographical centers
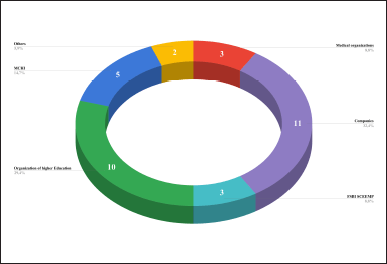
The majority of dITSs were developed with the participation of commercial organizations (11 out of 23, 47.8%), higher education institutions (6 out of 23, 30.4%), and the Moscow Center for Innovative Technologies in Healthcare (2022); 5 out of 23, 21.7% (MCHI). Most of the dITSs were proposed with the participation of the MCHI (5 out of 23, 21.7%) and FSBI “SCEEMP” (The Ministry of Health of the Russian Federation; 3 out of 23, 13%). A total of 34 developers were found (4 of them occur more than once). Commercial organizations (11 out of 34, 32.4%), higher education institutions (10 out of 34, 29.4%), and the MCHI (5 out of 34, 14.7%) were most often involved in the development (Fig. 5).
The main geographical center of innovation is the capital of Russia, Moscow (it occurs 19 out of 34 times, 55.9%) (Fig. 6).
CONCLUSION
When distributing dITSs through the PhPL, we faced the fact that most of the dITSs do not apply to the lifecycle, since they are associated with the optimization of DI RC in medical organizations. In this regard, it seems reasonable to consider the lifecycle of DI as an add-on to the PhPL in further research.
Also, during the screening of resources, we noticed that scientists pay much attention to discussing the problems and prospects of ITs, putting forward proposals and methods, while almost no experience in the use of already implemented and pilot ITSs to optimize DI RC in Russia is observed. This indicates that such innovations are hard-to-find in research papers and, if found, they are insignificantly described. Therefore, it is more productive to seek them through project aggregators and accelerators and business or investment reports. This is confirmed by the successful result of our predictive decision to conduct several search queries on Google—almost half (10 out of 23, 43.5%) of innovative dITSs were found this way. In the future, it is necessary to understand the reason for the lack of dITSs at the stage of disposal and waste management. To do this, we suppose to study the information structure and the needs of specialists in this field. Perhaps, this line of business needs optimization and innovation.
After all, based on the results of the review, one may see the drivers of the innovation vector in the studied issue for the next few years. These include traceability and control of DI to improve the quality of PhTMC and automation of RWD analysis with the help of AI to make faster and better medical, pharmaceutical, and management decisions.
LIMITATIONS
The review design is a “systematized review” according to review typology by Grant and Booth (2009). Thus, quality assessment was not included. This sets the stage for further studies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Center for drug evaluation and research FDA. Advancing health through innovation: new drug therapy approvals 2021. 2022. Available via https://www.fda.gov/media/155227/download (Accessed 23 November 2022).
Andreev DP, Kozlovich AV. Conceptualisation of an information ana-lytical database of reference standards. Bull Sci Cent Expert Eval Med Prod, 2019; 9(1):49–53; https://doi.org/10.30895/1991-2919-2019-9-1-49-53.
Armstrong A. Top 10 pharma R&D budgets in 2021. 2022. [Online]. Available via https://www.fiercebiotech.com/special-reports/top-10-pharma-rd-budgets-2021#b2772f88-9b62-4d75-8ab1-e42fd0e92c5d (Accessed 23 November 2022).
Assis P. Pharmaceutical R&D spending trends in 2022. 2022. [Online]. Available via https://www.prescouter.com/2022/06/pharmaceutical-rd-spending-trends-in-2022/ (Accessed 23 November 2022).
Atradius. Industry trends pharmaceuticals. 2022. Available via https://group.atradius.com/documents/industry-trends-pharmaceuticals-2022.pdf (Accessed 23 November 2022).
Buntz B. Pharma’s top 20 R&D spenders in 2021. 2022. [Online]. Available via https://www.drugdiscoverytrends.com/pharmas-top-20-rd-spenders-in-2021/ (Accessed 23 November 2022).
Chesnokova NN, Kononova SV. The use of information technology in pharmaceutical consulting. Remedium J Russ Market Med Med Equip, 2019; 6:34–7; https://doi.org/10.21518/1561-5936-2019-6-34-37.
Chitika. The value of Google result positioning. 2013. Available via https://research.chitika.com/wp-content/uploads/2022/02/chitikainsights-valueofgoogleresultspositioning.pdf (Accessed 26 November 2022).
Citeline. Pharma R&D annual review 2021. Citeline, 2021. Available via https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/2021/files/infographic/pharmard_whitepaper.pdf (Accessed 23 November 2022).
Congressional Budget Office. Research and development in the pharmaceutical industry. Congressional Budget Office, 2021. Available via https://www.cbo.gov/publication/57126 (Accessed 23 November 2022).
Google. Ranking results. n.d. Available via https://www.google.com/search/howsearchworks/how-search-works/ranking-results/ (Accessed 26 November 2022).
Google Help. Search result page. n.d. Available via https://support.google.com/faqs/answer/7049588?hl=en (Accessed 26 November 2022).
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J, 2009; 26(2):91–108; https://doi.org/10.1111/j.1471-1842.2009.00848.x.
Gruzina EV. K voprosu razvitiya elektronnoy torgovli v usloviyakh infor-matsionnoy revolyutsii i nauchno-tekhnicheskogo progressa [To the issue of digital commerce development in the context of the information revolution and scientific and technological progress]. In: Collection of Scientific Articles of the 8th International Youth Scientific Conference “Budushcheye nauki -2020”, 2020, Vol. 1, pp 118–24.
Karpov OE, Nikitenko DN. Automation of the medical supply system. Database of medicinal means of a multidisciplinary medical organization. Vrach Inform Tehnol (Physicians and IT), 2018; 3:29–44. (Accessed 28 November 2022).
Koshechkin K?. Creation of an information system for managing the activities of testing laboratories of an expert institution in the sphere of medicinal products circulation. Bull Sci Cent Expert Eval Med Prod, 2018; 8(2):103–8; https://doi.org/10.30895/1991-2919-2018-8-2-103-108.
Koshechkin KA, Preferansky NG, Preferanskaya NG. The use of blockchain-technology for maintaining the state register of medicines. Vrach Inform Tehnol (Physicians and IT), 2019; 3:58–64. (Accessed 28 November 2022).
Kurbesov AV, Kalugyan KKH. Relevance of use of technology blockchain in questions of provision of medicines of citizens. Vrach Inform Tehnol (Physicians and IT), 2018; 2:23–8.
Lebedev GS, Fartushniy EN, Shaderkin IA, Klimenko HS, Ryabkov IV, Kozhin PB, Koshechkin KA, Radzievskiy GP, Fomina IV. Bilding of the medical decision support system on the basis of providing medicine based on evidence-based medicine. Russ J Telemed E-Health, 2019; 5(1):8–16.
LLC. Markirovka lekarstv (Digital marking of medicines). 2018. Available via https://???????????.??/business/projects/medicines/ (Accessed 29 November 2022).
LLC. “Pervyy elektronnyy retsept” (“First digital prescription”). Moy retsept (My prescription). 2020. Available via https://???-??????.??/about (Accessed 29 November 2022).
Loginovskaya OA, Kolbatov VP, Sukhov RV, Ryavkina MS, Kolbin AS. New technologies in electronic pharmacovigilance systems for marketing authorisation holders. Saf Risk Pharmacother, 2022; 10(3):230–9; https://doi.org/10.30895/2312-7821-2022-10-3-230-239.
Lysykh EA, Ekusheva EV, Chefranova ZHYU, Zhuravleva EO, Rubinskiy AV. Secondary prevention of ischemic stroke in gerontological practice by using digital technologies. Sci J Curr Prob Health Care Med Stat, 2021; 3:205–15; https://doi.org/10.24412/2312-2935-2021-3-205-215.
Mikulic M. Total number of novel drugs approved by CDER from 2008 to 2021. [Online]. Available via https://www.statista.com/statistics/817534/annual-novel-drug-approvals-by-cder/ (Accessed 23 November 2022).
Moscow Center for Innovative Technologies in Healthcare. Projects piloted. 2022. Available via https://medtech.moscow/en#project (Accessed 29 November 2022).
Pyatigorskaya NV, Nikolenko NS, Beregovykh VV, Ishmukhametov AA. Razrabotka modeli kompleksnykh auditov farmatsevticheskoy sistemy kachestva [The development of the model of complex audits of pharmaceutical quality system]. Russian Academy of Sciences, Moscow, Russia, 2020.
Russian Community. Meditsina innovatsiy: kak MedTech-startapy mogut izmenit sovremennuyu meditsinu [Innovation medicine: how MedTech startups can change modern medicine]. 2021. Available via https://znanierussia.ru/library/video/medicina-innovacij-kak-medtech-startapy-mogut-izmenitsovremennu-125 (Accessed 29 November 2022).
Salimjanova IG, Dyachuk AV. Innovation circuit in healthcare in the context of digital transformation. Bull St. Petersburg State Econ Univ, 2021; 1:122–8.
Suetina TA, Kitaeva EA, Kitaev MR, Abdulganieva ZA. Mobile app “The diary of self-control”. Vrach Inform Tehnol (Physicians and IT), 2019; 1:6–11.
Svistunov AA, Tarasov VV. Etapy zhiznennogo tsikla lekarstvennogo sredstva [The pharmaceutical product lifecycle]. Laboratoriya znaniy (The Laboratory of Knowledge), Moscow, Russia, 2020.
Sychev DA. Information technologies’ role in optimizing the application of drugs in clinical practice: a view of a clinical pharmacologist. S Russ J Ther Pract, 2021; 2(4):30–2; https://doi.org/10.21886/2712-8156-2021-2-4-30-32.
Tezina NN. Platform approach to creating regional e-health systems. Vrach Inform Tehnol (Physicians and IT), 2020; 1:35–8; https://doi.org/10.37690/1811-0193-2020-s1-35-38.
The Eurasian Economic Union. Report on priorities and long-term forecast of scientific and technical development. 2022. Available via https://eec.eaeunion.org/upload/clcr/doklad_8.2.1.pdf (Accessed 22 November 2022).
Trapeznikova NA, Rostova NB. Information technologies for promoting rational use of medicines. Bull Smolensk State Med Acad, 2022; 21(2):211–9; https://doi.org/10.37903/vsgma.2022.2.27.
United Nations Industrial Development Organization. The role of science, technology and innovation policies in the industrialization of developing countries. Lessons from east Asian countries. United Nations Industrial Development Organization, 2021. Available via https://www.unido.org/sites/default/files/files/2022-03/STI_Policies.pdf (Accessed 22 November 2022).
Vaganova V, Sokolova K. “Glavnoye opaseniye—eto zavisaniye servisa”: kak apteki RT budut vydavat lekarstva po QR-kodu? [The main fear is the hang-up of the service: how will pharmacies in Tatarstan issue medicines according to QR-code?]. 2022. [Online]. Available via https://www.business-gazeta.ru/article/551746 (Accessed 29 November 2022).
Vardomatskaya LP, Lilyukhin AM. Digital economy tools in the con-text of their impact on pharmaceutical efficiency. State Municipal Manage Scholar Notes, 2020; 1(2):112–7; https://doi.org/10.22394/2079-1690-2020-1-2-112-117.