INTRODUCTION
Diabetes mellitus, or diabetes, is a chronic metabolic syndrome that has as its primary sign plasma glucose levels above those considered normal and is directly related to problems in the production or functioning of the hormone insulin (Centers for Disease Control and Prevention (CDC), 2020; National Institute of Diabetes and Digestive and Kidney Diseases, 2018; Nelson and Cox, 2008; Powers and D’Alessio, 2012). The lack of adequate control of the disease can trigger complications of various types at the cardiovascular, ocular, renal, neurological, and other levels, in addition to the possibility of exacerbating comorbidities that, in the worst case, could lead to the death of the patient (International Diabetes Federation (IDF), 2019). By 2021, it was estimated that around the world, 573 million adults between 20 and 79 years of age had diabetes, which corresponds to approximately 10% of the population in this age range and was the cause of death of 6.7 million people during that same year (International Diabetes Federation, 2021). Due to the high prevalence of the disease and the costs associated with its treatment, there is a gap in access to therapies, especially in developing countries and those with the greatest social inequality (International Diabetes Federation, 2021; International Diabetes Federation (IDF), 2019, 2011). For this reason, it is important to seek therapeutic alternatives that facilitate access for all people suffering from the onslaught of this disease.
One of the possible alternatives for the treatment of diabetes is the use of phytotherapeutics. It is possible to find evidence of the employ of various plant species with high potential as adjuvants in the management of the disease (Arulselvan et al., 2014; Choudhury et al., 2018; Furman et al., 2019; Governa et al., 2018; Saadeldeen et al., 2020; Xu et al., 2018). Application of plant species in therapeutics, the obtaining and application of herbal extracts represent an area of special importance due to the possibility of selectively separating the compounds responsible for the biological activity of the herbal material without going to their strict purification, which would imply higher costs; in this way, a product of easy access for patients and health systems would be obtained (Camel, 2014; Harbourne et al., 2013; Hussain et al., 2019; World Health Organization (WHO), 2013).
When working with an herbal extract as an active ingredient, one relevant challenge is nature as a multicomponent system and its variable composition, such as low bioavailability, physicochemical instability, low water solubility, and potential toxicity (Capasso et al., 2000; Falzon and Balabanova, 2017; Ramzan and Li, 2015). To overcome these challenges, nanotechnology becomes a promising alternative that looks forward to the optimization of the herbal extracts’ effect thanks to its nanometric size and versatility, which favors the vectorization of the system to the site of action (Hu et al., 2015; Xiao et al., 2017). Thus, it is possible to improve its effectiveness, consequently leading to a positive impact on safety, reducing toxicity, side effects, and adverse events (Gunasekaran et al., 2014; McClements, 2020; Mosaddik et al., 2018; Muller and Keck, 2004; Paroha et al., 2020; Xiao et al., 2017). In addition, this kind of system can be designed to be used in different routes of administration, facilitating and favoring the delivery of multicomponent systems (Musicanti and Gasco, 2016).
Nanotechnology-based systems contribute in large numbers to contemporary applications in nanomedicine. The available information is extensive, making it difficult to recognize the latest advances in this field, specifically related to herbal products. This topic is broad due to the different species with the potential to be employed in diabetes and applying such technologies. Thus, understanding the current state of the art regarding these topics, recognizing what has been studied and, therefore, what perspectives to contribute to these advances, becomes a challenge for all who work in this field of knowledge. The influence of nanotechnological applications in diabetes treatment has already been reviewed by other authors (Marella and Tollamadugu, 2018; Paul et al., 2021; Zolkepli et al., 2022); however, their analysis is focused on the description of existing systems and their potential as therapeutic agents.
This article seeks to contribute to the understanding of state of the art regarding the application of nanotechnology in systems for the delivery of substances of plant origin as extracts or specific compounds, with potential application in the treatment of diabetes and some of its complications, as well as the influence of some of the variables involved in its procurement and development, from a pharmaceutical technology point of view.
METHODOLOGY
A systematic search was performed in the referral databases Scopus, PubMed, and Web of Science and the databases Science Direct, Springer Link, SAGE, Scielo, Wiley, and Taylor & Francis, until July 2022, using a search equation that included the terms “nanotechnology”, “herbal extracts” and “diabetes”. For each term of interest, the equations in Table 1 were applied.
The search was limited to research articles in Spanish and English. From this first filter, 4,922 articles were obtained, which were pre-selected by title, eliminating those results that did not refer to nanotechnology-based systems and those related to nanosystems made from inorganic materials. Subsequently, the selected articles were reviewed in the abstract, classifying them by type of herbal ingredient, type of nanosystem, therapeutic application, materials used, and tests performed, among other characteristics, which led to a final selection. In this way, those articles related to the study of nanosystems with herbal extracts or their derivatives, with application in the treatment of diabetes and some of its complications, were taken as working articles, thus obtaining a total of 86 articles. Finally, an exhaustive review was made of the selected articles based on the inclusion criteria, from which it was possible to extract the key information for comparisons by constructing a database that facilitated the centralization of information for the corresponding analysis.
 | Table 1. General search equation employed to obtain information about the topic of interest from several databases. [Click here to view] |
RESULTS AND DISCUSSION
Bibliographic statistics
Based on the results, a preliminary descriptive statistical analysis was performed to illustrate the contribution of each database consulted to the central theme: Nanosystems of extracts and/or natural products with application in diabetes, where it was found that the Taylor & Francis database allowed for the largest number of articles to be retrieved when applying the corresponding search equation, with 1,693 findings, followed by Scopus and Web of Science. However, when applying the inclusion and exclusion criteria mentioned in the methodology, it was found that the database that contributed the most articles to the present analysis corresponds to Scopus, with 49% of the total number of articles analyzed (Fig. 1).
Additionally, to elucidate the landscape of study in nanosystems and their application in diabetes, a behavior of chronological randomness is observed, with a positive trend, with 2019 being the year of greatest contribution (Fig. 2). This trend is evidence that nanotechnology is a topic of scientific interest and that this interest tends to increase in recent years. Since its origin, its use has expanded in different areas; for example, at the pharmaceutical level, it has meant a potential alternative for drug delivery systems, improved production, shelf life, and bioavailability (Demetzos, 2016). In this sense, nanosystems have a potential application in diabetes therapy, which may be related to the progress of nanotechnologies in recent years (Hulla et al., 2015).
Herbal extracts and compounds of natural origin with nanotechnological applications in the treatment of diabetes and some complications
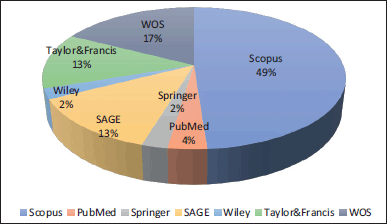 | Figure 1. Percentage of contribution to the total number of articles selected for final review by consulted database. [Click here to view] |
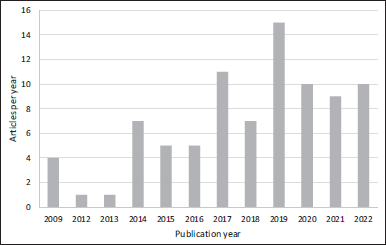 | Figure 2. Chronologic evolution of nanotechnology applied for herbal products with diabetes application based on the number of articles published per year since 2009. [Click here to view] |
In this review, it was possible to observe that the plant parts most used to obtain extracts or compounds of therapeutic interest in this field correspond to leaves, followed by seeds and fruits, which come mainly from the plant families Zingiberaceae, Asphodelaceae, Myrtaceae, Lamiaceae, and Ranunculaceae (Fig. 3). The use of extracts occurs in only 36% of the cases studied, with purified compounds of natural origin representing most of the substances used in nanotechnology-based systems for the treatment of diabetes (84%); these data are illustrated in Table 2 (Bonifácio et al., 2014; Kesarwani and Gupta, 2013). Although this trend may be due to the technological disadvantages of working with a multicomponent system as opposed to a purified compound, working with extracts may represent an alternative of great interest due to the ease of obtaining the product required as an active ingredient, which may even have an impact on the accessibility of these substances and the reduction of production costs, doing work with herbal extracts a topic of interest for further research into nanotechnology applications.
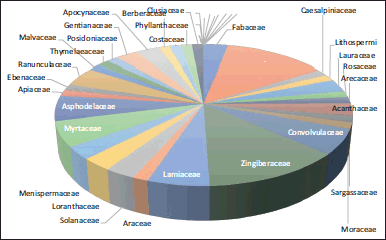 | Figure 3. Principal botanic families studied to extract natural products with applications in nanotechnology and diabetes. [Click here to view] |
To obtain the extracts with this therapeutic interest, it is evident that, maceration and Soxhlet techniques predominate over other technologies in the studies analyzed. Both are characterized by being simple and affordable and do not use much equipment and materials for their execution. In these techniques, the constant contact with the extraction solvent and the plant material allows for various compounds to be obtained without affecting their stability. The advantage of maceration over Soxhlet is the extraction of thermolabile substances due to the possibility of controlling the temperature of the technique (Kuete, 2017).
The Zingiberaceae family offers the highest proportion of substances studied for the above-mentioned purposes, mainly because turmeric is obtained from species belonging to this family, which is the most researched substance of natural origin for diabetes applications. Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), a polyphenol responsible for its characteristic coloration, is predominant in this spice extracted from the turmeric rhizome, possibly due to the number of conjugated bonds at the structural level, which in turn gives it an advantage when analyzing encapsulation in nanotechnology-based systems. This compound has been attributed to anti-inflammatory, antimicrobial, antioxidant, anticancer, and useful properties in treating liver disease and even metabolic diseases such as diabetes. However, curcumin has a low bioavailability due to its low solubility in water, being classified as class II according to the biopharmaceutical classification system, which affects its potential to be used in the treatment of liver disease and even in metabolic diseases such as diabetes (Hu et al., 2015), affecting the potential of its therapeutic action (Hewlings and Kalman, 2017), and makes it a model of interest for studies to improve these characteristics.
As shown in Figure 3, compounds from the Asteraceae and Lamiaceae families have also been studied importantly, with silymarin and rosmarinic acid being substances from these families, respectively, of therapeutic interest in diabetes. Silymarin is a complex compound consisting of silybin A, B, isosilybin A, and other flavonolignans. It is recognized for its potential uses as a hepatoprotective, anticancer, immunomodulator, and as renal, pancreatic, and neurological protector (Karimi et al., 2011). Regarding its application in diabetes, its use has been associated with treating complications such as retinopathy, neuropathy, and nephropathy. This is associated with different mechanisms; on the one hand, it reverses metabolic damage and improves the activity of mitochondrial respiration and, in turn, reduces the hydrolysis of glucose-6-phosphate, i.e., there is inhibition of gluconeogenesis and glycogenolysis (Stolf et al., 2017). Rosmarinic acid is also known for its antiviral activity and hepatic, pancreatic, and neurological protection (Guan et al., 2022).
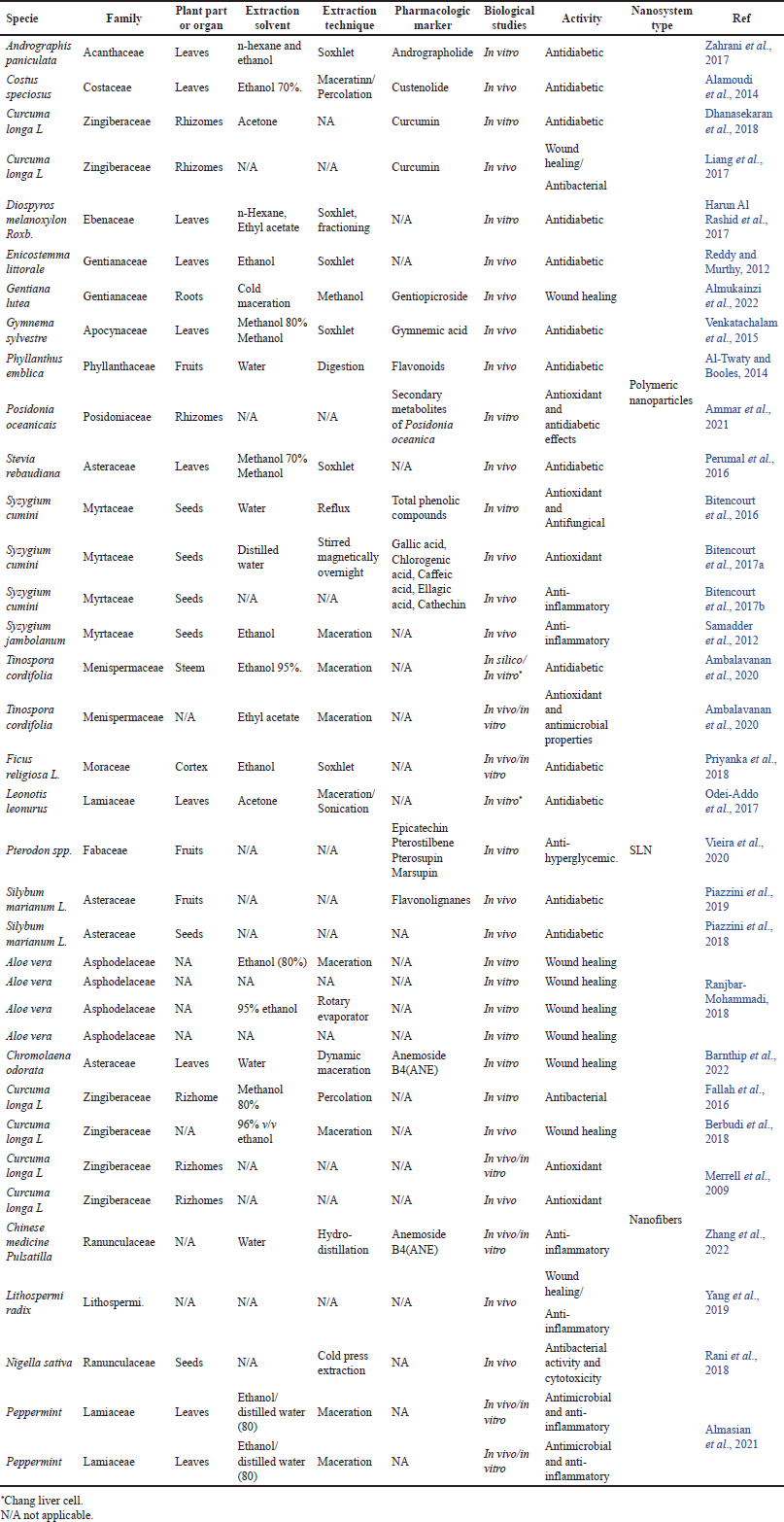 | Table 2. Extracts and natural compounds included in nanosystems in diabetes treatment and their complications. [Click here to view] |
Nanosystems studied for the dosing of herbal products: applications in diabetes and some of its complications
The search for therapeutic alternatives for treating metabolic diseases such as diabetes has been increasing, as previously exposed, as well as the interest in novel systems that improve existing therapies. Information previously compiled by other authors regarding nanotechnology as a resource to face this challenge has allowed us to go deeper into the subject and to continue building viable technological alternatives (Hu et al., 2022; Zolkepli et al., 2022). According to the state of the art, among the most studied nanosystems in the treatment of diabetes and its complications, based on natural extracts or compounds, are polymeric nanoparticles, solid lipid nanoparticles Solid lipid nanoparticles (SLN), and nanofibers.
Polymeric nanoparticles and SLN
Nanoparticles are colloidal solid particles mainly characterized by their size. According to their structure, they can generally be classified as nanospheres and nanocapsules (Desai and Madjar, 2008). These types of systems present technological advantages in the delivery of bioactive substances by improving their stability and serving as modified release systems (Patra et al., 2018). Another relevant advantage is the nanometric size, which ensures better penetration of biological barriers depending on the selected route of administration and affects the direction conferred to the system, improving the selectivity for therapeutic targets (Desai and Madjar, 2008).
Polymeric nanoparticles are characterized by using polymers as the forming material for delivery systems, with or without stabilizing agents, bearing in mind that they should always be biocompatible (Sharma, 2019).
Obtaining nanoparticles using polymers is based on the self-assembly of micelles formed by the polymers, from the presence of hydrophilic portions and lipophilic portions in their structure, as well as by the formation of a polymeric matrix, which can lead to the formation of nanocapsules or nanospheres (Fattal and Vaulthier, 2007; Kumari et al., 2010; Nasir et al., 2015; Thassu et al., 2007).
Lipid solid nanoparticles are systems characterized by the presence of biocompatible and biodegradable lipids in their structure, which are solid at room and body temperature (Musicanti and Gasco, 2016). Due to the nature of their structural components, these types of systems are widely used for the delivery of compounds of lipophilic nature, helping to overcome some of the limitations of natural extracts. Due to the characteristics of the materials used, it is considered that they have almost zero toxicity levels and present greater affinity with biological barriers; additionally, they are easy to produce on a large scale (Doktorovová et al., 2016). Among the main limitations of this type of system are the low loading capacity and possible instabilities during storage, which have been improved by using other types of agents during their formulation (Musicanti and Gasco, 2016).
Nanoparticles applied to herbal antidiabetic formulations are narrowly related to the enhancement of stability of the active substance and administration route. Conversely, the use of nanoparticles is suitable and safe for almost any route of administration due to their size (Chenthamara et al., 2019). Most of the analyzed systems are proposed for the oral route due to the administration benefits in chronic therapeutic. As Table 2 describes, many of the pharmacologic markers studied for diabetes treatment are phenolic compounds. Those substances are characterized by differential chemical and physical stability (Osorio-Tobón, 2020), requiring formulations that protect the active ingredient and ensure their delivery to the expected site. The inclusion of those substances in nanoparticles has shown an important enhancement of stability and vectorization of therapies, allowing the reduction of dose, avoiding possible secondary effects, and controlling delivery.
Influence of obtention variables in nanoparticles properties
Polymeric nanoparticles and SLN have common study characteristics due to their physical state and similar obtention processes. One example is that, for both types of nanoparticles, the most used preparation method corresponds to solvent emulsion-evaporation (Table 3) (Fattal and Vaulthier, 2007; Mora-Huertas et al., 2010). The preference for this method may be due to its versatility and the low influence of other types of factors in addition to those involved in the formation of emulsions, which decrease the possible interactions between the components of the extracts and the materials of the formulation. According to the data observed, it can be inferred that the particle sizes expected after loading with herbal substances for this method are generally greater than 100 nm.
Another outstanding method in elaborating this type of nanosystem corresponds to nanoprecipitation, related to solvent displacement. This consists of the physicochemical application of solvent/non-solvent precipitation, two terms related to structural component solubility (Iván Martínez-Muñoz and Elizabeth Mora-Huertas, 2022). The systems obtained by this technique mostly present particle sizes larger than 200 nm.
In these two types of systems, the particle size behavior would be expected to be consistent with that analyzed by Manne et al. (2020), who established that the amount of substance loaded during the obtention process and the particle size have a directly proportional relationship. Although there is not enough data to corroborate this information for the systems analyzed, Figure 4 relates the encapsulation efficiency with the particle size of each system, inferring that these parameters do not have a specific relationship and suggesting that, above all, the encapsulation efficiency may depend on factors other than particle sizes, such as the materials and the production method.
The size of the nanosystem is also related to the technique employed, as observed in Figure 5; however, the use of an extract or a pure compound of natural origin as loading substances in these nanosystems presents great influence, as observed in the case of SLNs (Table 3). The largest sizes (defined as greater than 200 nm) were evident in those systems that used a pure compound isolated from a vegetative source. This could be related to its loading capacity, favored in turn by the type of intermolecular interactions between the active substance and the structural materials of the nanoparticle, which would facilitate its inclusion regarding what would be a multicomponent system, such as extracts, in which each of the substances involved in the encapsulation must have some type of interaction, and may differ according to their nature, and which for this reason may hinder this process. Figure 6 shows the main materials used to develop nanosystems, in which chitosan predominates. This finding is consistent with the search for interactions with the material to be encapsulated since chitosan has characteristics that can be modified by external factors such as pH, degree of acetylation, temperature, and polymer crystallinity. Likewise, its chemical structure allows modifications to obtain polymers with different properties and behaviors, thus improving the affinity to encapsulate the material (Aranaz et al., 2021). Additionally, the nanosystems prepared with the assistance of ultrasound are the ones that presented smaller sizes compared to those obtained with other technologies, proposing this agitation technique as an optimal mechanism for the control of particle size and polydispersity.
Materials and their influence on diabetes treatment
As for polymeric nanoparticles, the most used natural polymers are cellulose, gelatin, pullulan, chitosan, alginates, and gliadin (Abasian et al., 2020; Chakravarthi et al., 2007; George et al., 2019). One of the main difficulties in working with these materials is achieving reproducibility due to variations in their chemical composition and the possibility of causing the immunogenic response, so synthetic polymer applications tend to become more attractive every day. The versatility of these synthetic polymers allows them to be designed according to the desired characteristics of the system, being able to control their dissolution behavior, permeability, degradation, and even conferring targeting characteristics according to the site of action to be achieved (Chakravarthi et al., 2007). Some examples of synthetic polymers used to obtain nanoparticles are polylactide (PLA), poly(lactide-co-glycolide) (PLGA), polyanhydrides, poly-ε-caprolactone, and polyphosphazene (Abasian et al., 2020; Asem and Malmström, 2018; Chakravarthi et al., 2007; Kumari et al., 2010; Torchilin, 2001). In addition, most of those materials do not interfere with diabetes treatment, considering their biodistribution and metabolization post-administration, and the reason why they are potential alternatives for use in formulations oriented to this pathology. PLA, polycaprolactone (PCL), and poly vinyl alcohol (PVA) are commonly hydrolyzed by some enzymes, and their degradation products enter the Krebs cycle to be transformed finally in CO2 and water (Su et al., 2019). Despite the main metabolic products of chitosan, the most studied polymer for the topic of interest is carbohydrates such as glucosamines; they have been associated with a lower risk of incident diabetes (Ma et al., 2020).
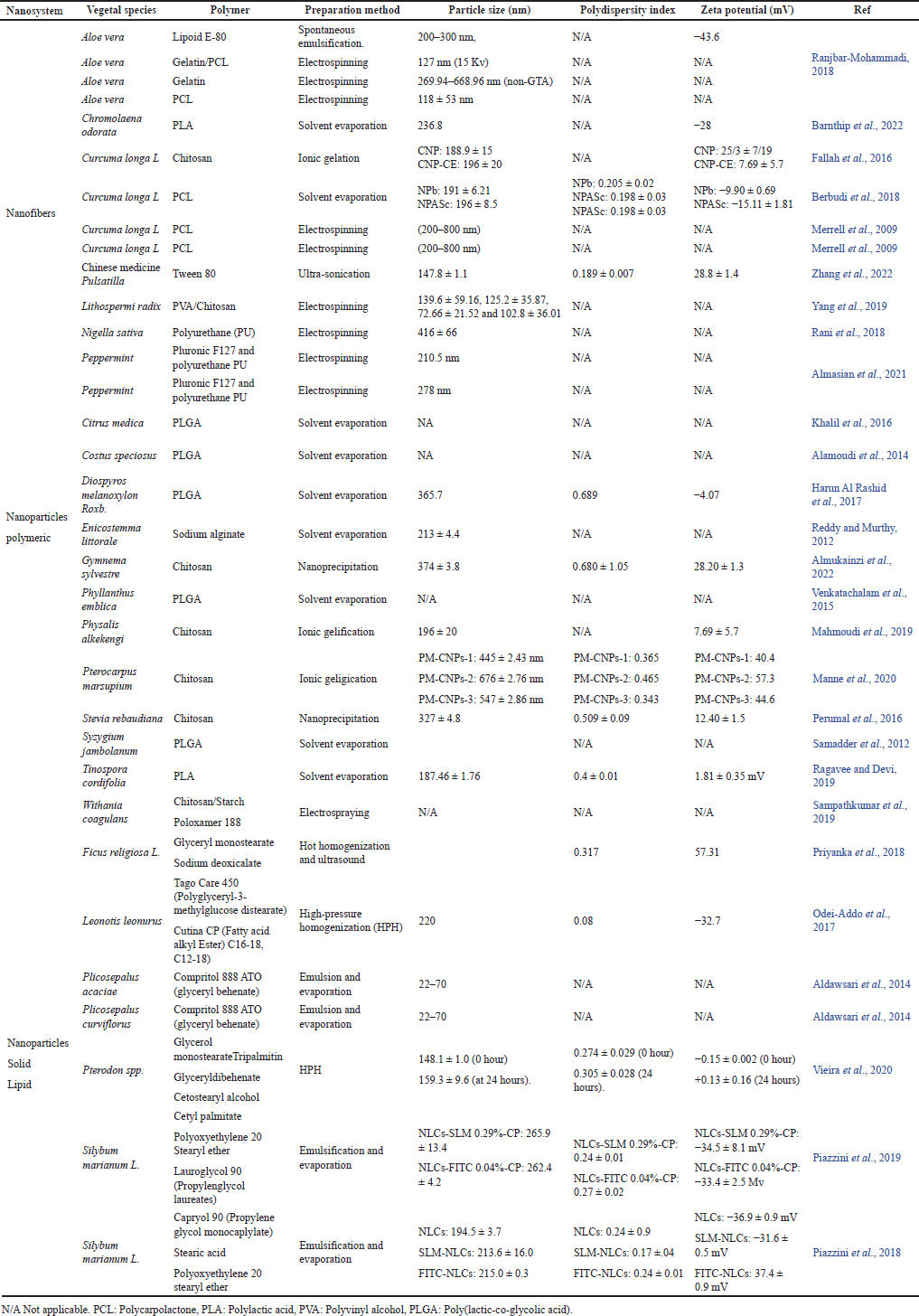 | Table 3. Herbal materials loaded nanosystems applied to diabetes and some complications PCL, PLA, PVA, PLGA. [Click here to view] |
For SLN, it is evident that the materials used are not unique and that, on the contrary, the spectrum of these is quite broad. Among these, there are different fatty acids and their corresponding salts, such as stearates and palmitates, which meet the primary characteristic of being solid at room temperature. Likewise, the systems are usually not composed of a single material but by the union of several materials with lipidic characteristics, some of them liquid at room temperature, which could correspond to the formation of nanostructured lipid carriers (NLC), which confers better encapsulation and stability characteristics (Chauhan et al., 2020). However, diabetic dyslipidemia is highly prevalent in patients with diabetes (International Diabetes Federation, 2021), and the use of lipids as materials for formulations that are focused on the treatment of this metabolic disorder could interfere with adequate disease management due to the changes in levels of those indicators, also for alterations in lipid metabolism (Athyros et al., 2018).
Nanofibers
Regarding nanosystems studied for the treatment of complications derived from diabetes, nanofibers stand out, which correspond to cylindrical nanomaterials with diameters between 10 and 100 nm and variable lengths. Different techniques can obtain these systems, electrospinning being the most widely used because it is considered the simplest and most affordable (Asem and Malmström, 2018). In this technique, a factor of significant influence on the characteristics of the nanofibers is the kilovolts (Kv) used. The higher the voltage, the smaller the diameter, which is directly related to the production quantity and the speed of the yarn flight (Niu et al., 2019). This type of nanosystem is a potential alternative for treating wounds derived from diabetes, most of which result in serious infections, so it seeks to evaluate not only their tissue regeneration activity but also their antimicrobial activity based on the structure and load materials.
The materials used for this type of system are polymeric, such as gelatin, PCL, and PLA, among others (Table 3), which have mechanical characteristics that allow them to be malleable and moldable. Wound healing applying these systems is focused on topic administration, where mentioned materials do not have an adequate permeation and thus do not affect diabetes treatment due to possible metabolism products (Su et al., 2019). Implementation of these systems could enhance the delivery of the active substances on the skin, allowing the increase of time of contact with the site of action.
Other examples of nanosystems
In addition, other types of nanosystems allow for the inclusion of natural compounds or extracts, including nanoemulsions (Ali et al., 2022; Prada et al., 2019), biosynthesized nanoparticles (I’tishom et al., 2021), nanogels (Berbudi et al., 2018; Salem et al., 2019; Sudhakar et al., 2015), and self-emulsifying systems (Kassem et al., 2020; Shiyan et al., 2022). There are more specific systems, such as nanovesicles or systems obtained by emulsification called nanocubosomal systems. This variety of systems is due to the specificity required by certain routes of administration or therapeutic targets and to the needs of herbal extracts or compounds of natural origin to improve their stability. Due to this reason, the existing studies related to this type of technology may not be so numerous compared to those mentioned above. However, this area of work is promising for developing systems that allow improving many of the formulation conditions, overcoming the disadvantages presented with the most common alternatives.
Models for the evaluation of therapeutic activity to treat diabetes
The activity evaluation models correspond to both in vitro and in vivo assays. The most outstanding in vitro assays for the systems studied were focused on the study of the antioxidant capacity of the material of natural origin present in the corresponding nanosystem, highlighting the application of the α-α-diphenyl-β- picrylhydrazyl “DPPH”, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) and oxygen radical antioxidant capacity (ORAC) assays. These types of studies allow us to demonstrate the effectiveness of the antidiabetic activity evaluated since there is a close relationship between oxidative processes and diabetes, such as metabolic syndrome. Oxidative stress triggers the loss of homeostasis between antioxidant systems and reactive oxygen species (ROS). This imbalance is not only one of the pathophysiological mechanisms of diabetes but is also responsible for the complications related to the disease. ROS are highly reactive compounds and can react with other macromolecules such as lipids, proteins, or DNA, leading to alterations at the cellular level. Studies also show that in type 2 diabetes mellitus, enzymes with antioxidant activity can be altered or reduced. An example of the impact of this imbalance is major depressive disorder (Burgos-Morón et al., 2019; Réus et al., 2019).
A general resume of different assays employed in the articles reviewed is available in Table 4. The most frequent method employed, in in vitro studies, is the (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide) (MTT) assay to evaluate cytotoxicity. Although this study is important for subsequent investigations due to exists a correlation between the application potential, toxicity, and the desired effect, of course, it is not exactly an assay to evaluate the antidiabetic activity. For this reason, it is necessary to employ other tests that allow complement information regarding diabetes as a model of disease, where the in vivo studies are the best alternative. For instance, studies by Sonaje et al. (2007). indicated that PGLA and PCL nanoparticles improved oral absorption and activity, using rats. Furthermore, studies that use animal models show the influence of the nanoencapsulation process over characteristics like pharmacokinetics or bioavailability. Chellampillai and Pawar (2011) found the enhancement on oral bioavailability of andrographolide when this is included in Eudragit® EPO nanoparticles, which was associated with changes in almost all pharmacokinetics parameters evaluated. These results support that this is a promising approach to follow-up pharmacokinetics parameters in evaluating new herbal nanosystems for diabetes treatment.
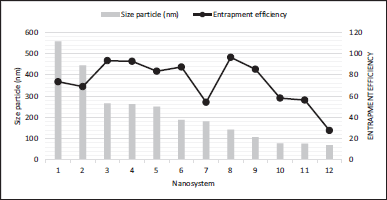 | Figure 4. Relationship between size particle and entrapment efficiency. Chitosan polymeric nanoparticle with Syzygium cumini extract, 2. Chitosan polymeric nanoparticle with Homalomena pineodora extract (PM-CNPs-1: 5% extract)72 3. SLN with Silybum marianum L. extract NLCs-SLM 0.29%-CP (cetyl palmitate), 4. SLN with Silybum marianum L. extract Fluorescent NLCs (FITC-NLCs-CP) 73 5. Polymeric nanoparticle with gentiopicroside formulation 5 (F5): 5% w/v PLGA, 0.4% W/V PVA, GPS: 60 mg, Organic/Aqueous phase volume ratio 1:574 6. PLA Polymeric nanoparticles with Tinospora cardifolia system obtained (1% w/v TG, 0.1% w/v Triton X-100, and 2% AlCl3 using magnetic stirrer for homogenization),75 7. SLNs with ethanolic extract of Ficus religiosa L. 76 PLGA polymeric nanoparticles with pelargonidin encapsulated77, 9. Eudragit RS100 polymeric nanoparticle with Phoenix dactylifera L. extract78, 10. SLN with berberine79, 11. Chitosan polymeric nanoparticle with Pterocarpus marsupium extract80, 12. Chitosan polymeric nanoparticle with Physalis alkekengi extract81. [Click here to view] |
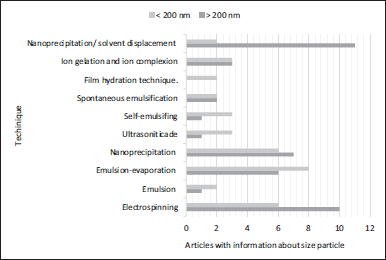 | Figure 5. Size particle obtained according to obtention technique. [Click here to view] |
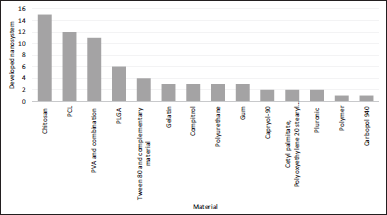 | Figure 6. Main materials employed in nanosystems production. [Click here to view] |
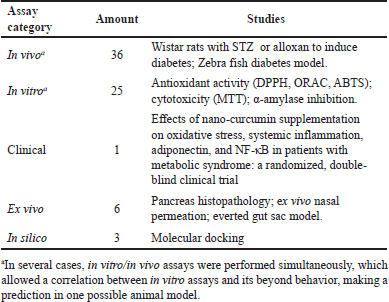 | Table 4. Biological assays employed in nanotechnology systems. [Click here to view] |
On the other hand, in vivo studies were mostly developed in Wistar rats in which diabetes was induced by chemical methods using streptozotocin (STZ) (Furman, 2021) or intraperitoneal alloxan. The use of zebrafish as an animal model was also found (Hayati et al., 2018). In most studies that include in vivo assays, it was possible to observe that changes in doses and pharmaceutical forms are allowed. Another important parameter is the number of groups included in the studies, which on average is six, corresponding to positive and negative controls, and the evaluation of raw extract compared with nanoparticle formulations at different doses to evaluate the effectiveness (Djamil et al., 2020). The wide variety of substances used as positive controls found between studies led to the proposal to standardize this parameter to grant comparison among prospective antidiabetic research.
CONCLUSION
The nanotechnology approach to formulating herbal systems for diabetes treatment is a current topic of study, proven by the growing number of publications about it during the last few years. There is a considerable variety of herbal materials studied with some nanotechnological application oriented to the treatment of diabetes and some of its complications, being the Zingiberaceae, Asphodelaceae, Myrtaceae, Lamiaceae, and Ranunculaceae the most representative botanic families included in these researches, using extracts or purified compounds obtained from some of those species, especially with the content of polyphenols, but not limited to those metabolites, despite the information regarding their effectiveness and mechanism of action not being described thoroughly, proposing a potential area of study. The approach to nanotechnological systems seeks to improve the stability of the compounds to be encapsulated and provide special characteristics that facilitate the administration of the formulation and the modification of some of its properties, like biopharmaceutical and pharmacokinetic parameters. Among the most widely studied systems with these applications are polymeric nanoparticles, SLN, and nanofibers, which in turn, show a wide spectrum of materials and techniques used to produce them, which responds to the great variety of substances found in nature with some biological activity useful in diabetes treatment, but found that the selection of materials could be a critical parameter, especially if they are part of some of the disease markers (as the lipids are) or their biodegradation could affect diabetes treatment due to produced metabolites. Between those systems, it is important to observe that techniques like emulsification-evaporation and nanoprecipitation are the most used to obtain them, reaching encapsulation efficiencies of about 89%. Similarly, other types of systems have been studied that can be adjusted to the specific needs of the route of administration or the therapeutic target, demonstrating that it is possible to use other types of technologies in the formulation of products of plant origin and broadening the scope of this kind of technologies. It is evident that the formulation challenges are persistent, such as the inclusion of substances of different natures in the same nanosystem, the improvement of encapsulation efficiencies, and even guaranteeing a synchronized release of the substances, among others, and that the development of new materials and systems plays an important role in the conception of novel, safe and effective phytotherapeutics, which also overcome the difficulties of working with this type of substances. Furthermore, there is evident difficulty in comparing the effectiveness between different systems due to the absence of standardized methods that allow the identification of the differences in terms of activity, despite the fact that most studies show an enhancement in some related characteristics and propose nanotechnological systems as a promising alternative to formulating phytotherapeutics to diabetes treatment.
ACKNOWLEDGMENTS
The authors acknowledge the funding of the Ministry of Science, Technology, and Innovation of the Republic of Colombia (Minciencias), through contract 187–2019, Project: Comparative evaluation of Physalis peruviana fruit extracts with antidiabetic activity, alone and included in nanostructured systems, with potential application in the development of phytotherapeutic products.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest related to the publication of this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abasian P, Ghanavati S, Rahebi S, Nouri Khorasani S, Khalili S. Polymeric nanocarriers in targeted drug delivery systems: a review. Polym Adv Technol, 2020; 31(12):1–16.
Alamoudi EF, Khalil WKB, Ghaly IS, Hassan NHA, Ahmed ES. Nanoparticles from of Costus speciosus extract improves the antidiabetic and antilipidemic effects against STZ-induced diabetes mellitus in albino rats. Int J Pharm Sci Rev Res, 2014; 29(1):279–88.
Aldawsari HM, Hanafy A, Labib GS, Badr JM. Antihyperglycemic activities of extracts of the mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z Naturforsch C J Biosci, 2014; 69(9–10):391–8.
Ali T, Hussain F, Naeem M, Khan A, Al-Harrasi A. Nanotechnology approach for exploring the enhanced bioactivities and biochemical characterization of freshly prepared Nigella sativa L. nanosuspensions and their phytochemical profile. Front Bioeng Biotechnol, 2022; 10:2–12.
Almasian A, Najafi F, Eftekhari M, Shams Ardekani MR, Sharifzadeh M, Khanavi M. Preparation of polyurethane/pluronic F127 nanofibers containing peppermint extract loaded gelatin nanoparticles for diabetic wounds healing: characterization, in vitro, and in vivo studies. Evid Based Complement Altern Med, 2021; 2021:3–5.
Almukainzi M, El-Masry TA, Negm WA, Elekhnawy E, Saleh A, Sayed AE, Khattab MA, Abdelkader DH. Gentiopicroside PLGA nanospheres: fabrication, in vitro characterization, antimicrobial action, and in vivo effect for enhancing wound healing in diabetic rats. Int J Nanomed, 2022; 17:1203–25.
Al-Twaty NH, Booles HF. Nano-encapsulated form of Phyllanthus embilica extract increases its therapeutic effects as antidiabetic and antioxidant in rats. Int J Pharm Sci Rev Res, 2014; 29(1):11–7.
Ambalavanan R, John AD, Selvaraj AD. Nano-encapsulated Tinospora cordifolia (Willd.) using poly (D, L-lactide) nanoparticles educe effective control in streptozotocininduced type 2 diabetic rats. IET Nanobiotechnol, 2020; 14(9):803–8.
Ammar NM, Hassan HA, Mohammed MA, Serag A, Abd El-Alim SH, Elmotasem H, El Raey M, El Gendy AN, Sobeh M, Abdel-Hamid AHZ. Metabolomic profiling to reveal the therapeutic potency of Posidonia oceanicana noparticles in diabetic rats. RSC Adv, 2021; 11(14):8398–410.
Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Caballero AH, Acosta N. Chitosan: an overview of its properties and applications. Polymers (Basel), 2021; 13:3256.
Arulselvan P, Ghofar HAA, Karthivashan G, Halim MFA, Ghafar MSA, Fakurazi S. Antidiabetic therapeutics from natural source: a systematic review. Biomed Prev Nutr, 2014; 4:607–17.
Asem H, Malmström E. Polymeric nanoparticles explored for drug-delivery applications. ACS Symp Series, 2018; 1296:315–31.
Athyros VG, Doumas M, Imprialos KP, Stavropoulos K, Georgianou E, Katsimardou A, Karagiannis A. Diabetes and lipid metabolism. Hormones, 2018; 17(1):61–7.
Barnthip N, Paosoi J, Pinyakong O. Concentration effect of Chromolaena odorata (Siam weed) crude extract on size and properties of gelatin nanofibers fabricated by electrospinning process. J Ind Text, 2022; 51(1):1499S–510S.
Bonifácio BV, da Silva PB, Aparecido dos Santos Ramos M, Maria Silveira Negri K, Maria Bauab T, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed, 2014; 9:1.
Berbudi A, Hasna Hasibah U, Faried A, Putri AC, Ersyad Hamda M. Administration of Curcuma longa extract topically does not accelerate skin excision wound closure in alloxan-induced diabetic mice model. Biomed Pharmacol J, 2018; 11(1):437–44.
Bitencourt PER, Cargnelutti LO, Stein CS, Lautenchleger R, Ferreira LM, Sangoi M, Boligon A, Duarte MMMF, Moresco RN, Cruz L, Zanette RA, Alves SH, Moretto MB. Anti-inflammatory action of seed extract and polymeric nanoparticles of Syzygium cumini in diabetic rats infected with Candida albicans. J Appl Pharm Sci, 2017a; 7(1):7–16.
Bitencourt PER, Cargnelutti LO, Stein CS, Lautenchleger R, Ferreira LM, Sangoi M, Denardi L, Borges RM, Boligon A, Moresco RN, Cruz L, Zanette RA, Alves SH, Moretto MB. Nanoparticle formulation increases Syzygium cumini antioxidant activity in Candida albicans infected diabetic rats. Pharm Biol, 2017b; 55(1):1082–8.
Bitencourt PER, Ferreira LM, Cargnelutti LO, Denardi L, Boligon A, Fleck M, Brandão R, Athayde ML, Cruz L, Zanette RA, Alves SH, Moretto MB. A new biodegradable polymeric nanoparticle formulation containing Syzygium cumini: phytochemical profile, antioxidant and antifungal activity and in vivo toxicity. Ind Crops Prod, 2016; 83:400–7.
Burgos-Morón E, Abad-Jiménez Z, de Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, Salom C, Jover A, Mora V, Roldan I, Solá E, Rocha M, Víctor VM. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med, 2019; 8:1385.
Camel V. Extraction methodologies in plants: general introduction. Encyclopedia of analytical chemistry. John Wiley & Sons, Ltd, Hoboken, NJ, pp 1–26, 2014.
Capasso R, Izzo AA, Pinto L, Bifulco T, Vitobello C, Mascolo N. Phytotherapy and quality of herbal medicines. Fitoterapia, 2000; 71:58–65.
Centers for Disease Control and Prevention (CDC). Diabetes [Online]. Available via https://www.cdc.gov/diabetes/index.html (Accessed 22 March 2020).
Chakravarthi SS, Robinson DH, De S. Nanoparticles prepared using natural and synthetic polymers. In: Thassu D, Deleers M, Pathak Y (eds.). Nanoparticulate drug delivery systems. 1st edition, Informa Healthcare USA Inc, Boca Raton, FL, vol. 1, pp 51–60, 2007.
Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharm Bull, 2020; 10:150.
Chellampillai B, Pawar AP. Improved bioavailability of orally administered andrographolide from pH-sensitive nanoparticles. Eur J Drug Metab Pharmacokinet, 2011; 35(3–4):123–9.
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res, 2019; 23(1):1–29.
Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LE, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med, 2018; 8:361–76.
Demetzos C. Pharmaceutical nanotechnology. Fund Pract Appl, 2016; (2):153–7.
Desai GJ, Madjar IY. Herbal medicine. Encyclopedia Glob Health, 2008; 1:831–5.
Dhanasekaran S, Rameshthangam P, Venkatesan S, Singh SK, Vijayan SR. In vitro and in silico studies of chitin and chitosan based nanocarriers for curcumin and insulin delivery. J Polym Environ, 2018; 26(10):4095–113.
Djamil R, Zaidan S, Rahmat D, Pratami D, Hakim F. Nanoemulsion of okra fruit extract as antidiabetic treatment. Int J Appl Pharm, 2020; 12(5).
Doktorovová S, Kova?evi? AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm, 2016; 108:235–52.
Fallah M, Bahrami SH, Ranjbar-Mohammadi M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J Ind Text, 2016; 46(2):562–77.
Falzon CC, Balabanova A. Phytotherapy: an introduction to herbal medicine. Prim Care Clin Off Pract, 2017; 44:217–27.
Fattal E, Vaulthier C. Drug delivery: nanoparticles. Encyclopedia Pharm Technol, 2007; 4:1183–200.
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc, 2021; 1:3–7.
Furman BL, Candasamy M, Bhattamisra SK, Veettil SK. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J Ethnopharmacol, 2019; 247:112264.
George A, Shah PA, Shrivastav PS. Natural biodegradable polymers-based nano-formulations for drug delivery: a review. Int J Pharm, 2019; 561:244–64.
Governa P, Baini G, Borgonetti V, Cettolin G, Giachetti D, Magnano AR, Miraldi E, Biagi M. Phytotherapy in the management of diabetes: a review. Molecules, 2018; 23:1–22.
Guan H, Luo W, Bao B, Cao Y, Cheng F, Yu S, Fan Q, Zhang L, Wu Q, Shan M. A comprehensive review of rosmarinic acid: from phytochemistry to pharmacology and its new insight. Molecules, 2022; 27(10):3292.
Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed, 2014; 4:S1–7.
Harbourne N, Marete E, Jacquier JC, O’Riordan D. Conventional extraction techniques for phytochemicals. In: Tiwari BK, Brunton NP, Brennan CS (eds.). Handbook of plant food phytochemicals: sources, stability and extraction. 1st edition, John Wiley & Sons, Ltd, Dublin, UK, pp 399–411, 2013.
Harun Al Rashid M, Deepak Bharadwaj PV, Mandal V, Pal M, Mandal SC, Amirthalingam Thandavarayan R. Preparation and characterization of PLGA loaded nanoparticles obtained from D. melanoxylon Roxb. leaves for their antiproliferative and antidiabetic activity. Int J Green Pharm, 2017; 11(3):S438.
Hayati F, Chabib L, Darma DD. Antihyperglycemia activity of self-nano emulsifying drug-delivery systems (SNEDDS) of Ipomoea reptans, Poir leaf ethanolic extract in zebrafish (Danio rerio). AIP Conf Proc, 2018; 2026:020026.
Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods, 2017; 6:92.
Hong K, Uhrig D, Mays JW. Living anionic polymerization. Curr Opin Solid State Mater Sci, 1999; 4:531–8.
Hu L, Shi Y, Li JH, Gao N, Ji J, Niu F, Chen Q, Yang X, Wang S. Enhancement of oral bioavailability of curcumin by a novel solid dispersion system. AAPS PharmSciTech, 2015; 16:1327–34.
Hu F, Sun DS, Wang KL, Shang DY. Nanomedicine of plant origin for the treatment of metabolic disorders. Front Bioeng Biotechnol, 2022; 9:1466.
Hulla JE, Sahu SC, Hayes AW. Nanotechnology: history and future. Hum Exp Toxicol, 2015; 34:1318–21.
Hussain MK, Saquib M, Faheem Khan M. Techniques for extraction, isolation, and standardization of bio-active compounds from medicinal plants. In: Swamy MK, Akhtar MS (eds.). Natural bio-active compounds: chemistry, pharmacology and health care practices, Springer-Verlag, Berlin, Germany, Vol. 2, pp 179–200, 2019.
International Diabetes Federation (IDF). IDF diabetes atlas. 10th edition, International Diabetes Federation, Brusseles, Belgium, 2021.
International Diabetes Federation (IDF). IDF diabetes atlas. 9th edition, International Diabetes Federation, Brusseles, Belgium, 2019.
International Diabetes Federation (IDF). Global diabetes plan 2011-2021. International Diabetes Federation, Brussels, Belgium, 2011.
I’tishom R, Wafa IA, Budi DS, Pratama NR. Oral delivery of purple sweet potato (Ipomoea batatas l.) extract-loaded carboxymethyl chitosan and alginate nanocapsule in streptozotocin-induced diabetic mice. Indian J Pharm Educ Res, 2021; 55(3):709–14.
Iván Martínez-Muñoz O, Elizabeth Mora-Huertas C. Nanoprecipitation technology to prepare carrier systems of interest in pharmaceutics: an overview of patenting. Int J Pharm, 2022; 614:121440.
Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci, 2011; 14:308–17.
Kassem AA, Abd El-Alim SH, Salman AM, Mohammed MA, Hassan NS, El-Gengaihi SE. Improved hepatoprotective activity of Beta vulgaris L. leaf extract loaded self-nanoemulsifying drug delivery system (SNEDDS): in vitro and in vivo evaluation. Drug Dev Ind Pharm, 2020; 46(10):1589–603.
Kesarwani K, Gupta R. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed, 2013; 3:253.
Khalil WKB, El-Bassyouni GT, Fahime Booles H. Nano-encapsulated form of Citrus medica for osteoporosis treatment in animal model. Int J Pharm Clin Res, 2016; 8(1):49–59.
Kuete V. Medicinal spices and vegetables from Africa: therapeutic potential against metabolic, inflammatory, infectious and systemic diseases. Academic Press, Cambridge, MA, 2017.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles-based drug delivery systems. Colloids Surf B Biointerfaces, 2010; 75:1–18.
Liang H, Friedman JM, Nacharaju P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif Cells Nanomed Biotechnol, 2017; 45(2):297–304.
Ma H, Li X, Zhou T, Sun D, Liang Z, Li Y, Heianza Y, Qi L. Glucosamine use, inflammation, and genetic susceptibility, and incidence of type 2 diabetes: a prospective study in UK Biobank. Diabetes Care, 2020; 43(4):719–25.
Mahmoudi R, Ardakani MT, Verdom BH, Bagheri A, Mohammad-Beigi H, Aliakbari F, Salehpour Z, Alipour M, Afrouz S, Bardania H. Chitosan nanoparticles containing Physalis alkekengi-l extract: preparation, optimization and their antioxidant activity. Bull Mater Sci, 2019; 42(3):1–6.
Manne AA, Vinay Viswanath K, Ajay Kumar G, Mangamuri U, Podha S. Pterocarpus marsupium Roxb. heartwood extract synthesized chitosan nanoparticles and its biomedical applications. J Genet Eng Biotechnol, 2020; 18:1–13.
Marella S, Tollamadugu NVKVP. Nanotechnological approaches for the development of herbal drugs in treatment of diabetes mellitus—a critical review. IET Nanobiotechnol, 2018; 12(5):549–56.
McClements DJ. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol Adv, 2020; 38:107287.
Merrell JG, Mclaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS, Phil M, Exp C, Author PP. Curcumin loaded poly(ε-Caprolactone) nanofibers: diabetic wound dressing with antioxidant and anti-inflammatory properties NIH public access author manuscript. Clin Exp Pharmacol Physiol, 2009; 36(12):1149–56.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm, 2010; 385:113–42.
Mosaddik A, Ravinayagam V, Elaanthikkal S, Fessi H, Badri W, Elaissari A. Development and use of polymeric nanoparticles for the encapsulation and administration of plant extracts. In: Filho VC (ed.). Natural products as source of molecules with therapeutic potential: research and development, challenges and perspectives. 1st edition, Springer Nature Switzerland AG, Basel, Switzerland, pp 391–463, 2018.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol, 2004; 113:151–70.
Musicanti C, Gasco P. Solid lipid nanoparticles (SLN). In: Bhushan B (ed.). Encyclopedia of nanotechnology. 2016th edition, Springer, Dordrecht, The Netherlands, 2016.
Nasir A, Kausar A, Younus A. A review on preparation, properties and applications of polymeric nanoparticle-based materials. Polym Plast Technol Eng, 2015; 54:325–41.
National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes for health professionals [Online]. 2018. Available via https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes (Accessed 19 February 2020).
Nelson DL, Cox MM. Hormonal regulation of fuel metabolism. In: Nelson DL, Lehninger AL, Cox MM (eds.). Lehninger’s principles of biochemistry. 5th edition, W.H. Freeman and Company, New York, NY, pp 922–30, 2008.
Niu H, Zhou H, Wang H. Electrospinning: an advanced nanofiber production technology. In: Fang J, Lin T (eds.). Energy harvesting properties of electrospun nanofibers, IOP Publishing, Bristol, UK, 2019.
Odei-Addo F, Shegokar R, Müller RH, Levendal RA, Frost C. Nanoformulation of Leonotis leonurus to improve its bioavailability as a potential antidiabetic drug. 3 Biotech, 2017; 7(5):1–9.
Osorio M, Martinez E, Naranjo T, Castro C. Recent advances in polymer nanomaterials for drug delivery of adjuvants in colorectal cancer treatment: a scientific-technological analysis and review. Molecules, 2020; 25:2270.
Osorio-Tobón JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol, 2020; 57(12):4299–315.
Paroha S, Dewangan RP, Dubey RD, Sahoo PK. Conventional and nanomaterial-based techniques to increase the bioavailability of therapeutic natural products: a review. Environ Chem Lett, 2020; 18:1767–78.
Pathak Y, Thassu D, Deleers M. Pharmaceutical applications of nanoparticulate drug-delivery systems. In: Thassu D, Deleers M, Pathak Y (eds.). Nanoparticulate drug delivery systems. 1st edition, Informa Healthcare USA, Inc, New York, NY, pp 185–212, 2007.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology, 2018; 16:1–33.
Paul RK, Kesharwani P, Raza K. Recent update on nano-phytopharmaceuticals in the management of diabetes. J Biomater Sci, 2021; 32(15):2046–68
Perumal V, Manickam T, Bang KS, Velmurugan P, Oh BT. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int J Biol Macromol, 2016; 92:63–9.
Piazzini V, Lemmi B, D’Ambrosio M, Cinci L, Luceri C, Bilia AR, Bergonzi MC. Nanostructured lipid carriers as promising delivery systems for plant extracts: the case of silymarin. Appl Sci (Switzerland), 2018; 8(7):1163.
Piazzini V, Micheli L, Luceri C, D’Ambrosio M, Cinci L, Ghelardini C, Bilia AR, Di Cesare Mannelli L, Bergonzi MC. Nanostructured lipid carriers for oral delivery of silymarin: improving its absorption and in vivo efficacy in type 2 diabetes and metabolic syndrome model. Int J Pharm, 2019:572:118838.
Powers AC, D’Alessio D. Páncreas endocrino y farmacoterapia de la diabetes mellitus e hipoglucemia. In: Brunton L, Chabner B, Knollman B (eds.). Goodman & Gilman Las Bases Farmacológicas de la Terapéutica. 12 Edición, McGraw-Hill, New York, NY, pp 1237–73, 2012.
Prada AL, Keita H, de Souza TP, Lima ES, Acho LDR, da Silva MJA, Carvalho JCT, Amado JRR. Cassia grandis Lf nanodispersion is a hypoglycemic product with a potent α-glucosidase and pancreatic lipase inhibitor effect. Saudi Pharm J, 2019; 27(2):191–9.
Priyanka K, Sahu PL, Singh S. Optimization of processing parameters for the development of Ficus religiosa L. extract loaded solid lipid nanoparticles using central composite design and evaluation of antidiabetic efficacy. J Drug Deliv Sci Technol, 2018; 43:94–102.
Ragavee A, Devi S. Nanoencapsulation of Tinospora cordifolia (Willd.) using poly (D, L-lactide) nanoparticles: yield optimization by response surface methodology and in silico modeling with insulin receptor tyrosine kinase. Pharmacogn Mag, 2019; 15(64):218.
Ramzan I, Li GQ. Phytotherapies—past, present, and future. In: Ramzan I (ed.). Phytotherapies: efficacy, safety, and regulation. 1st edition, John Wiley & Sons, Inc, Hoboken, NJ, pp 1–17, 2015.
Rani R, Dahiya S, Dhingra D, Dilbaghi N, Kim KH, Kumar S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem Biol Interact, 2018; 295:119–32.
Ranjbar-Mohammadi M. Characteristics of Aloe vera incorporated poly(ε-caprolactone)/gum tragacanth nanofibers as dressings for wound care. J Ind Text, 2018; 47(7):1464–77.
Reddy NP, Murthy BP. Development, characterization, efficacy, and repeated dose toxicity of nanoemulsified ethanolic extract of enicostemma littorale in streptozotocin-induced diabetes rats. Int J Phytomed, 2012; 4:70.
Réus GZ, Carlessi AS, Silva RH, Ceretta LB, Quevedo J. Relationship of oxidative stress as a link between diabetes mellitus and major depressive disorder. Oxid Med Cell Longev, 2019; 2019
Saadeldeen FSA, Niu Y, Wang H, Zhou L, Meng L, Chen S, Sun-Waterhouse D, Waterhouse GIN, Liu Z, Kang W. Natural products: regulating glucose metabolism and improving insulin resistance. Food Sci Hum Wellness, 2020; 9:04–5
Salem HF, Kharshoum RM, Abou-Taleb HA, Naguib DM. Nanosized nasal emulgel of resveratrol: preparation, optimization, in vitro evaluation and in vivo pharmacokinetic study. Drug Dev Ind Pharm, 2019; 45(10):1624–34.
Samadder A, Das S, Das J, Paul A, Khuda-Bukhsh AR. Ameliorative effects of Syzygium jambolanum extract and its poly (lactic-co-glycolic) acid nano-encapsulated form on arsenic-induced hyperglycemic stress: a multi-parametric evaluation. JAMS J Acupunct Meridian Stud, 2012; 5(6):310–8.
Sampathkumar K, Riyajan S, Tan CK, Demokritou P, Chudapongse N, Loo SCJ. Small-intestine-specific delivery of antidiabetic extracts from Withania coagulans using polysaccharide-based enteric-coated nanoparticles. ACS Omega, 2019; 4(7):12049–57.
Sharma M. Transdermal and intravenous nano drug delivery systems: present and future. Applications of targeted nano drugs and delivery systems: nanoscience and nanotechnology in drug delivery. Elsevier, Amsterdam, The Netherland, pp 499–550, 2019.
Shiyan S, Suryani RP, Mulyani LN, Pratiwi G. Stability study of super saturable catechin-self nano emulsifying drug delivery system as antidiabetic therapy. Biointerface Res Appl Chem, 2022; 12(5):5811–20.
Sonaje K, Italia JL, Sharma G, Bhardwaj V, Tikoo K, Kumar MNVR. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm Res, 2007; 24(5):899–908.
Stolf AM, Cardoso CC, Acco A. Effects of silymarin on diabetes mellitus complications: a review. Phytother Res, 2017; 31:366–74.
Su C, Liu Y, Li R, Wu W, Fawcett JP, Gu J. Absorption, distribution, metabolism and excretion of the biomaterials used in nanocarrier drug delivery systems. Adv Drug Deliv Rev, 2019; 143:97–114.
Sudhakar K, Madhusudana Rao K, Subha MCS, Chowdoji Rao K, Sadiku ER. (Temperature-responsive poly (N-vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des Monomers Polym, 2015; 18(8):705–13.
Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol, 2013; 19:29–43.
Thassu D, Pathak Y, Deleers M. Nanoparticulate drug-delivery systems: an overview. In: Thassu D, Deleers M, Pathak Y (eds.). Nanoparticulate drug delivery systems. 1st edition, Informa Healthcare USA, Inc, New York, NY, 1–32, 2007.
Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release, 2001; 73:137–72.
Venkatachalam P, Thiyagarajan M, Sahi SV. Fabrication of bioactive molecules loaded chitosan nanoparticles using Gymnema sylvestre leaf extracts and its antidiabetic potential in experimental rat model. J Bionanosci, 2015; 9(5):363–72.
Vieira R, Severino P, Nalone LA, Souto SB, Silva AM, Lucarini M, Durazzo A, Santini A, Souto EB. Sucupira oil-loaded nanostructured lipid carriers (NLC): lipid screening, factorial design, release profile, and cytotoxicity. Molecules, 2020; 25(3):685.
World Health Organization. WHO traditional medicine strategy: 2014-2023. World Health Organization, Geneva, Switzerland, 2013.
Xiao J, Cao Y, Huang Q. Edible nanoencapsulation vehicles for oral delivery of phytochemicals: a perspective paper. J Agric Food Chem, 2017; 65:6727–35.
Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: pharmacology and mechanisms. Pharmacol Res, 2018; 130:451–65.
Yang BY, Hu CH, Huang WC, Ho CY, Yao CH, Huang CH. Effects of bilayer nanofibrous scaffolds containing curcumin/lithospermi radix extract on wound healing in streptozotocin-induced diabetic rats. Polymers, 2019; 11(11):1745.
Zahrani K, Imansari F, Utami TS, Arbianti R. Release profile of Andrographis paniculata leaf extract nanocapsule as α-glucosidase inhibitors. IOP Conf Ser Mater Sci Eng, 2017; 214(1):012020.
Zhang H, Zhang M, Wang X, Zhang M, Wang X, Li Y, Cui Z, Chen X, Han Y, Zhao W. Electrospun multifunctional nanofibrous mats loaded with bioactive anemoside B4 for accelerated wound healing in diabetic mice. Drug Deliv, 2022; 29(1):174–85.
Zhao L, Skwarczynski M, Toth I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater Sci Eng, 2019; 5:4937–50.
Zolkepli H, Widodo RT, Mahmood S, Salim N, Awang K, Ahmad N, Othman R. A review on the delivery of plant-based antidiabetic agents using nanocarriers: current status and their role in combatting hyperglycemia. Polymers (Basel), 2022; 14:2991.