INTRODUCTION
Diabetes mellitus (DM), which is notable for improper lipid, lipoprotein, and glucose metabolism, has emerged as one of the most common illnesses and is on the rise globally. The Centre for Disease Control and Prevention has recently released the 2022 National Diabetes Statistics Report, as per the report estimates more than 130 million adults are living with diabetes or pre-diabetes in the United States. The prevalence of DM and impaired fasting blood glucose in India was 9.3% and 24.5%, respectively [1]. Among those with DM, 45.8% were aware, 36.1% were on treatment and 15.7% had it under control. It is projected that by 2025 the number of cases of diabetes in India would be 69.9 million with a vast majority still undiagnosed. Given that controlling diabetes can be done with a variety of current treatments, such as insulin injections and oral medications like hypoglycemic pills, the majority of them suffer from a variety of drawbacks, such as being costly or coming with side effects like hepatic toxicity, weight gain, hypoglycemia, gastrointestinal (GI) disturbances, and GI hemorrhage. Because of their accessibility, efficacy, and historical, cultural, and religious preferences, medicinal herbs continue to be a widely used alternative medicine. According to estimates from the WHO, 65%–80% of the world’s population lives in underdeveloped nations due to budgetary limitations, a lack of access to modern medications, and relies primarily on naturally occurring items for their initial medical care. The global market for medicinal plants expanded from US $23 billion in 2002 to more than US $83 billion in 2008. Only 10% of the world’s (2.5 million) species have had the therapeutic potential of their plants and herbs examined since World War I, irrespective of the fact that there has been extensive research done on the problem since that time. This implies that there are still many effective medications that have not yet been explored.
As oxygen is crucial to the survival of life on earth, a small amount of oxygen is converted to numerous free radicals as it is utilized in various living processes. In addition to the above-mentioned pollutants, endogenous ones generated by metabolic processes are also exposed, as are various chemicals and the climate. Reactive oxygen species (ROS) such as superoxide anion (O2•-), hydroxyl (HO•), and peroxyl (ROO•) radicals, as well as reactive nitrogen species (RNS) such as peroxynitrite anion (ONOO-) and nitric oxide (NO•) radical, are present in the exposed oxidants; overall harmful effects occur when these species attack human cells and tissues, leading to cancer [2]. Natural antioxidants in high quantities are frequently found in plants. Antioxidants can stop a chain reaction by scavenging free radicals and preventing oxidation processes [3]. The phytochemicals found in plants, such as phenols, flavanols, carotenoids, and vitamins C and E, can be employed to remove excess radicals from the human body [4].
Ecbolium linneanum, is an indigenous Indian plant that naturally grows along India’s eastern coast. Also, it can be found in tropical Asia and Africa. There have been numerous traditional uses for E. linneanum, including the treatment of jaundice, menorrhagia, rheumatism, inflammation, etc. Root juice is used to treat premenstrual colic and as an anti-helminthic [5]. The plant is being used for gout, dysuria, and stricture with a leaf decoction. The plant’s leaves and roots are used to cure malignancies and used to treat cardiovascular diseases. The extracts from various plant parts, particularly leaf extract, are high in polyphenolic substances such as phenols and flavonoids [6]. Plant components are thought to provide a variety of beneficial health impacts, such as antioxidant properties, which prevent cell damage from ROS and RNS, which can be free radicals, singlet oxygen, and hydroperoxides. Cell damage caused by free radicals is one of the primary causes of aging and several degenerative disorders of aging, such as stress, cancer, cardiovascular disease, immune system degeneration, DM and inflammation, etc. Free radicals can be scavenged or quenched by the body’s enzymes, such as glutathione peroxidase and superoxide dismutase, to defend the body from negative impacts. These enzymes might be assisted in doing so by phytochemicals that exist in plants. Numerous assays, such as the DPPH, 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid, ferric ion reducing antioxidant power (FRAP), and CUPRAC tests, have been used to evaluate the antioxidant capabilities of plant extracts. These methods have shown varying results among the examined plants and laboratories.
The leaves of E. linneanum have been demonstrated to be a possible hypoglycemic source in an earlier investigation [7]. Therefore, we broadened our study to include in vitro assays on target enzyme activity, such as α-amylase, to assess the antidiabetic potentials of leaf extracts. In vitro, testing was used to ascertain the extracts’ anti-oxidant properties. Phytocompounds identified by gas chromatography-mass spectroscopy (GC-MS) and high-resolution liquid chromatography-mass spectrometry (HR-LCMS) investigations and were further analyzed virtually for binding interactions with α-amylase using the Protein Data Bank (PDB) crystal structure (1b2y).
MATERIALS AND METHODS
Chemicals
Folin ciocalteu (FC) reagent, gallic acid, quercetin, 2,2-Diphenyl-1-picrylhydrazyl) (DPPH), ascorbic acid (ABA), hydrogen peroxide (H2O2), phosphate buffer, potassium ferricyanide, trichloroacetic acid, ferric chloride (FeCl3), etc., were purchased from Merck India, Mumbai. The α-amylase was purchased from Sigma-Aldrich, India. All the solvents and reagents used were of analytical grade and obtained from Merck India, Mumbai.
Plant material
In the first week of September 2018, E. linneanum leaves were acquired as raw material from the wastelands of Guntur, Andhra Pradesh, India. The collected leaves were recognized by Dr. Madhav Shetty, a professor in the Department of Botany at Sri Venkateshwara University in Tirupati, Andhra Pradesh, India. The leaves were scrubbed until there was no longer any sign of foreign matter in distilled water, detergent, and tap water. To air dry, the leaves were kept at room temperature in the shade. The dried leaves were freed from the moisture and processed into a powder using a sterile electrical blender. The powdered substance was then kept in an airtight container for later use [8].
Extraction
A Soxhlet apparatus was used to get the crude leaf extracts of petroleum ether (PEEL), ethyl acetate (EAEL), and methanolic (MEEL) from 180 g of coarse powder throughout 72 hours. By utilizing a rotary evaporator, the crude filtrate was concentrated. The resultant extracts were kept in a refrigerator at 4ºC for later use [8].
Phytochemical analysis
To determine the presence of a variety of secondary metabolites in leaf extracts qualitative phytochemical testing was conducted. The earlier investigations presented that the MEEL was comprised of rich amounts phytochemicals, such as alkaloids, glycosides, phenols, and flavonoids [8].
Total phenolic content (TPC)
The FC colorimetric method [9] was used to assess the TPC of E. linneanum leaf extracts. About 100 μl of the diluted extracts, 500 μl of the FC reagent, and 400 μl of 7.5% (w/v) saturated aq. Na2CO3 were combined thoroughly. Then, it was homogenized and incubated for 30 minutes at 40ºC. After incubation, each sample’s absorbance was measured at 765 nm using methanol as the blank. The calibration curve approach was used to ascertain the TPC using gallic acid as the standard. Each test was run in triplicate, and the results were expressed as gallic acid equivalents (GAE) in mg per g of dry extract (mg GAE/g) [9].
Total flavonoid content (TFC)
TFC of E. linneanum leaf extracts was assessed by the aluminum chloride (AlCl3) colorimetric technique [10]. In this method, 125 μl of sodium nitrite solution (5%), 250 μl of each solvent extract, and 1,250 μl of distilled water were combined. The contents were then let to stand for 6 minutes at room temperature. 150 μl of 10% AlCl3 solution was then added, and the mixture was left to stand for another 6 minutes. Following that, 275 μl of distilled water was used to dissolve 500 μl of sodium hydroxide solution (4%). The mixture was completely combined and left to stand at room temperature for 30 minutes. At 510 nm, the absorbance was measured in comparison to a reagent blank that contained methanol. The measurement of total antioxidant capacity was performed using quercetin as a reference (QE) per mg of dry extract (μg QE/mg) [11].
In vitro antidiabetic activity
The in vitro antidiabetic activity was assessed by performing α-amylase inhibitory assay. In which the sample solution (100 μg/ml) of all the solvent extracts was made by using phosphate buffer (pH-6.8). 500 μl of test solution was added with α-amylase (0.5 mg/ml) solution. The resulting solution was incubated for 10 minutes at 25ºC [12]. Further procedure was followed to estimate the inhibitory effect on enzyme activity taking acarbose as a reference standard. The inhibitory effect on the α-amylase activity of solvent extracts was statistically expressed in terms of their IC50 values [13].
Antioxidant activity
The solvent extracts were subjected to screen for their possible antioxidant capabilities by DPPH, H2O2, and FRAP methods.
DPPH free radical-scavenging assay
The free radical scavenging assay of the extracts (PEEL, EAEL, and MEEL) utilizes the stable free radical of DPPH according to Yeo and Shahidi [14] DPPH methanolic solution (1 ml, 0.2 mM) was added to the extract solution (1 ml, 2.5–100 μg/ml). The mixture was vortexed thoroughly and incubated for 30 minutes at room temperature. The absorbance was measured at 517 nm with methanol as a blank. ABA as a positive control, the DPPH activity was measured by using the below equation:
Radical scavenging activity (% inhibition) = (Abs. blank–Abs. sample)/Abs. blank × 100
IC50 value of solvent extract was calculated from the regression line [14].
H2O2 radical scavenging assay
The H2O2 scavenging assay was carried out by following the standard procedure described by Ruch et al. [15]. A solution of H2O2 (43 mM) was prepared in phosphate buffer (0.1 M, pH—7.4). The three extracts (PEEL, EAEL, and MEEL) at different concentrations (1 ml, 2.5–100 μg/ml) in 3.4 ml phosphate buffer were added to 0.6 ml of H2O2 solution (43 mM). The absorbance of the mixture was measured at 230 nm [16].
H2O2 scavenging activity (% inhibition) = (Abs. blank–Abs. sample)/Abs. blank × 100
The IC50 value of solvent extracts was measured from the regression line of the % of remaining H2O2 radical with the sample concentration.
FRAP assay
The method described by Gohari et al. [17] was used to measure the reducing power of the PEEL, EAEL, and MEEL. 1 ml of test sample solution at various concentrations (2.5–100 μg/ml) was combined with 2.5 ml of potassium ferricyanide (1% w/v), 2.5 ml of phosphate buffer (0.2 M, pH–6.6) and 2.5 ml of 10% trichloroacetic acid, which was then incubated for 20 minutes at 50ºC. After the mixture was centrifuged for 10 minutes at 3,000 rpm, 2.5 ml of the upper layer was removed and combined with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1%). The calibration curve approach was used to determine the % inhibition using the standard ABA (10 g/ml). A spectrophotometer was used to measure each absorbance in triplicate at 700 nm [17].
Ferric reducing power (% inhibition) = (Abs. blank–Abs. sample)/Abs. blank × 100
The IC50 of solvent extracts was analyzed from the regression line of the % of remaining ferric ion radical at the test concentration of extract.
GC-MS and HR-LCMS study
Conventional methods for identifying bioactive phytoconstituents require a variety of procedures, including extraction, chromatographic separation, and spectroscopic characterization. Nevertheless, despite substantial time and effort, most researchers only attempt to characterize a small number of known phytochemicals due to the lack of appropriate phytochemical standards. To identify and simplify efforts to understand their action on the target, high throughput, and high-resolution approaches must be used to reveal the complex chemistry of bioactive crude extracts. Extensive research has been conducted to better understand the medicinal applications of the Ecbolium genus. However, despite its usage as a replacement constituent for E. linneanum, efforts toward knowing the chemistry of the plant have remained significantly low. The preliminary phytochemical study confirmed that the MEEL comprised diverse classes of phytoconstituents like alkaloids, flavonoids, phenols, saponins, etc. Besides, it displayed significant activity in in vitro and in vivo studies. Thus, as per the findings of qualitative phytochemical tests and biological efficacies, the MEEL was subjected to GC-MS and HR-LC-electrospray ionization (ESI)-MS/MS analysis to determine the presence of diverse phytochemicals [18].
GC-MS analysis
All the probable phytochemicals that are separated from the sample will be detected as a spectral emissary by this approach. After injecting the sample into the GC-MS device’s port, the sample is vaporized and then separated using an analyzer. Each component is generating a clean, identifiable peak, which was recorded digitally on a graph. The analysis was performed at CSIR-Indian Institute of Chemical Technology, Habsiguda, Hyderabad. The data was recorded on a combined gas chromatogram system (Agilent GC-MS5977B) and mass spectrometer, fitted with an HP-5 MS fused silica column (5% phenyl methyl siloxane 30.0 m × 250 μM, film thickness 0.25 μM), interfaced with 5675C Inert MSD with Triple-Axis detector.
HR-LCMS analysis
In HR-LCMS analysis, the phytoconstituents were profiled based on the retention time (Rt), m/z values, NIST library hits, and metabolite class. The metabolite analysis in MEEL was performed by HR-LCMS, UHPLC-PDA detector-ESI-QTOP-MS (Agilent Technologies, USA). With the aid of mass spectra and distinctive mass fragmentation patterns, compounds were discovered. For the chromatographic separation, Hypersil GOLD C18 (2.1 × 100 mm 3-μ) column was used with a gradient solvent system, (A) water with 0.1% formic acid and (B) 90 % acetonitrile with 10% water + 0.1% formic acid, A 95% B 5% for 1 minute, B 100% for 2–30 minutes, A 95% B 5%, for 30–35 minutes at 0.3 ml/minute flow rate with pressure maintained at 1,200 bar. The mass spectral data were acquired in both ESI-positive and negative ionization modes. MS was acquired over the m/z range of 100–1,200 at a mass resolution of 22,000 full-width half at maximum [19].
 | Table 1. TPC and TFC. [Click here to view] |
 | Figure 1. % Inhibition α-amylase activity of leaf extracts and Acarbose. PEEL—Petroleum ether extract; EAEL—Ethyl acetate extract; MEEL—Methanol extract. [Click here to view] |
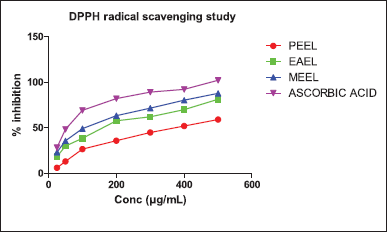 | Figure 2. % Inhibition of DPPH radical scavenging of leaf extracts and ABA. PEEL—Petroleum ether extract; EAEL—Ethyl acetate extract; MEEL—Methanol extract. [Click here to view] |
In silico absorption, distribution, metabolism, and excretion (ADME) study
The ADME properties of discovered metabolites in GC-MS and HR-LCMS were assessed using an online tool (SwissADME), to choose the most promising compounds with minimal risk of drug attrition in the later studies. The metabolites with the most reliable ADME properties have been taken into consideration for docking study [20].
 | Figure 3. % Inhibition of H2O2 radical scavenging of plant extracts and ABA. PEEL—Petroleum ether extract; EAEL—Ethyl acetate extract; MEEL—Methanol extract. [Click here to view] |
 | Figure 4. % Inhibition by FRAP of plant extracts compared with ABA. PEEL—Petroleum ether extract; EAEL—Ethyl acetate extract; MEEL—Methanol extract. [Click here to view] |
Docking study
The X-ray crystal structure of the α-amylase (PDB ID: 1b2y) [21] protein co-crystallized with acarbose was retrieved from a protein data bank (rcsb.com/pdb database). The protein was refined by eliminating water molecules, adding polar hydrogen. The co-crystal ligand was extracted using the Discovery studio visualizer 2021 program and saved in pdb format. The protein was checked for any missing amino acid residues, and the Ramachandran plot was used to check for any structural problems. The created protein file in pdb format was converted to pdbqt format using the macromolecule option in the Autodock tool of the PyRx virtual screening application 0.8. The target compounds 2D structures were drawn and their energy was minimized in 3D structure using ChemDraw software tools and saved as pdb files. The saved .pdb files were subjected to energy minimization (force field-off), and then generated conformers (AutoDock pdbqt files) using the open babel tab in PyRx software. The macromolecule (protein pdbqt file) and ligands were chosen using Vina Wizard to do the docking study (Autodock pdbqt files) by drawing a grid box around the area where the co-crystal ligand exhibits iterations with amino acids, the active site of the protein was defined to dock ligands to proteins. The potential compounds with a high binding affinity against the target protein were identified as the ligands with the lowest binding energies. The binding interactions were visualized using the Discovery Studio Visualizer 2021 program.
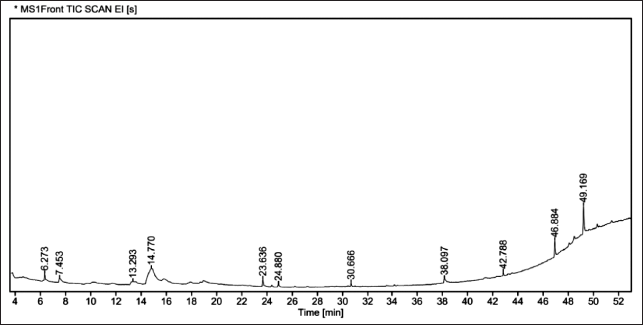 | Figure 5. GC chromatogram of MEEL. [Click here to view] |
 | Table 2. List of phytochemicals identified by GC-MS analysis in MEEL. [Click here to view] |
 | Figure 6. LC chromatogram (ESI positive mode) of MEEL. [Click here to view] |
 | Table 3. List of phytochemicals identified by HR-LCMS analysis in MEEL. [Click here to view] |
RESULTS AND DISCUSSION
Phytochemical analysis
The initial phytochemical analysis showed that each solvent leaf extract contained a distinctive variety of phytoconstituents. Saponins and tannins were the primary components of all three solvent extracts. However, the ratio of other phytoconstituents varied [7].
TPC and TFC
In Table 1, the amounts of TPC and TFC were shown as mg/g. When compared to the PEEL and EAEL, the MEEL comprised the highest levels of TPC and TFC. The phytochemical content of MEEL was thought to be high, so the plant extract was tested for its ability to combat diabetes and free radical damage [9,11].
In vitro antidiabetic activity
The α-amylase inhibiting activity of leaf extracts (PEEL, EAEL, and MEEL) was determined in vitro using the standard method. In this investigation, a dose-dependent increase in α-amylase inhibitory activity was observed. The extract demonstrated 21.60% ± 0.2354 inhibition at a concentration of 100 μg/ml, and 59.31% ± 0.352 at a concentration of 1,000 μg/ml (Fig. 1). The extract revealed an IC50 of 684.94 ± 3.96 μg/ml, while acarbose’s IC50 was 322.50 ± 4.5 μg/ml [13].
Antioxidant activity
The antioxidant capabilities of three solvent extracts determined by DPPH, H2O2, and FRAP assays were presented in the following,
DPPH radical scavenging assay
MEEL inhibited (87.78% ± 0.16 ***) the DPPH radical significantly more than the other two extracts. The % radical scavenging inhibition of MEEL was nearly alike to that of ABA (102.19% ± 0.13%) (Fig. 2).
H2O2 radical scavenging assay
The H2O2 radical scavenging assay was tested at 25–500 μg/ml of three solvent extracts, in which MEEL exhibited the highest (77.22% ± 0.43%) radical scavenging activity at 500 μg/ml. The PEEL and EAEL extracts were moderately potent at 500 μg/ml (Fig. 3).
 | Table 4. Chemical classes of phytochemicals identified by GC-MS and HR-LCMS analysis. [Click here to view] |
 | Table 5. ADME profile of identified phytochemicals as per in-silico study. [Click here to view] |
FRAP assay
The FRAP assay was tested at 25–500 μg/ml of three solvent extracts. In this study, the MEEL exhibited 76.56% ± 0.27% of inhibition at 500 μg/ml, which was a similar impact observed in H2O2 radical scavenging assay. The PEEL and EAEL extracts were found less potent at 500 μg/ml (Fig. 4).
GC-MS and HR-LCMS study
GC-MS analysis
Based on the m/z and Rt of each fraction from the column, the plant compound was searched in the NIST library for the likely components there in the leaf extract (Fig. 5). The GC-MS reports stated the existence of bioactive metabolites in varied proportions such as l-Gala-l-ido-octose (46.19%), γ-Sitosterol (17.34%), Peiminine (6.7%), and 4H-Pyran-4-one, 2, 3-dihydro-3,5-dihydroxy-6-methyl (4.49%) etc (Table 2). The majority of compounds have been reported to poses antioxidant, anti-inflammatory, and hypoglycemic properties.
HR-LCMS analysis
The m/z values were in the range of 135 to 724 for the majority of separated compounds in MEEL. The HR-LCMS study could provide information on the distribution of various polar to non-polar phytochemicals. ESI positive and negative ion modes were applied to identify all types of small molecules. The representative base peak LC 8chromatogram of MEEL was depicted in Figure 6.
Table 3 which summarized the tentative metabolites characterized from MEEL includes their Rt, experimental m/z, mass, proposed metabolites, molecular formula, etc. The HR-LCMS study revealed that the distribution of several (57) bioactive compounds, such as (+/−)-3-[(2-methyl 3-furl)thiol]-2-butanone, 8-hydroxy-2-chlorodibenzofuran, sinapoyl malate, and other phytochemicals. It reveals that there was a considerable number (57) of metabolites present in the MEEL as only a few metabolites have been focused on in the present study.
The present study also revealed the pharmacological richness of E. linneanum wherein 30 bioactive chemical compounds were identified. Examples include steroid compounds like 23-acetoxysoladulcidine and Rhodexin A, flavonoids like 2?, 3?-Di-O-p-coumaroylafzelin, and alkaloids like pheophorbide a and pyropheophorbide a. Out of 57 identified phytoconstituents, 21 bioactive compounds had good oral bioavailability, and certain compounds were promising and supported Lipinski’s rule with zero violations in an in silico SwissADME study. Around 28 phytoconstituents listed in Table 4 were selected and performed in silico prediction using acarbose as the standard.
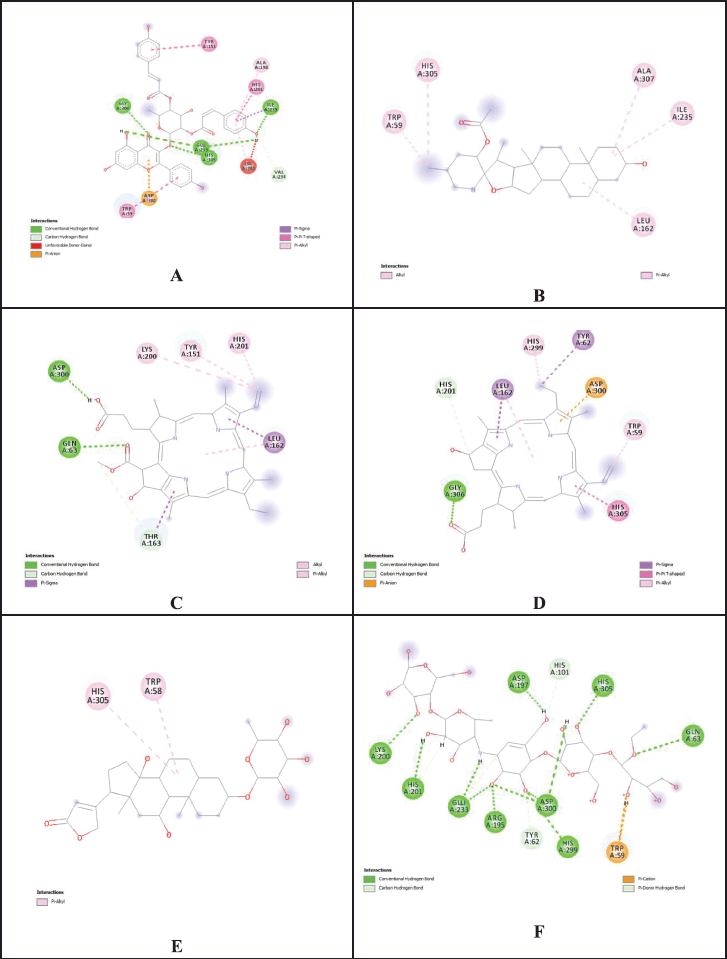 | Figure 7. 2D interactions of phytochemicals and reference standard with best docking scores at active cites of 1b2y. (A = 2?,3?-Di-O-p-coumaroylafzelin; B = 23-Acetoxysoladulcidine; C = Pheophorbide a; D = Pyropheophorbide a; E = Rhodexin A; F = Acarbose). [Click here to view] |
 | Table 6. Binding energy of phytochemicals in docking study against 1b2y. [Click here to view] |
 | Table 7. Binding interactions of phytochemicals at the active cite of 1b2y. [Click here to view] |
In silico ADME study
Online tools, such as the Swiss ADME free version, were used to evaluate drug-likeness and other pharmacokinetic parameters. A few compounds were found to have strictly adhered to Lipinski rule 5. Table 5 displayed the ADME profile including GI absorption, BBB permeability, and Lipinski rule violations.
In silico docking study
Since α-amylase (PDB ID: 1b2y) is one of the key enzymes that facilitates the release of glucose into the bloodstream, it was selected as a target for in silico docking study. One of the potential approaches to regulating blood sugar involves inhibiting its enzyme activity. Thirty compounds were chosen based on their m/z values, and their properties were predicted in silico with acarbose as the reference compound. The in silico results demonstrated that the 23-acetoxysoladulcidine (−9.6 kcal/mol), rhodexin A (−9.6 kcal/mol), pheophorbide a (−9.5 kcal/mol), and pyropheophorbide a (−9.5 kcal/mol) were significant binding affinities against target (Fig. 7). The binding energies and active-site interactions that are crucial to their binding of ligands screened has been indicated in Tables 6 and 7.
It tends to be of utmost importance to use medicinal herbs as antioxidants and in the treatment of diabetes. This is said to be a result of the safety stigma linked to plants, the high cost, the abundance of adverse effects, and the lack of orthodox medications. The advantages of the secondary metabolites that these plants make to defend themselves from outside invaders are being highlighted in an increasing number of studies. MEEL is abundant in alkaloids, phenols, flavonoids, steroids, and tannins, according to a phytochemical study. For their capacity as antioxidants, tannins, phenols, and flavonoids are widely known. GC-MS and HR-LCMS analysis revealed about 67 phytoconstituents, in which few compounds (Ex. γ-sitosterol) have been found to be anti-diabetic activity [22]. Among these 21 compounds met the drug-likeness and other pharmacokinetic parameters when tested in silico. Only 6 of the 28 compounds tested by docking studies had good binding energy. According to the GC-MS and HR-LCMS metabolite profiling results, the volatile compounds γ-sitosterol, and pheophorbide A have been reported to be hypoglycemic.
CONCLUSION
The experimental investigations on PEEL, EAEL, and MEEL suggested that the leaves of the plant were enriched with several biologically active compounds. The MEEL contains a high concentration of flavonoids and phenols, which may account for its high antioxidant activity. In vitro, antidiabetic testing revealed that MEEL significantly inhibited α-amylase activity when compared to acarbose. It has also been reported for the first time on HR-LCMS-based identification of possible phytochemicals, and the profile showed 57 compounds, of which 21 compounds showed significant physiochemical and pharmacokinetic profiles. Among these 2?,3?-Di-O-p-coumaroylafzelin, 23-acetoxysoladulcidine, pheophorbide a, pyro pheophorbide a, and rhodexin, etc. The in silico analysis of the discovered phytochemical compounds predicted that they would have excellent bioavailability and good binding energies. The GC-MS analysis demonstrated the distribution of a few bioactive compounds. According to the literature, one of the compounds γ-Sitosterol has antihyperglycemic activity, and have significant antioxidant activity. Additional research is needed to isolate the more abundant compound in the MEEL by using different methods and to continue further studies for the identified compounds. In future isolation of responsible phytochemicals and their in vivo hypoglycemic and antioxidant assessment may lead to the development of effective novel natural agents.
ABBREVIATIONS
EAEL: Ethyl acetate extract of E .linneanum; FC: Folin ciocalteu; GC-MS: Gas chromatography-mass spectroscopy; HR-LCMS: High-resolution liquid chromatography-mass spectrometry; MEEL; Methanolic extract of E. linneanum; PEEL: Petroleum ether extract of E. linneanum; TPC: Total phenolic content; TFC: Total flavonoid content.
ACKNOWLEDGMENTS
The authors thank the authorities of KL College of Pharmacy, Koneru Lakshmaiah Education Foundation, Guntur, Andhra Pradesh, India for providing the infrastructure to conduct the research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report..
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
COMPETING INTERESTS
The authors declare that they have no competing interests.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Mathur P, Leburu S, Kulothungan V. Prevalence, awareness, treatment and control of diabetes in India from the countrywide national NCD monitoring survey. Public Health Front. 2022;10:748157. doi: https://doi.org/10.3389/fpubh.2022.748157
2. Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging beyond correlation. Aging Cell. 2002;1:117–23. doi: https://doi.org/10.1046/j.1474-9728.2002.00015.x
3. Larson RA. The antioxidants of higher plants. Phytochem Lett. 1988;27:969–73. doi: http://dx.doi.org/10.1016/0031-9422(88)80254-1
4. Huang MT, Ho CT, Lee CY. Phenolic compounds in food and their effects on health II. Washington, DC: American Chemical Society; 1992.
5. Dipankar C, Murugan S, Devi PU. Review on medicinal and pharmacological properties of Iresine herbstii, Chrozophora rottleri and Ecbolium linneanum. Afr J Tradit Complement Altern Med. 2011;8(5S):124–9. doi: https://doi.org/10.4314%2Fajtcam.v8i5S.6
6. Mishra T, Goyal AK, Mondal P, Sen A. Free radical scavenging activity of ornamental and edible cultivars of Canna found in Eastern India. NBU J Plant Sci. 2011;5(1):41–5. doi: https://ir.nbu.ac.in/handle/123456789/4442
7. Nallapaty S, Malothu N, Pasupula R, Ketha A. Antidiabetic potential of leaf extracts of Ecobolium linneanum Kurz in streptozotocin-induced diabetic rats. J Pharm Pharmacogn Res. 2022;10(3):496–507. doi: http://dx.doi.org/10.56499/jppres21.1319_10.3.496
8. Nallapati S, Kulandaivelu U, GSNK Rao, Panda SP, Alavala RR. Antidiabetic activity of Chrozophora rottleri leaves extracts in streptozotocin induced diabetic rats. Int J Pharm Res. 2019;11(4):978–88. doi: https://doi.org/10.31838/ijpr/2019.11.04.049
9. Onder FC, Ay M, Sarker SD. Comparative study of antioxidant properties and total phenolic content of the extracts of Humulus lupulus L. and quantification of bioactive components by LC–MS/MS and GC–MS. J Agric Food Chem. 2013;61(44):10498–506. doi: https://doi.org/10.1021/jf4031508
10. Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50(17):4959–64. doi: https://doi.org/10.1021/jf0255937
11. Saeed N, Khan MR, Shabbir M. Antioxidant activity total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: https://doi.org/10.1186/1472-6882-12-221
12. Narkhede MB, Ajimire PV, Wagh AE, Mohan M, Shivashanmugam AT. In vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. Asian J Plant Sci. 2011;1(2):101–6.
13. Miller N, Joubert E. Critical assessment of in vitro screening of α-glucosidase inhibitors from plants with acarbose as a reference standard. Planta Med. 2022;88(12):1078–91. doi: https://doi.org/10.1055/a-1557-7379
14. Yeo J, Shahidi F. Revisiting DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay as a useful tool in antioxidant evaluation: a new IC100 concept to address its limitations. J Food Bioact. 2019;7:36–42. doi: https://doi.org/10.31665/JFB.2019.7196
15. Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–8. doi: https://doi.org/10.1093/carcin/10.6.1003
16. Muthu S, Durairaj B. Evaluation of antioxidant and free radical scavenging activity of Annona muricata. Eur J Exp Biol. 2015;5(3):39–45.
17. Gohari AR, Hajimehdipoor H, Saeidnia S, Ajani Y, Hadjiakhoondi A. Antioxidant activity of some medicinal species using FRAP assay. J Med Plants. 2011;10(37):1–7.
18. Kala SC, Ammani K. GC–MS analysis of biologically active compounds in Canthium parviflorum Lam. leaf and callus extracts. Int J Chemtech Res. 2017;10:1039–58.
19. Naaz I, Ali SA. Identification and characterization of bioactive compound berberine in the Berberis vulgaris root extract using HR-LC-MS analysis. J Anal Pharm Res. 2018;7(4):467–70. doi: https://doi.org/10.15406/japlr.2018.07.00268
20. Malathi K, Ramaiah S. Ethyl iso-allocholate from medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: a molecular docking and dynamics study. Indian J Pharm Sci. 2017;78(6):780–8. doi: https://doi.org/10.4172/pharmaceutical-sciences.1000184
21. Nursamsiar N, Mangande MM, Awaluddin A, Nur S, Asnawi A. In silico study of aglycon curculigoside A and its derivatives as α-amilase inhibitors. Indonesian J Pharm Sci Tech. 2020;7(1):29–37. doi: http://dx.doi.org/10.24198/ijpst.v7i1.23062
22. Tripathi N, Kumar S, Singh R, Singh CJ, Singh P, Varshney VK. Isolation and identification of γ-Sitosterol by GC-MS from the leaves of (Decne). Curr Bioact Compd. 2013;4(1):25–7. doi: http://dx.doi.org/10.2174/1874847301004010025
23. Santhi V, Sivakumar V, Mukilarasi M, Kannagi A. Antimicrobial substances of potential biomedical importance from Babylonia zeylanica. J Chem Pharm Res. 2013;5(9):108–15.
24. Yu X, Zhao M, Liu F, Zeng S, Hu J. Identification of 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products Int Food Res J. 2013;51(1):397–403. doi: http://dx.doi.org/10.1016%2Fj.foodres.2012.12.044
25. Marzouk MM, Elkhateeb A, Latif RR, Abdel-Hameed ES, Kawashty SA, Hussein S. C-glycosyl flavonoids-rich extract of Dipcadi erythraeum Webb & Berthel. bulbs: phytochemical and anticancer evaluations. J Appl Pharm Sci. 2019;9(6):94–8. doi: http://dx.doi.org/10.7324/JAPS.2019.90613
26. McGinty D, Letizia CS, Api AM. Fragrance material review on 2-hexadecen-1-ol, 3, 7, 11, 15-tetramethyl. Food Chem Toxicol. 2010;48:S101–2. doi: https://doi.org/10.1016/j.fct.2009.11.023
27. Santos CC, Salvadori MS, Mota VG, Costa LM, de Almeida AA, de Oliveira GA, et al. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci J. 2013;2013:949452. doi: https://doi.org/10.1155/2013/949452
28. Sivakumaran G, Prabhu K, Rao MR, Jones S, Sundaram RL, Ulhas VR, et al. Gas chromatography-mass spectrometry analysis of one Ayurvedic oil, Ksheerabala Thailam. Drug Invent Today. 2019;11:2661–5.
29. Eldamaty HS, Elbasiouny H, Elmoslemany AM, Abd El-Maoula LM, El-Desoky OI, Rehan M, et al. Protective effect of wheat and barley grass against the acute toxicological effects of the concurrent administration of excessive heavy metals in drinking water on the rats liver and brain. Appl Sci. 2021;(11):5059. doi: https://doi.org/10.3390/app11115059
30. Lee JH, Kim SC, Kim KH, Kim WK, Lee MJ, Yoo BC, et al. Decreased cellular levels of palmitic amide are linked to 5-fluorouracil resistance in human colon cancer cells. Hepatogastroenterology. 2014;61(130):343–8.
31. Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, et al. A. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood. 2007;109(2):560–5. doi: https://doi.org/10.1182/blood-2006-06-029934
32. Feldstein A, Chang FH, Kucharski JM. Tryptophol 5-hydroxytryptophol and 5-methoxytryptophol induced sleep in mice. Life Sci. 1970;9(6):323–9. doi: https://doi.org/10.1016/0024-3205(70)90220-1
33. Yannai S. Dictionary of food compounds with CD-ROM. Boca Raton, FL: Crc Press; 2012.
34. Liu T, Liang W, Tu G. Perlolyrine: a β-carboline alkaloid from Codonopsis pilosula. Planta Med. 1988;54(05):472–3. doi: https://doi.org/10.1055/s-2006-962513
35. Ohishi K, Toume K, Arai MA, Sadhu SK, Ahmed F, Mizoguchi T, et al. Ricinine: a pyridone alkaloid from Ricinus communis that activates the Wnt signaling pathway through casein kinase 1α. Bioorg Med Chem. 2014;22(17):4597–601. doi: https://doi.org/10.1016/j.bmc.2014.07.027
36. Kapadia GJ, Shukla YN, Basak SP, Sokoloski EA, Fales HM. The melosatins-a novel class of alkaloids from Melochia tomentosa. Tetrahedron. 1980;36(17):2441–7. doi: https://doi.org/10.1016/0040-4020(80)80221-3
37. Erdogan Orhan I, Tareq Hassan Khan M. Flavonoid derivatives as potent tyrosinase inhibitors–a survey of recent findings between 2008-2013. Curr Top Med Chem. 2014;14(12):1486–93. doi: https://doi.org/10.2174/1568026614666140523120741
38. Chandrakumar NS, Yonan PK, Stapelfeld A, Savage M, Rorbacher E, Contreras PC, et al. Preparation and opioid activity of analogs of the analgesic dipeptide 2, 6-dimethyl-L-tyrosyl-N-(3-phenylpropyl)-D-alaninamide. J Med Chem. 1992;35(2):223–33. doi: https://doi.org/10.1021/jm00080a005
39. Arnone A, Cardillo R, Meille SV, Nasini G, Tolazzi M. Isolation and structure elucidation of clavilactones AC, new metabolites from the fungus Clitocybe clavipes. J Chem Soc Perkin Trans. 1994;(15):2165–8.
40. Mochalski P, Leja M, Gasenko E, Skapars R, Santare D, Sivins A, et al. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J Breath Res. 2018;12(4):046005. doi: https://doi.org/10.1088/1752-7163/aacbfb
41. Scott-Thomas AJ, Syhre M, Pattemore PK, Epton M, Laing R, Pearson J, et al. 2-Aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med. 2010;10(56):1–10. doi: https://doi.org/10.1186/1471-2466-10-56
42. Strohalm H, Dregus M, Wahl A, Engel K. Enantioselective analysis of secondary alcohols and their esters in purple and yellow passion fruits. J Agric Food Chem. 2007;55(25):10339–44. doi: https://doi.org/10.1021/jf072464n
43. Takahashi A, Kusano G, Ohta T, Nozoe S. The constituents of Lactarius flavidulus Imai. Chem Pharm Bull. 1988;36(7):2366–70. doi: https://doi.org/10.1248/cpb.36.2366
44. Marshall JH, Wilmoth GJ. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147(3):900–13. doi: https://doi.org/10.1128/jb.147.3.900-913.1981
45. Chung N, Mao C, Heitman J, Hannun YA, Obeid LM. Hytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J Biol Chem. 2001;276(38):35614–21. doi: https://doi.org/10.1074/jbc.m105653200
46. Yoshikawa M, Yamaguchi S, Kunimi K, Matsuda H, Okuno Y, Yamahara J, et al. Stomachic principles in ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyl digalactosyl glycerols, ginger glycolipids A, B, and C, from Zingiberis rhizoma originating in Taiwan. Chem Pharm Bull. 1994;42(6):1226–30. doi: https://doi.org/10.1248/cpb.42.1226
47. Garg SK, Gupta SR, Sharma ND. Glucosides of Apium graveolens. Planta Med. 1980;38(04):363–5.
48. Sheng F, Zhang L, Wang S, Yang L, Li P. Deacetyl ganoderic acid F inhibits LPS-induced neural inflammation via NF-κB pathway both in vitro and in vivo. Nutrients. 2019;12(1):85. doi: https://doi.org/10.3390%2Fnu12010085
49. Umebayashi C, Yamamoto N, Nakao H, Toi Y, Chikahisa-Muramatsu L, Kanemaru K, et al. Flow cytometric estimation of cytotoxic activity of rhodexin A isolated from Rhodea japonica in human leukemia K562 cells. Biol Pharm Bull. 2003;26(5):627–30. doi: https://doi.org/10.1248/bpb.26.627
50. Adams M, Efferth T, Bauer R. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards CCRF-CEM leukaemia cells and multi-drug resistant CEM/ADR5000 cells from Artemisia argyi. Planta Med. 2006;2(09):862–4. doi: https://doi.org/10.1055/s-2006-947165
51. Jing N, Liu X, Jin M, Yang X, Hu X, Li C, et al. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food Funct. 2020;11(8):6971–86. doi: https://doi.org/10.1039/D0FO01282C
52. Meng X, Wang W, Xie Z, Li P, Li Y, Guo Z, et al. Neomycin biosynthesis is regulated positively by AfsA-g and NeoR in Streptomyces fradiae CGMCC 4.7387. Sci China Life Sci. 2017;60:980–91. doi: http://dx.doi.org/10.1007/s11427-017-9120-8
53. Murae T, Tsuyuki T, Ikeda T, Nishihama T, Masuda S, Takahashi T. Bitter principles of Picrasma ailanthoides planchon. Nigakilactones A, B, C, D, E and F. Tetrahedron. 1971;27(7):1545–55.
54. Inouye H, Ueda S, Aoki Y, Takeda Y. Studies on monoterpene glucosides and related natural products. XVII. The intermediacy of 7-desoxyloganic acid and loganin in the biosynthesis of several iridoid glucosides. Chem Pharm Bull. 1972;20(6):1287–96.
55. Martin RA, Edgington LV. Antifungal, phytotoxic, and systemic activity of fenapanil and structural analogs. Pestic Biochem Physiol. 1982;17(1):1–9.
56. Wongsinkongman P, Brossi A, Wang HK, Bastow KF, Lee KH. Antitumor agents. Part 209: pheophorbide-a derivatives as photo-independent cytotoxic agents. Bioorg Med Chem. 2002;10(3):583–91. doi: https://doi.org/10.1016/s0968-0896(01)00305-4
57. Matroule JY, Carthy CM, Granville DJ, Jolois O, Hunt DW, Piette J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene. 2001;20(30):4070–84. doi: https://doi.org/10.1038/sj.onc.1204546
58. Parish LC, Parish JL. Retapamulin: a new topical antibiotic for the treatment of uncomplicated skin infections. Drugs Today. 2008;44(2):91–102. doi: https://doi.org/10.1358/dot.2008.44.2.1153446
59. Wiese M, D’Agostino PM, Mihali TK, Moffitt MC, Neilan BA. Neurotoxic alkaloids: saxitoxin and its analogs. Mar Drugs. 2010;8(7):2185–211. doi: https://doi.org/10.3390%2Fmd8072185
60. Do CT, Pollet B, Thévenin J, Sibout R, Denoue D, Barrière Y, et al. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta. 2007;226:1117–29. doi: https://doi.org/10.1007/s00425-007-0558-3