INTRODUCTION
The human eye is a complex organ with a unique structure and physiology that prevents medications from reaching specific ophthalmic areas. The challenging task is to increase medication residence time and ensure proper ocular penetration [1]. The issues with conventional ocular drug delivery include short contact times of the drug in the eye and poor ocular bioavailability (BA) as a result of solution drainage, tears turnover, and dilution or lacrimation [2]. Due to different anatomical and physiological barriers, only 1% or even less of the administered dose of the drug reaches the intraocular tissues, which lowers the absorption of the drugs. Topical dosage forms need to maintain a balance between lipophilicity and hydrophilicity as well as longer contact times for better therapeutic outcomes [3]. All these drawbacks of conventional methods can be overcome by nanotechnology. By delivering precise medications to specified locations and targets, nanotechnology provides numerous advantages in the treatment of chronic human diseases [4]. The emergence of nanoformulation results in the ability to surpass ocular barriers, improve drug residence time on the cornea surface, boost permeability and BA of the medication, reduce degradation of unstable drugs, and be well tolerated by the patient compared to the conventional drug [5]. With a compound annual growth rate of 6.9% from 2023 to 2030, the market for ocular drug delivery in India is expected to increase from its estimated value of 0.9 billion USD in 2022 to 1.5 billion USD by 2030 [6]. It has been established that nanosized formulations can improve medication retention/permeation and extend drug release in ocular tissue [7]. Some common eye ailments or diseases include glaucoma, cataracts, ocular surface infections, inflammation, dry eye syndrome, diabetic retinopathy (DR), diabetic macular edema, age-related macular degeneration (AMD), and retinal vein occlusion [8]. Ocular drug delivery systems are employed to administer drugs to the eyes for the treatment of various disorders that affect vision [9,10]. Various dosage forms for ophthalmic preparations include liquid, semi-solid, or solid for example eye drops, suspensions, ointments, creams, emulsions, contact lenses, and ocular inserts. The anterior region of the eye absorbs medication through eye drops, but short residence time prevents the proper absorption of drugs from eye drops [11]. The ocular residence time can be greatly improved by the creams and ointments (semi-solid), however, they may cause blurred vision [12]. Ocular gels are one technique to extend the residence time of drugs at the precorneal level and, their BA. Ocular gels are formed of a 3-D cross-linked polymer or a colloidal network submerged in a fluid [13]. The in-situ gelling system is among the most intriguing methods for lengthening the period that medications remain on the surface of the eye [14]. Temperature, pH, and ions are some examples of internal stimuli that can trigger gelation [15]. The easy method of preparation, simple administration, and exact dose distribution are some advantages of the in-situ gelling system [16]. Drugs can be delivered to ocular targets using nanoparticulate drug delivery devices [17]. The current study focuses on the applications of nanogels in the treatment of various ocular diseases. It provided a thorough description of nanogels from various angles, including ideal characteristics, approaches, applications, upcoming trends, formulation challenges, and future prospects.
Ideal characteristic of in-situ gels for ocular drug delivery
The successful treatment of a disease depends on the ideal requirement from the formulation. A perfect in-situ gelling drug delivery formulation should meet the requirements listed below. Some crucial factors that must be required in ocular drug delivery are discussed here [18,19].
Gelation (transition of phase from sol-gel)
Under physiological circumstances or in the presence of the trigger for gelation, the formulation should be supplied as a solution that is converted immediately into a gel to avoid precorneal drainage [18].
Optimal pH
The pH of the formulation should be the same as that of eye pH, i.e., formulations that have slight changes in pH either acidic or alkaline can irritate or harm ocular tissues [20].
Clarity
The formulation must be transparent, clear, and colorless. Normal vision should not be hampered by it. Any contaminant, such as particles, ought to be absent because they could irritate the ocular tissues [21].
Adhesiveness
The formulation must adhere to the eye’s precorneal surface to provide longer residence time and absorption [22].
Isotonicity
Isotonicity should be maintained to avoid eye discomfort or tissue damage [22].
Ocular tolerance
The polymers in the formulation should be well-tolerated and biocompatible with the ocular tissues. There should not be any tissue damage brought on by the formulation, including inflammation, redness, edema, or any other unfavorable side effects [23].
Reproducibility
The formulation must exhibit the same characteristics on repeated preparation and massive manufacturing. An in-situ gelling device is best for consistent administration when it is a free-flowing liquid [23].
Rheological properties
The two most important requirements for an in-situ gelling system are viscosity and gel strength. The formulation must have the right amount of viscosity for convenient administration and a fast transition from sol to gel. In addition, the gel that is produced must reside over time without disintegrating or degrading. In-situ gels usually show pseudoplastic flow. It is preferable to utilize viscoelastic fluids with high viscosities at low shear rates and low viscosities under high shear rates because certain areas endure extremely high shear rates and tissue movement [23].
Drug content
The formulation must have high drug content without any chemical degradation or unfavorable interactions with the polymers or other excipients [22].
OCULAR GELS
Ocular gels are simple viscous dosage forms that remain unchanged after injection. They can reside for a longer time in the eye in comparison to normal liquid eye drops. However, they are difficult to administer and can result in vision impairment, crusting on the eyelids, and tearing while preventing accurate and reproducible drug delivery [24]. Therefore, ocular gels are primarily used as tear substitutes in the treatment of dry eye [25]. The designing of a novel strategy for safe, easy, and efficient ocular drug delivery is a serious matter that necessitates innovative approaches [26]. In recent years, several novel techniques utilizing various tactics have been created to enhance the ocular delivery system [27]. The ocular in-situ gel has drawn the most attention over the past few years. The in-situ gel system is designed as a liquid preparation that can be injected into the eyes and, when exposed to the physiologic environment of the body, transforms into an in-situ gel [28]. This increases the precorneal residence time of the formulation and improves the ocular BA of the drug [29]. Table 1 highlighted some ocular liquid gels.
IN-SITU OCULAR GELS
Drug delivery systems known as in-situ gels undergo in-situ gelation after being injected into the body and release the drug over a prolonged or regulated period [30]. This gelation is caused by stimuli like temperature, pH, and other factors [18]. In-situ, ocular gels are low-viscosity liquids that transform into viscoelastic gels in the conjunctival cul-de-sac as a result of conformational changes in polymers in reaction to the physiological environment [31]. In-situ gels are liquids in the rest and get converted quickly into the gel in the eye’s cul-de-sac in reaction to environmental changes to generate viscoelastic gels [32]. The fluid system of the eye creates a solution or weak gel before instillation in the eye and before a strong gel forms, therefore, the rate at which in-situ gel develops is essential [17]. In-situ gels can be formulated using both natural and artificial polymers [33]. A wide variety of drug molecules and materials of therapeutic advantages such as antibiotics (ofloxacin, ciprofloxacin, and gatifloxacin), beta-blockers (timolol and carteolol), Non-steroidal anti-inflammatory drugs (NSAIDs) (ketorolac tromethamine and indomethacin), pilocarpine hydrochloride, puerarin, recombinant Recombinant Human Epidermal Growth Factor (rhEGF), and antivirals (acyclovir) has been delivered through in-situ gelling systems. Table 2 summarizes some marketed ocular in-situ gels and their applications.
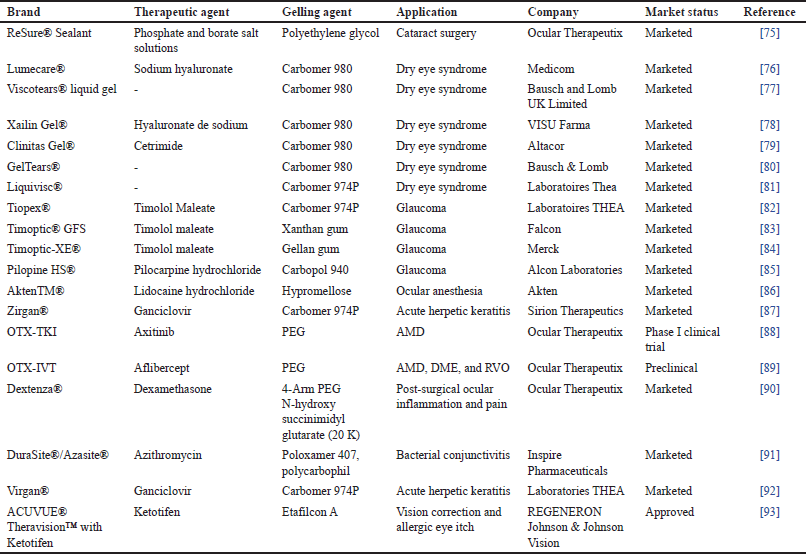 | Table 1. List of ocular hydrogels. [Click here to view] |
Approaches of in-situ gelling system
The situ gelling system works on the stimuli response [19]. The major stimuli for in-situ gels are temperature, pH, and ions.
Temperature-induced gelling system
Temperature-induced in-situ gels are liquid at room temperature (20°C–25°C) and get converted to the gel due to the higher temperature (35°C–37°C), at the application location. At a critical temperature, such as the lower critical solution temperature or upper critical solution temperature, temperature-sensitive hydrogels either go through a volume phase transition or a sol-gel phase transition. The polymer gradually dissolves and micellar aggregation increases, at high temperatures to give sol-to-gel transformation (the entanglement of the polymeric network) [34,35].
pH-induced gelling system
pH-induced in-situ gels are polymeric dispersions in aqueous systems that spontaneously gel after application at the target site in response to a change in pH [36]. The polymer undergoes inter-diffusion due to electrostatic, hydrophobic, and hydrogen bonding interactions at a particular pH [37]. The phase transition of the carbopol solution occurs at a pH range of 4.0–7.4 due to the ionization of the carbopol polymer.
Ion-activated gelling system
The sol-to-gel transition is triggered in this form of in-situ hydrogel by the presence of monovalent or divalent cations such as Na+, K+, Ca2+, and Mg2+ ions. The formulation is administered as a liquid solution into the cul-de-sac, and the electrolytes of the tear fluid, particularly Na+, Ca2+, and Mg2+cations, induce gelation of the polymer. Cross-linking occurs between cations and negatively charged polysaccharides [38].
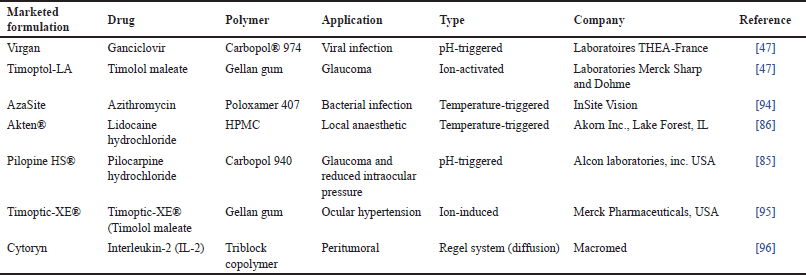 | Table 2. List of approved ocular in-situ gels. [Click here to view] |
OCULAR NANOGELS
Compared to pure gel, nanogels combine the properties of nanoparticles (NPs) and gel to provide high adhesiveness and BA [39]. Various drugs have been incorporated into gels for ocular administration as given in Table 3. NPs are nanosized particles of biodegradable components. Nanogels can be created using a variety of synthetic and natural polymers, including Poly Lactic Acid (PLA) and poly(lactic-co-glycolic acid (PLGA), as well as natural polymers such as chitosan and alginate [40]. Both hydrophilic and hydrophobic drugs, as well as charged solutes and other diagnostic tools, can be administered using nanogels. The drug loading in nanogels is relatively high because of the functional groups present in the polymeric network. The drug may be integrated into the matrix or bonded to the surface. For a decade, nanotechnology has demonstrated promising outcomes for the delivery of ocular medications [41]. The minute size of NPs is a very positive trait for their potential to reduce irritation in ocular tissue and to sustain drug delivery without needing repeated doses [42]. The size and surface characteristics of NPs are the primary factors influencing the penetration of NPs into the eye [33]. The capacity of modern nanotechnology-based carriers to entrap both hydrophilic and lipophilic medicines, good ocular permeability, prolonged residence time, good drug stability, and high BA are some key advantages to adopting this technique for ocular delivery [43,44].
NPs with a size between 200 and 2,000 nm are capable of being taken up effectively and kept by the tissues where they have been kept for up to 2 months [24]. The incorporation of NPs with hydrogel provides the desired drug levels at the retina [45].
IN-SITU OCULAR NANOGEL
The term “nanoparticle-loaded in-situ gel” refers to the combination of two drug delivery techniques, namely NPs and in-situ gel [4]. Both integrating the drug during NP creation or adsorbing the drug after NP formation by incubating them in drug solution are effective ways to achieve drug loading in NPs [46]. The incorporation strategy is more effective than the latter one since it traps a significant amount of the medication. For ocular administration, drug-loaded NPs are added to the gels [47]. The transport of drugs into the eye is limited by the blood–retinal barrier, corneal barrier, and tear film barrier [48]. It is significantly more difficult to deliver drugs to the posterior segment because of the shape and complicated physiology of the eye. Typical ophthalmic formulations cannot give and maintain an acceptable drug concentration in the retina [21]. In-situ, gelling systems are gels that are administered as a solution and change into gels at the ocular surface. In-situ gelling systems often experience reversible sol-gel phase transitions [22]. The idea of a NP has significantly grown in popularity during the past few decades. Recently, there has been an increase in interest in producing nanogels, which provide researchers with vast and new prospects for a variety of biological and pharmacological applications by fusing the benefits of NPs and hydrogels [49]. One might achieve optimal drug loading and controlled release for effective peroral, rectal, vaginal, ophthalmic, and transdermal drug administration by modifying a biodegradable nano gel technology and fine-tuning its composition [50]. Also, the basic components can be easily purchased and the nanogels can be generated on an industrial scale, offering a high cost-benefit ratio. Most importantly, to lessen the undesirable toxicity, nano gel particles can be quickly eliminated from the body after therapy by renal excretion and/or enzymatic destruction. Nano-gelling systems are excellent at speeding up corneal penetration and increasing ocular BA. These nanogels are replete with NPs [51]. Various research focused on NPs-loaded ocular in-situ gels has been given in Table 4. The limitations of nanotechnology include large-scale manufacturing, and limiting the dosages of medications [52].
Application of in-situ nanogels in ocular drug delivery
NPs loaded in-situ gels for bacterial keratitis
Antibiotics administered topically can cure ophthalmic infections more effectively. Various strategies, such as microparticles, gels, micelles, liposomes, gel-forming solutions, NPs, and nanoemulsions (NEs), have been used to extend the stay of drugs in the cornea. Reducing nasolacrimal drainage is one of the most promising methods for lengthening the residence time at the ocular surface [53]. Often, this is accomplished by raising the vehicle’s viscosity. Moreover, ocular inserts may offer improved drug residency at the ocular surface. However, the foreign body experience from the ocular implants results in low patient compliance [54]. As an alternative, in-situ, gel-forming solutions have emerged as one of the most promising methods for lengthening the period that drugs remain in the eye without having any negative side effects [11]. When applied in the form of solutions, in-situ gel-forming substances go through a sol-to-gel transition once they reach the eye. Several mechanisms, such as changes in pH (pH-sensitive gel), changes in temperature (thermosensitive gel), and ionic strength, can cause the gel to develop (ionic gelation) as shown in Figure 1 [55]. Polymeric solutions that transform from a solution to a gel when subjected to temperature variations are known as thermoresponsive gelling systems. At body temperature, some organic and synthetic polymers exhibit thermoresponsive gelling characteristics. In a research chitosan-based thermoresponsive hydrogels were introduced into rabbit eyes for the sustained administration of latanoprost and ferulic acid [56].
Hydrocortisone-loaded PLGA NPs included in thermoresponsive in-situ gel showed increased ocular BA [57]. Increased precorneal residence duration and enhanced ocular BA were found for sparfloxacin-loaded PLGA-NPs integrated with pH-sensitive in-situ gel [35]. Ganciclovir prodrug-loaded PLGA-NPs in thermoresponsive PLGA-PEG in-situ gel showed improved efficacy for corneal keratitis [58]. Polyacrylic acid (PAA) is a frequently used safe and mucoadhesive ocular polymer because of its nonabrasive and flexible nature. Poloxamers are biocompatible synthetic amphiphilic polymers with good thermoresponsive properties [59]. Furthermore, poloxamer solutions can create transparent gels that do not hinder normal eyesight. The Food and Drug Administration (FDA) has authorized the use of poloxamer 407 as an “inactive ingredient” in a variety of products, including intravenous, solutions, suspensions, inhalation, and ophthalmic formulations [60].
NPs loaded in-situ gels for the treatment of cataract
Cataract is the most common ocular condition and the major cause of blindness in people worldwide. In cataracts excessive lens and tissue collapse are caused by crystallin aggregation. Genetic predisposition, aging, toxic chemicals, exposure to UV light, oxidative stress, metabolic problems, hereditary mutations, and diabetes are some risk factors for the development of cataracts. Surgery is the most effective method of cataract prevention in clinical settings because there are currently no anti-cataract medications on the market. However, recent studies have shown that lanosterol (Lan), a crucial early rate-limiting step in the biosynthesis of cholesterol, interfered with the aggregation of D-crystalline by interacting with the hydrophobic dimerization interface in individuals and it was a significant factor in the prevention of cataract development in animal models [61]. The application of drugs in the form of in situ nanogels would be a promising approach for the treatment of cataracts.
NPs loaded in-situ gels for the treatment of glaucoma
Retinal ganglion cells loss, excavation of the optic disc, and progressive visual field loss are symptoms of glaucoma, an age-related optic neuropathy [3]. The primary risk factor for the onset and progression of glaucoma is intraocular pressure. Although all medical and surgical treatments lower intraocular pressure. However, in certain glaucoma patients, uncontrollable intraocular pressure (IOP) continues to cause rapid vision loss even after therapeutic IOP reduction [62]. Innovative drug delivery techniques, such as in-situ gels filled with NPs, have greatly increased the medication’s ocular BA [63]. In-situ, gel-containing NPs loaded with dorzolamide hydrochloride offered a more intensive glaucoma treatment and higher patient compliance because it requires fewer applications daily than traditional eye drops [64].
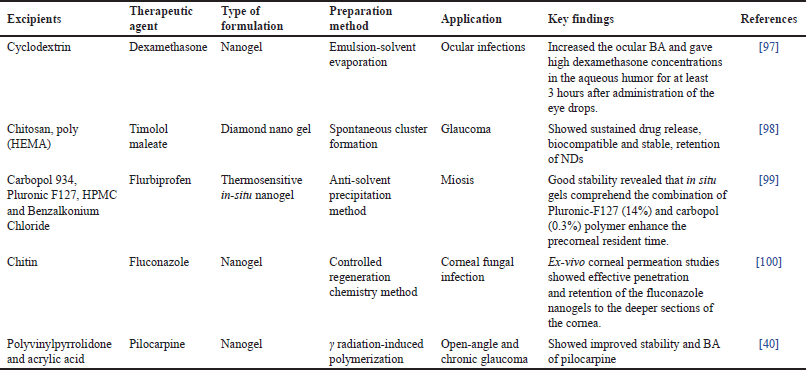 | Table 3. Nanogel for ocular delivery. [Click here to view] |
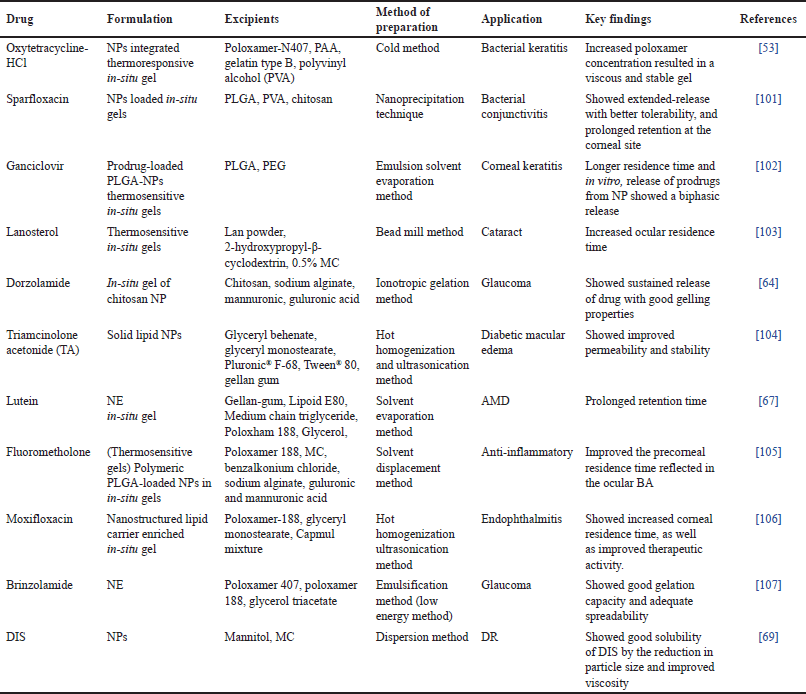 | Table 4. List of NPs loaded ocular in-situ gels. [Click here to view] |
NP-loaded in-situ gel for the treatment of AMD
AMD, the world’s leading cause of blindness, affects millions of people over 65. As the population ages, it is predicted that this degenerative sickness will become more common, creating an unmet medical need [65]. Although the pathogenic mechanism underlying AMD is not yet fully understood, oxidative stress is known to play a substantial role in AMD pathology. The retina uses a lot of oxygen, making it vulnerable to damage from oxidative stress [66]. Lutein is a carotenoid with a molecular weight of 568.85. It is soluble in organic solvents and has high lipophilicity despite having a low solubility in water (0.000732 mg/ml, log P 7.8). Because of its ability to quench singlet oxygen and eliminate free radicals, lutein is commonly used as an antioxidant to protect the retina from harm caused by oxidative stress. In addition, lutein can filter out UV and blue rays, which are harmful to the retina. Furthermore, the amount of lutein in the macular region is related to the pathogenesis of AMD since it is the main component of human retinal macular pigment. The optical density of the macular pigment can be increased in patients’ retinas to prevent and treat AMD. Lutein, in general, can treat AMD either by reducing reactive oxygen species, increasing the optical density of the macular pigment, and/or reducing the quantity of damaging light that enters the retina. To reduce the precorneal loss caused by the medication, an in-situ gel was created [67]. Cell-penetrating peptides are short, less than 30 amino acid peptides that quickly and without the need for receptors bind to cells. A homologous domain generates penetratin, a peptide that enters cells. According to studies, it has a strong ability to enter the eye and can carry medications to the retina. Stearyl penetratin (ste-penetratin) was added to the NE to boost penetration using a noninvasive delivery approach. To increase the period of corneal retention and let the penetratin work to its full potential, an ion-responsive in-situ gel of penetratin-NE was developed [67].
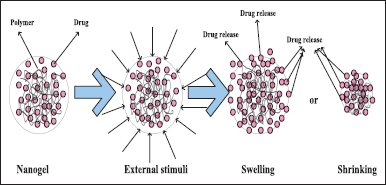 | Figure 1. Mechanism of stimuli-responsive nanogel. [Click here to view] |
NPs loaded in-situ gel for the treatment of DR
Major global public health concerns today include the treatment and prevention of diabetes mellitus (DM), which is predicted to affect 4.4% of patients by 2030. Prothrombotic conditions with poor coagulation, platelet dysfunction, and endothelial dysfunction develop in DM patients. Nephropathy, neuropathy, and microvascular issues are the early signs of diabetes complications. DM is characterized by DR. DR is one of the most prevalent microvascular issues in persons with diabetes [68]. It is distinguished by retinal capillary cell death, malfunction of the glia and neurons, artery blockage, vascular leakage, and visual loss that may result in blindness. The alcoholic syndrome is treated with disulfiram (DIS), a dimer of diethyldithiocarbamate. The administration of DIS to the retina has been demonstrated to be effective in treating DR’s retinal impairment. To further enhance the ocular BA, NPs and an in-situ gelling method were combined. It was found that increased the ocular BA by using methylcellulose (MC) as the in-situ gelling agent. These high ocular BAs enable medicines to reach the retina [69].
Retinoblastoma
The most frequent primary malignant intraocular tumor in children, retinal blastoma (Rb), arises from retinal stem cells. Most children survive Rb with the help of systemic chemotherapy, but they frequently lose their vision or require ocular enucleation [70]. The administration of local anticancer drugs would be helpful to raise the local drug concentration and reduce chemotherapy’s negative side effects concerning the pure availability of the target tumor by systemic chemotherapy [70]. The current study describes a novel hydrogel implant that can deliver low molecular weight hydrophilic anticancer medicines topotecan and vincristine at therapeutically effective quantities. The proposed hydrogel implant consists of two layers: an inner hydrophilic layer made of 2-hydroxyethyl methacrylate (HEMA) that acts as a reservoir for the chemotherapy agent and an outer hydrophobic layer made of 2-ethoxy ethyl methacrylate (EOEMA) that serves as a barrier to guard the surrounding vascularized tissue from the cytotoxicity of the chemotherapy agents that are being delivered. Studies conducted on enucleated pig eyes showed that the medicines may diffuse past the sclera and enter the vitreous humor. The sorption, release, and transport characteristics of HEMA-based hydrogels were investigated, demonstrating the possibility of modifying the drug loading capacity and diffusion by the level of crosslinking. The gels made from EOEMA turned out to be impervious to drug sorption and diffusion. Unloaded hydrogels had excellent biocompatibility according to a chorioallantoic membrane assay. In vitro tests revealed considerable cytotoxicity of drug-loaded hydrogels against an Rb cell line after 2 days for topotecan-loaded hydrogels and at least 6 days for vincristine-loaded hydrogels. For the local administration of active drugs to the eyeball for the treatment of Rb and other ocular illnesses, the bi-layered hydrogel implant can be viewed as promising.
UPCOMING TRENDS IN NANOGELS
In various biomedical applications, from cancer to neurological illnesses, nanogels constitute an innovative system for adjustable drug release and targeted therapy. To improve the processes and provide advanced nanomaterials, researchers have spent a lot of time investigating the design of these nanocarriers [71]. These NPs’ swelling behavior is what sets them apart. The hydrophilic characteristic of the finished nanonetwork, which can incorporate a large quantity of water or biological fluids while preserving its structural integrity, is largely due to the polymers utilized in Nanogel production, which absorb water. However, nanogels have recently been investigated for use in a variety of sectors besides healthcare. The potential use of nanogels as catalysts, adsorbents, or sensing materials for environmental applications serves as a clear instance of this. Nanogels have been proven to be particularly helpful in environmental applications for the removal of organic contaminants, due to their huge surface area, porosity, and encapsulating capabilities [72]. Nanogels have sparked a lot of interest in targeted drug delivery. Targeting cancer cells in the body with effective formulation and functionalization of the particles helps prevent numerous negative effects of other treatments [57]. Cardiovascular disorders can also be treated with nanogel compositions [73].
CHALLENGES AND FUTURE PROSPECTIVE OF OCULAR NANOGELS
Nanogels are emerging as a promising approach to treating various ocular diseases. Table 5 highlights some patents on this emerging drug delivery system. The eye is a very sensitive and very useful part of the human body. Therefore, attention should be given to the safety of any dosage form administered to the eye. The unreacted monomers or remaining surfactants in the nano gel preparation could be hazardous. Researchers need to assess the possible toxicity of nanogels and the materials utilized to prepare nanogels during repeated and prolonged application in the eye before their clinical use and commercial production. Most of the biocompatibility and safety studies of nanogels are performed on animal eyes. However, the structure of animal eyes is quite different from human eyes. Unfortunately, the results of these preclinical studies are sometimes not validated in humans. Most preclinical findings concluded no signs of toxicity and altered pharmacological effects of nanogels in the eyes. Nanogels have a great ability to get stimulated by external stimuli such as pH, light, enzymes, redox reactions, temperature, enzymes. However, the stimuli-responsive characteristics can cause significant problems if the drug does not deliver to the site of action. There are possibilities that nanogels may degrade in an undesired area before reaching their target site. This can cause off-target delivery of drugs. Nanogels are known to give prolonged drug release; therefore, this off-target drug delivery may cause significant adverse effects. Besides the toxicity issues the particle size and polydispersity of nanocarriers, as well as problems with polymer degradation, are additional drawbacks of nanogels. The problems associated with the handling and storage of nanogels are one significant challenge. It is a major problem, particularly for temperature-responsive nanogels with gelation temperatures lower than 37°C. The nano gel can get converted from sol-to-gel state in the needle or syringe making the administration difficult. The instability of nanogels can affect the shelf life of the formulation.
 | Table 5. Patents on ocular in-situ gel. [Click here to view] |
Nanogels are frequently prepared using organic/inorganic solvents and synthetic polymers. Therefore, toxic solvents must be fully eliminated from the formulation. Special attention must be paid to the toxicity of the degraded polymers in the eyes. The polymers should be modified for ideal characteristics such as bioadhesion, biocompatibility, and biodegradability. It is also preferable to carefully plan the nanogel formulations to reduce any hazardous effects brought on by the degradation of polymers of nanogels. The ideal feature of nanogels includes the ability to load multiple therapeutic moieties and the controlled release of the therapeutic agents. The rheology of nanogels must be ideal for handling and storage. The prolonged retention of nanogels in the patient’s body, and storage stability are other crucial properties required from nanogels. Particle sizes of 50–200 nm and polydispersity index of NPs below 0.7 are additional requirements [23,74].
CONCLUSION
The combined benefits of hydrogels and NPs to increase ocular BA and therapeutic efficacy and lessen systemic absorption and toxicity, are seen in-situ nanogels. Traditional ophthalmic formulations have faced various difficulties with topical medication administration because of the anatomical and physiological barriers of the eye. Nevertheless, a lot of work has been put into finding safer and more efficient therapeutic agents up to this point, and new ocular drug delivery techniques are constantly being investigated. The release of ocular medications can be prolonged and the frequency of administration is reduced by encapsulating them in nanogels, which improves patient compliance. The safety, efficacy, stability, and patient acceptance of the formulation greatly depend on the quality control testing of the formulation. Ocular problems such as glaucoma, retinoblastoma, cataracts, AMD, dry eye syndrome, DR, and bacterial keratitis may eventually lead to vision loss or blindness if they are not properly treated. The limitations of conventional ocular delivery systems, such as their low therapeutic efficacy and unfavorable side effects from invasive surgery or systemic exposure, can be greatly overcome by nanotechnology. However, the challenges associated with the scale-up of NPs from laboratory to large-scale production, and their ocular safety need to be addressed.
ACKNOWLEDGMENT
The authors conveyed special thanks to Mr. Jitender Joshi, Chancellor, and Prof. (Dr.) Dharam Buddhi, Vice Chancellor of Uttaranchal University, for their research-associated encouragement.
AUTHOR CONTRIBUTIONS
The investigation, conceptualization, and writing of Shalu Verma; Nidhi Nainwal. Review and editing, Shalu Verma. Supervision, Nidhi Nainwal. All the authors have contributed to the preparation of the manuscript, accepted the entire content, and approved its submission. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomedicine. 2007;2:11–21.
2. Kandpal N, Nainwal N, Ale Y, Semwal Y, Jakhmola V, Padiyar N. Proniosomes: a pro vesicular system in ocular drug delivery. J Adv Biotechnol Exp Ther. 2023;6:622. doi: https://doi.org/10.5455/jabet.2023.d154
3. Wang R, Gao Y, Liu A, Zhai G. A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances. J Drug Target. 2021;29:687–702. doi: https://doi.org/10.1080/1061186x.2021.1878366
4. Gorantla S, Waghule T, Rapalli VK, Singh PP, Dubey SK, Saha RN, et al. Advanced hydrogels based drug delivery systems for ophthalmic delivery. Recent Pat Drug Deliv Formul. 2020;13:291–300.
5. Kagkelaris K, Panayiotakopoulos G, Georgakopoulos CD. Nanotechnology-based formulations to amplify intraocular bioavailability. Ther Adv Ophthalmol. 2022;14:251584142211123. doi: https://doi.org/10.1177/25158414221112356
6. Spherical Insights LLP. Global ocular drug delivery system market size to grow $ 140.5 billion by 2030 | CGR 7.35%. GlobeNewswire News Room 2023. Available from: https://www.globenewswire.com/en/news-release/2023/04/27/2655880/0/en/Global-Ocular-Drug-Delivery-System-Market-Size-To-Grow-140-5-Billion-By-2030-CGR-7-35.html
7. Fang G, Yang X, Wang Q, Zhang A, Tang B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater Sci Eng C. 2021;127:112212.
8. Lau CML, Yu Y, Jahanmir G, Chau Y. Controlled release technology for anti-angiogenesis treatment of posterior eye diseases: current status and challenges. Adv Drug Deliv Rev. 2018;126:145–61.
9. Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188.
10. Mazzotta C, Ferrise M, Gabriele G, Gennaro P, Meduri A. Chemically-boosted corneal cross-linking for the treatment of keratoconus through a riboflavin 0.25% optimized solution with high superoxide anion release. J Clin Med. 2021 Mar 23;10(6):1324. doi: https://doi.org/10.3390/jcm10061324.
11. Dubald M, Bourgeois S, Andrieu V, Fessi H. Ophthalmic drug delivery systems for antibiotherapy—a review. Pharmaceutics. 2018;10:10.
12. Fangueiro JF, Veiga F, Silva AM, Souto EB. Ocular drug delivery—new strategies for targeting anterior and posterior segments of the eye. Curr Pharm Des. 2016;22:1135–46.
13. Bhowmik M. Study of thermo-sensitive in-situ gels for ocular delivery. Sci Pharm. 2011;79:351–8.
14. Singh M, Dev D, Prasad DN. A recent overview: in-situ gel smart carriers for ocular drug delivery. J Drug Deliv Ther. 2021;11:195–205.
15. Zafar M, Ijaz M, Iqbal T. Efficient Au nanostructures for NIR-responsive controlled drug delivery systems. Chem Papers. 2021;75:2277–93. doi: https://doi.org/10.1007/s11696-020-01465-y
16. Gote V, Sikder S, Sicotte J, Pal D. Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther. 2019;370:602–24.
17. Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014;11:1647–60.
18. Huang W, Zhang N, Hua H, Liu T, Tang Y, Fu L, et al. Preparation, pharmacokinetics and pharmacodynamics of ophthalmic thermosensitive in-situ hydrogel of betaxolol hydrochloride. Biomed Pharmacother. 2016;83:107–13.
19. Kurniawansyah IS, Sopyan I, Wathoni N, Fillah DL, Praditya RU. Application and characterization of in-situ gel. Int J Appl Pharm. 2018;10:34.
20. Bisht R, Mandal A, Jaiswal JK, Rupenthal ID. Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;10:1–21. doi: https://doi.org/10.1002/wnan.1473
21. Majeed A, Khan NA. Ocular in-situ gel: an overview. J Drug Deliv Ther. 2019;9:337–47.
22. Wu Y, Liu Y, Li X, Kebebe D, Zhang B, Ren J, et al. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J Pharm Sci. 2019;14:1–15.
23. Wu Y, Tao Q, Xie J, Lu L, Xie X, Zhang Y, et al. Advances in nanogels for topical drug delivery in ocular diseases. Gels. 2023;9:292.
24. Halasz K, Kelly SJ, Iqbal MT, Pathak Y, Sutariya V. Micro/nanoparticle delivery systems for ocular diseases. Assay Drug Dev Technol. 2019;17:152–66.
25. Deepthi S, Jose J. Novel hydrogel-based ocular drug delivery system for the treatment of conjunctivitis. Int Ophthalmol. 2018;39:1355–66.
26. Kumari B. Ocular drug delivery system: approaches to improve ocular bioavailability. GSC Biol Pharm Sci. 2019;6:001–10.
27. Kumar N, Pahuja S, Sharma R. Occular Drug Delivery System – A Review. International Journal of Drug Delivery Technology 2019;9. https://doi.org/10.25258/ijddt.9.2.1.
28. Bai L, Lei F, Luo R, Fei Q, Zheng Z, He N, et al. Development of a thermosensitive in-situ gel formulations of vancomycin hydrochloride: design, preparation, in vitro and in vivo evaluation. J Pharm Sci. 2022;111:2552–61.
29. Shi H, Wang Y, Bao Z, Lin D, Liu H, Yu A, et al. Thermosensitive glycol chitosan-based hydrogel as a topical ocular drug delivery system for enhanced ocular bioavailability. Int J Pharm. 2019;570:118688.
30. Agrawal AK, Das M, Jain S. In-situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402.
31. Makwana SB, Patel VA, Parmar SJ. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016;6:1–6.
32. Adeyeye MC, Davis VL, Kotreka UK. In-situ gel ophthalmic drug delivery system of estradiol or other estrogen for prevention of cataracts. US 2011/0082128 A1. 2011.
33. Al-Kinani AA, Zidan G, Elsaid N, Seyfoddin A, Alani AWG, Alany RG. Ophthalmic gels: past, present and future. Adv Drug Deliv Rev. 2018;126:113–26.
34. Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in-situ gel system. Drug Deliv. 2007;14:507–15.
35. Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010 Apr;6(2):324–33. doi: https://doi.org/10.1016/j.nano.2009.10.004. Epub 2009 Oct 23.
36. Wu H, Liu Z, Peng J, Li L, Li N, Li J, et al. Design and evaluation of baicalin-containing in-situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int J Pharm. 2011;410:31–40.
37. Kurniawansyah IS, Rahmi F, Sopyan I. pH triggered in-situ gelling ophthalmic drug delivery system. Int J Drug Deliv Technol. 2018;8:1–5.
38. Vijaya C, Goud KS. Ion-activated in-situ gelling ophthalmic delivery systems of azithromycin. Indian J Pharm Sci. 2011:73(6):615–20.
39. Gautam D, Pedler MG, Nair DP, Petrash JM. Nanogel-facilitated in-situ delivery of a cataract inhibitor. Biomolecules. 2021;11:1150.
40. Abd El-Rehim HA, Swilem AE, Klingner A, Hegazy E-SA, Hamed AA. Developing the potential ophthalmic applications of pilocarpine entrapped into polyvinylpyrrolidone–poly(acrylic acid) nanogel dispersions prepared by γ radiation. Biomacromolecules. 2013;14:688–98.
41. Pillai AR, Prajapati B, Dharamsi A. Protein nanoparticles laden in-situ gel for topical ocular drug delivery. Curr Drug Deliv. 2023;20: 38–51.
42. Åhlén M, Tummala GK, Mihranyan A. Nanoparticle-loaded hydrogels as a pathway for enzyme-triggered drug release in ophthalmic applications. Int J Pharm. 2018;536:73–81.
43. Zoratto N, Forcina L, Matassa R, Mosca L, Familiari G, Musarò A, et al. Hyaluronan-cholesterol nanogels for the enhancement of the ocular delivery of therapeutics. Pharmaceutics. 2021;13:1781.
44. Silva M, Calado R, Marto J, Bettencourt A, Almeida A, Gonçalves L. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar Drugs. 2017;15:370.
45. Abdel-Rashid RS, Helal DA, Omar MM, El Sisi AM. nanogel loaded with surfactant-based nanovesicles for enhanced ocular delivery of acetazolamide. Int J Nanomed. 2019;14:2973–83.
46. Almeida H, Amaral M, Lobao P, Frigerio C, Sousa Lobo J. Nanoparticles in ocular drug delivery systems for topical administration: promises and challenges. Curr Pharm Des. 2015;21:5212–24.
47. Sheshala R, Kok Y, Ng J, Thakur R, Dua K. In-situ gelling ophthalmic drug delivery system: an overview and its applications. Recent Pat Drug Deliv Formul. 2015;9:242–53.
48. Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J, et al. Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul. 2020;13:246–54.
49. Mittal N, Kaur G. In situgelling ophthalmic drug delivery system: Formulation and evaluation. J Appl Polym Sci. 2013;131:n/a-n/a. https://doi.org/10.1002/app.39788.
50. Kesarla R, Tank T, Vora PA, Shah T, Parmar S, Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in-situ gel. Drug Deliv. 2015;23:2363–70.
51. Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for the treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol. 2019;71:1209–21.
52. Vaneev A, Tikhomirova V, Chesnokova N, Popova E, Beznos O, Kost O, et al. Nanotechnology for topical drug delivery to the anterior segment of the eye. Int J Mol Sci. 2021;22:12368.
53. Abbas MN, Khan SA, Sadozai SK, Khalil IA, Anter A, Fouly ME, et al. Nanoparticles loaded thermoresponsive in-situ gel for ocular antibiotic delivery against bacterial keratitis. Polymers. 2022;14(6):1135.
54. Pandey M, Choudhury H, Abdul-Aziz A, Bhattamisra SK, Gorain B, Su JST, et al. Advancement on sustained antiviral ocular drug delivery for herpes simplex virus keratitis: recent update on potential investigation. Pharmaceutics. 2020;13:1.
55. Kendre PN, Satav TS. Current trends and concepts in the design and development of nanogel carrier systems. Polym Bull. 2018;76:1595–617. doi: https://doi.org/10.1007/s00289-018-2430-y
56. Cheng Y-H, Tsai T-H, Jhan Y-Y, Chiu AW, Tsai K-L, Chien C-S, et al. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr Polym. 2016;144:390–9.
57. Yang X, Trinh HM, Agrahari V, Sheng Y, Pal D, Mitra AK. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech. 2015;17:294–306.
58. Colin J, Hoh HB, Easty DL, Herbort CP, Resnikoff S, Rigal D, et al. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea. 1997 Jul;16(4):393–9.
59. Al Khateb K, Ozhmukhametova EK, Mussin MN, Seilkhanov SK, Rakhypbekov TK, Lau WM, et al. In-situ gelling systems based on pluronic F127/pluronic F68 formulations for ocular drug delivery. Int J Pharm. 2016;502:70–9.
60. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709–28.
61. Nagai N, Umachi K, Otake H, Oka M, Hiramatsu N, Sasaki H, et al. ophthalmic in-situ gelling system containing lanosterol nanoparticles delays collapse of lens structure in Shumiya cataract rats. Pharmaceutics. 2020;12:629.
62. Yadav KS, Rajpurohit R, Sharma S. Glaucoma: current treatment and impact of advanced drug delivery systems. Life Sci. 2019;221:362–76.
63. Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther. 2016;32:67–82.
64. Katiyar S, Pandit J, Mondal RS, Mishra AK, Chuttani K, Aqil Mohd, et al. In-situ, gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym. 2014;102:117–24.
65. Delplace V, Payne S, Shoichet M. Delivery strategies for treatment of age-related ocular diseases: from a biological understanding to biomaterial solutions. J Control Release. 2015;219:652–68.
66. Mitchell P, Liew G, Gopinath B, Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–59.
67. Ge Y, Zhang A, Sun R, Xu J, Yin T, He H, et al. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin Drug Deliv. 2020;17:603–19.
68. Zhang C, Xu T, Zhang D, He W, Wang S, Jiang T. Disulfiram thermosensitive in-situ gel based on solid dispersion for cataract. Asian J Pharm Sci. 2018;13:527–35.
69. Deguchi S, Ogata F, Yamaguchi M, Minami M, Otake H, Kanai K, et al. In-situ gel incorporating disulfiram nanoparticles rescues the retinal dysfunction via ATP collapse in Otsuka Long–Evans Tokushima fatty rats. Cells. 2020;9:2171.
70. Cocarta AI, Hobzova R, Sirc J, Cerna T, Hrabeta J, Svojgr K, et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater Sci Eng C. 2019;103:109799.
71. Mauri E, Giannitelli SM, Trombetta M, Rainer A. Synthesis of nanogels: current trends and future outlook. Gels. 2021;7:36. doi: https://doi.org/10.3390/gels7020036
72. Shoueir KR, Sarhan AA, Atta AM, Aki MA. Macrogel and nanogel networks based on crosslinked poly (vinyl alcohol) for adsorption of methylene blue from aqua system. Environ Nanotechnol Monit Manag. 2016;5:62–73. doi: https://doi.org/10.1016/j.enmm.2016.03.001
73. Cheraghi M, Namdari M, Daraee H, Negahdari B. Cardioprotective effect of magnetic hydrogel nanocomposite loaded N,α-L-rhamnopyranosyl vincosamide isolated from Moringa oleifera leaves against doxorubicin-induced cardiac toxicity in rats: in vitro and in vivo studies. J Microencapsul. 2017;34:335–341.
74. Stawicki B, Schacher T, Cho H. Nanogels as a versatile drug delivery system for brain cancer. Gels. 2021 May 26;7(2):63.
75. Masket S, Hovanesian JA, Levenson J, Tyson F, Flynn W, Endl M, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40:2057–66. doi: https://doi.org/10.1016/j.jcrs.2014.03.034
76. Stewart M. Sodium hyaluronate sodium hyaluronate eye drops and gel | Patient. 2021. Available from: https://patient.info/medicine/sodium-hyaluronate-for-dry-eyes-artelac-blink-intensive-clinitas-hycosan-hylo-forte-xailin-ha
77. Brodwall J, Alme G, Gedde-Dahl S, Smith J, Lilliedahl NP, Kunz PA, et al. A comparative study of polyacrylic acid (viscotears) liquid gel versus polyvinyl alcohol in the treatment of dry eyes. Acta Ophthalmol Scand. 1997 Aug;75(4):457–61. doi: https://doi.org/10.1111/j.1600-0420.1997.tb00413.x.
78. Saeed N, Qazi Z, Butt NH, Siddiqi A, Maheshwary N, Athar Khan M. Effectiveness of sodium hyaluronate eye gel in patients with dry eye disease: a multi-centre, open label, uncontrolled study. Pak J Med Sci. 2013 Jul;29(4):1055–8. doi: https://doi.org/10.12669/pjms.294.3487.
79. Robben J. A brief overview presented at dry eye university of inflammation in chronic dry eye disease. J Dry Eye Dis. 2020;3:e17–8. doi: https://doi.org/10.22374/jded.v3i(sp1).23
80. Wilson CG, Zhu YP, Frier M, Rao LS, Gilchrist P, Perkins AC. Ocular contact time of a carbomer gel (GelTears) in humans. Br J Ophthalmol. 1998 Oct;82(10):1131–4. doi: https://doi.org/10.1136/bjo.82.10.1131.
81. Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014 Jul 31;8:1419–33. doi: https://doi.org/10.2147/OPTH.S65263.
82. Stewart M. Timolol eye drops for glaucoma (Timoptol, Tiopex) | Patient. 2022. Available from: https://patient.info/medicine/timolol-eye-drops-for-glaucoma-timoptol-tiopex-eysano
83. Shedden A, Laurence J, Tipping R. Efficacy and tolerability of timolol maleate ophthalmic gel-forming solution versus timolol ophthalmic solution in adults with open-angle glaucoma or ocular hypertension: a six-month, double-masked, multicenter study. Clin Ther. 2001;23:440–50. doi: https://doi.org/10.1016/s0149-2918(01)80048-5
84. Schenker HI, Silver LH. Long-term intraocular pressure-lowering efficacy and safety of timolol maleate gel-forming solution 0.5% compared with timoptic XE 0.5% in a 12-month study. Am J Ophthalmol. 2000;130:145–50. doi: https://doi.org/10.1016/S0002-9394(00)00458-X
85. Jain N, Verma A, Jain N. Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits. Drug Deliv. 2020 Dec;27(1):888–99. doi: https://doi.org/10.1080/10717544.2020.1775726.
86. Busbee BG, Alam A, Reichel E. Lidocaine hydrochloride gel for ocular anesthesia: results of a prospective, randomized study. Ophthalmic Surg Lasers Imag Retina. 2008;39:386–90. doi: https://doi.org/10.3928/15428877-20080901-03
87. Chou TY, Hong BY. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Ther Clin Risk Manag. 2004;10:665–81. doi: https://doi.org/10.2147/TCRM.S58242
88. Elhayek RF, Jarrett T, Lattrell Z, Takach S, Jarrett PK, McGrath M, et al. Efficacy of a 6 month sustained hydrogel delivery system for tyrosine kinase inhibitors in a VEGF induced retinal leakage model. Investig Ophthalmol Vis Sci. 2017 Jun 23;58(8):1968.
89. Kang-Mieler JJ, Rudeen KM, Liu W, Mieler WF. Advances in ocular drug delivery systems. Eye. 2020;34:1371–9.
90. Lee A, Blair HA. Correction to: dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs. 2020 Aug;80(12):1265. doi: https://doi.org/10.1007/s40265-020-01366-0. Erratum for: Drugs. 2020 Jul;80(11):1101–8.
91. Friedlaender MH, Protzko E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol. 2007 Mar;1(1):3–10.
92. Su C-C, Wang I-J, Chen W-L, Lin C-P, His B, Hu F-R. Topical ganciclovir treatment in patients with cytomegalovirus endotheliitis receiving penetrating keratoplasty. Clin Expe Ophthalmol. 2012;41:339–47. doi: https://doi.org/10.1111/j.1442-9071.2012.02888.x
93. Pereira-da-Mota AF, Phan C-M, Concheiro A, Jones L, Alvarez-Lorenzo C. Testing drug release from medicated contact lenses: the missing link to predict in vivo performance. J Control Release. 2022;343:672–702. doi: https://doi.org/10.1016/j.jconrel.2022.02.014
94. Jain D. Newer trends in in situ gelling systems for controlled ocular drug delivery. J Anal Pharm Res. 2016;2. doi: https://doi.org/10.15406/japlr.2016.02.00022
95. Ako-Adounvo AM, Nagarwal RC, Oliveira L, Boddu SH, Wang XS, Dey S, et al. Recent patents on ophthalmic nanoformulations and therapeutic implications. Recent Pat Drug Deliv Formul. 2014;8(3):193–201.
96. Kumbhar AB, Rakde AK, Chaudhari PD. In situ gel forming injectable drug delivery system. Int J Pharm Sci Res. 2013;4:597–609.
97. Moya-Ortega MD, Alves TFG, Alvarez-Lorenzo C, Concheiro A, Stefánsson E, Thorsteinsdóttir M, et al. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int J Pharm. 2013;441:507–15.
98. Kim HJ, Zhang K, Moore L, Ho D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano. 2014 Mar 25;8(3):2998–3005. doi: https://doi.org/10.1021/nn5002968. Epub 2014 Feb 12.
99. Maddiboyina B, Jhawat V, Desu PK, Gandhi S, Nakkala RK, Singh S. Formulation and evaluation of thermosensitive flurbiprofen in situ nano gel for the ocular delivery. J Biomater Sci Polym Edition. 2021;32:1584–97. doi: https://doi.org/10.1080/09205063.2021.1927460
100. Mohammed N, Rejinold NS, Mangalathillam S, Biswas R, Nair SV, Jayakumar R. Fluconazole loaded chitin nanogels as a topical ocular drug delivery agent for corneal fungal infections. J Biomed Nanotechnol. 2013;9:1521–31.
101. Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18:499–505.
102. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2021;6:1973–87.
103. Nagai N, Minami M, Deguchi S, Otake H, Sasaki H, Yamamoto N. An in-situ gelling system based on methylcellulose and tranilast solid nanoparticles enhances ocular residence time and drug absorption into the cornea and conjunctiva. Front Bioeng Biotechnol. 2020;8:764.
104. Tatke A, Dudhipala N, Janga K, Balguri S, Avula B, Jablonski M, et al. In-situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: tear kinetics and ocular disposition studies. Nanomaterials. 2018;9:33.
105. Gonzalez-Pizarro R, Carvajal-Vidal P, Halbault Bellowa L, Calpena AC, Espina M, García ML. In-situ forming gels containing fluorometholone-loaded polymeric nanoparticles for ocular inflammatory conditions. Colloids Surf B Biointerfaces. 2019;175:365–74.
106. Zafar A, Alsaidan OA, Imam SS, Yasir M, Alharbi KS, Khalid M. Formulation and evaluation of moxifloxacin loaded bilosomes in-situ gel: optimization to antibacterial evaluation. Gels. 2022;8:418.
107. Gupta P, Yadav KS. Formulation and evaluation of brinzolamide encapsulated niosomal in-situ gel for sustained reduction of IOP in rabbits. J Drug Deliv Sci Technol. 2022;67:103004.
108. Xia E, Smerbeck RV. Reversible gelling system for ocular drug delivery. US 2002/0114778 A1. 2002.
109. Lin HR, Sung KC. Ophthalmic drug delivery formulations and method for preparing the same. US 6511660 B1. 2003.
110. Chandavarkar NM, Jindal KC, Malayandi R. In-situ gel forming solution for ocular drug delivery. WO 2011018800 A3. 2011.
111. Banerjee R, Carvalho E. Nanoparticulate in-situ gels of TPGS, gellan and PVA as vitreous humor substitutes. US 8343471 B2. 2013.
112. Clyde L. Schultz, Ponte Vedra. Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases. US20050208102A1. 2005.
113. Coffey and Martin, Ophthalmic gel compositions. WO2013043387A1. 2013