INTRODUCTION
Sponges have recently attracted high interest from researchers because they contain microorganisms that have not been identified from the marine environment but which have the potential as new chemicals (Brinkmann et al., 2017; Calcinai et al., 2017; Mahfur et al., 2022b; Pallela dan Ehrlich, 2016). Sponges contain chemical compounds that have many pharmacological activities, such as antiviral, antifungal, antibacterial, and antimalarial, anti-inflammatory, and neurosuppressive effects (Carroll et al., 2019; Mahfur et al., 2022a; Novanna et al., 2018; Shady et al., 2019; Zhang et al., 2021). Although exploration of sponges as a source of active compounds can interfere with the survival of sponges due to their scarcity, this can be prevented by isolating microorganisms that have a symbiosis with sea sponges, including fungi (Brinkmann et al., 2017; Handayani dan Artasasta, 2017; Hikmawan et al., 2018).
Fungal symbionts in sponges can produce the same compounds as sponges because genetic transfer occurs from the sponge to the fungal body. Fungal symbiosis with sponges can occur in the sponge body cells’ cytoplasm (Casertano et al., 2020; Pejin dan Karaman, 2017; Thacker dan Freeman, 2012). Previous research showed that symbiosis between fungi and sponges has the potential to be a source of compounds that produce antibacterial and cytotoxic effects (Brinkmann et al., 2017; Setyowati et al., 2017). Fungi found in symbiosis with sponges are generally Aspergillus fumigatus, Trichoderma reesei, Aspergillus flavus, Aspergillus nomius, and Penicillium sp. (Handayani and Aminah, 2017; Setyowati et al., 2018).
Rhabdastrella sp. is a sponge that belongs to the Ancorinidae family. It is native to the Indo-West Pacific and tropical oceanic waters of Asia (Manalundong and Uy, 2021; Mioso et al., 2017). Most of the compound groups isolated from Rhabdastrella sponges are triterpenoids which have been shown to have anticancer activity (Lai et al., 2021; Trang et al., 2022). To the best of our knowledge, fungal symbionts in Rhabdastrella sp. have never been studied. However, based on the activity of the sponge isolate, it is possible that the sponge symbiont also has anticancer and antibacterial activity. In this study, the symbiont sponge extract will be tested for antibacterial activity against Escherichia coli, Staphylococcus aureus, and Streptococcus pyogenes.
Recently, antimicrobial activities of medicinal sources have been investigated to ascertain claims of therapeutic properties (Sallau et al., 2016, 2022; Uttu et al., 2021, 2015). The bacteria E. coli, S. aureus, and S. pyogene are types of bacteria that are often found as sources of disease in humans (Affi Kakou et al., 2021; Rustamova et al., 2020; Valentin et al., 2011). They also belong to a type of bacteria that is already resistant to several existing antibacterials (Abdelfattah et al., 2016; Asif, 2016). Therefore, is necessary to look for alternative sources of new antibacterials, one of which can be from Rhabdastrella sp.
This study aims to determine the type of fungus symbiotic with the sponge Rhabdastrella sp., and the compounds and the antibacterial activity in the symbiont fungus.
MATERIALS AND METHODOLOGY
Sampling and cultivation of symbiont fungus
Rhabdastrella sp. samples were collected by scuba diving at a depth of around 12 meters on Gili Layar Island in Sekotong, West Lombok, Indonesia. Sponges were identified at the Marine Natural Product Laboratory at Diponegoro University in Semarang, Indonesia. The sponges Rhabdastrella sp. were sterilized with 70% ethanol for 30 seconds and were then cut into small pieces. Afterward, they were cultivated on Sabouraud’s Dextrose Agar Saline with the addition of 1 ml ciprofloxacin and put in the incubator at a temperature of 25°C for 14 days (Setyowati et al., 2018).
Purification and identification of symbiont gungus
The purification process was carried out based on the morphological appearance of each different colony (Hidayat et al., 2021). This process was carried out by taking one more dominant isolate growing on the media and planting it in new media until a pure isolate was obtained (Setyowati et al., 2017). Identification was carried out by observing the symbiont fungus macroscopically, microscopically, and molecularly based on partial locus genetic analysis in the internal transcribed spacer (ITS). Molecular identification began with DNA extraction from a 7-day-old fungus with a Zymo Research extractor. DNA amplification was carried out using the polymerase chain reaction (PCR) which involved the thermal cycler method with ITS 1 and ITS4 as DNA regions (Setyowati et al., 2018). PCR product quality was seen using electrophoresis on 1% agarose. The results of the PCR visualization were analyzed using PT at 1st Base Laboratories Sdn Bhd, Malaysia. DNA sequences were analyzed for homology using the basic local alignment search tool (www.ncbi.nlm.nih.gov). Phylogenetic trees were analyzed using MEGA 7.0 software, while statistical analysis used the neighbor-joining method with 1,000 bootstrap replication fields (Larasati et al., 2021).
Fermentation and extraction of fungal secondary metabolites
Fermentation was carried out by inoculating pure fungal on an Sabouraud dextrose broth (SDB) medium and incubating it at 37°C for 10 days with a shaker. The fermented symbiont fungus was separated between the supernatant and mycelia. The supernatant obtained was 90 ml and extracted with a 1:1 ratio of ethyl acetate. The ethyl acetate extract obtained was concentrated with a rotary evaporator at 60°C (Setyowati et al., 2017).
Identification of metabolite compound profiles
Identification of secondary metabolites used thin layer chromatography (TLC) with a mobile phase of chloroform: methanol 9:1. The results of the TLC analysis were seen in visible light with UV light 254 and 366 nm and sprayed with a reagent. The identification of compounds used gas chromatography-mass spectra (GC-MS) (Shimadzu GCMS-QP2010SE). Injection in GC-MS with GC-MSQP-2010 ultra running conditions was carried out in a column oven with a temperature of 50°C and an injection with a temperature of 250°C. The carrier gas used was helium with a pressure of 100 kPa and a flow rate of 89.3 ml/minute (Kanjana et al., 2019). The grouping and writing of identification results followed previous research (Ibrahim et al., 2021; Risikat et al., 2022).
Antibacterial activity test
The antibacterial method used well diffusion on Muller Hinton agar media with three bacteria: E. coli ATCC25922, S. aureus ATCC25923, and S. pyogenes ATCC19615. Bacterial suspension used 0.5 McFarland standard whose concentration was 1.5 × 108 CFU/ml. The testing was performed with extract concentrations of 50; 25; 12.5; and 6.25 mg/ml, positive control (ciprofloxacin) and negative control (ethyl acetate). The petri dish was incubated at 37°C for 1 × 24 hours. After incubation, the clear zone was measured from each test (Handayani and Aminah, 2017).
RESULTS AND DISCUSSION
Symbiont fungus cultivation
The results of the cultivation showed the presence of fungal growth. The Rhabdastrella sp. sponges obtained two fungus isolates with white (MIC 6B1) and green (MIC 6B2) characteristics (Fig. 1). The growth of fungi was evident, with several microorganisms collaborated in the filter feeder animal tissue, and in this case, the fungus throve. The symbiotic relationship between the fungi and sponges is mutualistic in which genetic transfer occurs from the body of the sponge to the body of the fungus (Chu et al., 2021; Gaino et al., 2014; Panggabean et al., 2022). Through this relationship, fungi can also produce secondary metabolites that are the same as or similar to the symbiotic sponge (Hong et al., 2022; Nguyen dan Thomas, 2018; Samirana et al., 2021).
 | Figure 1. (a) Sponge symbiont fungus Rhabdastrella sp.: (1) dominant fungi MIC 6B1 and (2) fungi MIC 6BA. (b) Results of MIC 6B1 purification. [Click here to view] |
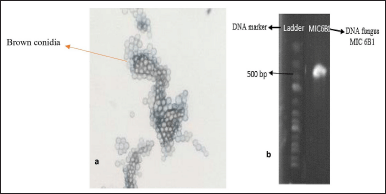 | Figure 2. (a) Results of microscopic identification. (b) Results of visualization of molecular PCR identification. [Click here to view] |
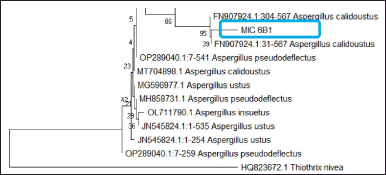 | Figure 3. Phylogentic tree of fungus MIC 6B1 (Aspergillus sp.). [Click here to view] |
Purification and identification of symbiont fungus
The purification process aims to obtain pure MIC 6B1 isolates based on their morphology (Setyowati et al., 2017). The symbiont fungus used for the purification is a fungus that is 14 days old because it is in the logarithmic/exponential phase range where fungal cell division occurs very quickly and constantly (Kjer et al., 2010; Rendowaty et al., 2017). The purification was carried out for the dominant fungus, which was MIC 6B1 with white mycelium (Fig. 1). The purification obtained green fungus with round colonies (Fig. 1). The dominant fungi which initially had white colonies turned green after 14 days of rearing. This can happen because of the process of adaptation to the environment in the growth medium (Höller et al., 2000). The results of the macroscopic identification of the colonies were round, green colonies with white on reverse and had a texture like cotton. The results of microscopic identification also showed the presence of round and pale brown conidia (Fig. 2). The MIC 6B1 fungal strains were identified by DNA sequencing (Fig. 2) and registered in GenBank (accession number OQ451587, www.ncbi.nlm.nih.gov). The isolates showed 93.90% homology and 74% query cover to Aspergillus calidoustus (Fig. 3). A maximum identity value of above 97% indicates that the isolate has a high degree of homology and is likely of the same species. Low percent homology makes the sample classified into Aspergillus sp. The fungus Aspergillus sp. has pale brown radiating conidia consisting of catenate conidia. Previous research conducted by Manuel et al. (2021) found that Aspergillus sp. isolates showed the growth of white cotton colonies which turned green over time.
 | Figure 4. Profile of secondary metabolites from ethyl acetate extract fungus Aspergillus sp. with mobile phase chloroform: methanol (9:1) (a) 254 UV, (b) 366 UV, and (c) Vanilin. As. Sulfat. [Click here to view] |
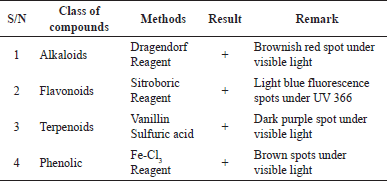 | Table 1. Results of classes of compounds identified in ethyl acetate extract of Aspergillus sp. [Click here to view] |
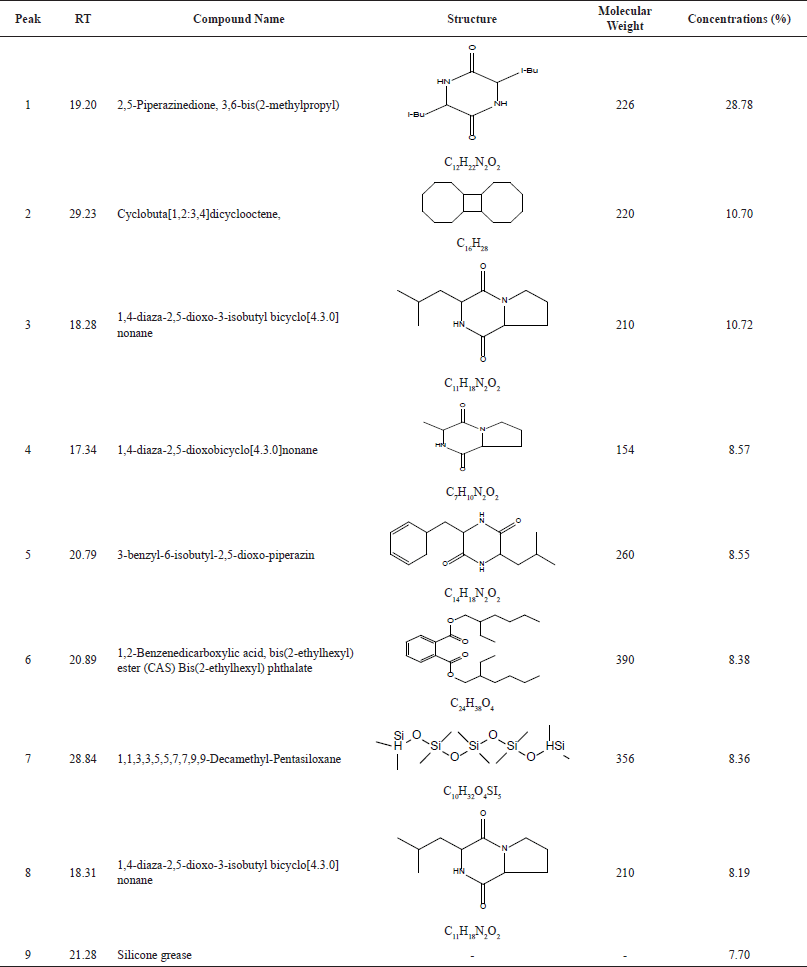 | Table 2. Result of compounds suggested by GC-MS. [Click here to view] |
Fermentation and extraction of fungal secondary metabolites
The fungus Aspergillus sp. from the sponge Rhabdastrella sp. was fermented in liquid media SDB saline to increase the production of secondary metabolites. Fungal growth during the fermentation period occurred from day 7 to day 14 in which there was an increase in secondary metabolites production. Meanwhile, starting on the 15th day, the growth of the fungus relatively decreased (Rendowaty et al., 2017; Zhang et al., 2019). In this study, harvesting was carried out on the 10th day of fermentation following research by Samirana et al. (2021) which revealed that fermentation on the 10th day produced more extracts and maximum secondary metabolites. At the extraction stage, the choice of solvent is an important factor in obtaining the optimal amount of extract. Ethyl acetate was chosen as a solvent because the sample used was a marine natural product containing salt. Therefore, ethyl acetate functions to separate salt and water, so the metabolites produced from the extraction process are a group of pure organic compounds without any other unwanted ingredients (Al-Saleem et al., 2022; Ibrahim et al., 2022; Kumar et al., 2018). The average yield produced in the extraction was 0.077% ± 001% w/v.
Identification of metabolite compound profiles
The TLC profile results with chloroform: methanol 9:1 mobile phase at 254 and 366 nm UV light and sprayed with reagents to determine the presence of secondary metabolites such as alkaloids, flavonoids, terpenoids, and phenols (Kaur et al., 2017) as seen in Figure 4 and Table 1. Research by Tanod et al. (2020) found that the fungus Aspergillus sp. as a sponge symbiont produced alkaloid, terpenoid, and phenolic compounds which produced antibacterial activity. The active compound of the ethyl acetate extract was identified quantitatively using the GC-MS instrument to determine the specific compound content in the ethyl acetate extract of the fungus Aspergillus sp. Table 2 shows the chemical compound of ethyl acetate extract of Aspergillus sp. Of all chemical components found in the ethyl acetat extract of Aspergillus sp., the biggest secondary metabolite compound was 2,5-piperazinedione, 3,6-bis(2-methylpropyl) with 28% composition. These compounds are likely to have contributed to the extract’s antifungal effects (Raut et al., 2021).
Antibacterial activity test
An antibacterial activity test was carried out to determine the potential of the ethyl acetate extract of Aspergillus sp. in inhibiting bacterial growth. The test was carried out on the Gram-negative bacteria E. coli ATCC25922, the Gram-positive bacteria S. aureus ATCC25923, and S. pyogenes ATCC19615. The ethyl acetate extract of Aspergillus sp. could inhibit the growth of the bacteria which was characterized by the formation of clear zones around the wells after 24 hours of incubation (Table 3). The antibacterial activity of Aspergillus sp. extract against E. coli, S. aureus, and S. pyogenes at a concentration of 50 mg/ml showed inhibition zones on bacterial media of 16.77 ± 1.73, 7.00 ± 0.41, and 6.41 ± 0.84 mm. The results of the test showed that the inhibition of ethyl acetate extract on E. coli bacteria was greater compared to S. aureus and S. pyogenes (Fig. 5). The ethyl acetate extract of the fungus Aspergillus sp. is known to contain organic compounds based on the identification of secondary metabolite profiles. These compounds have chemical components that contain nitrogen atoms in their structure, so they are classified as alkaloid compounds that have antibacterial activity (Beesoo et al., 2017; Casertano et al., 2020). The natural alkaloid antibacterial mechanism shows that alkaloids can disrupt bacterial cell membranes, affect DNA function, and inhibit protein synthesis. Thus, natural alkaloids are potentially active against various bacteria, including E. coli, S. aureus, and S. pyogenes (Gao et al., 2019; Liu et al., 2020; Yan et al., 2021). The other compounds in the extract, such as flavonoids, phenols, and terpenoids, also have antibacterial activity. Flavonoids have an antibacterial mechanism of inhibiting nucleic acid synthesis, inhibiting the function of the cytoplasmic membrane, and inhibiting the energy metabolism of bacterial cells (Beesoo et al., 2017; Xie et al., 2014). The antibacterial mechanism of terpenoid compounds is by destroying the bacterial cell membrane which will affect the basic properties of bacteria and as an inhibitor of protein synthesis which can achieve an antibacterial effect by blocking every process of the protein synthesis pathway (Huang et al., 2022). The antibacterial mechanism of phenolic compounds is by modifying the permeability of cell membranes, the changes in various intracellular functions induced by the binding of phenolic compounds to enzymes, and thus, phenolic compounds can increase their antimicrobial activity by supporting their interactions with cell membranes (Bouarab et al., 2019).
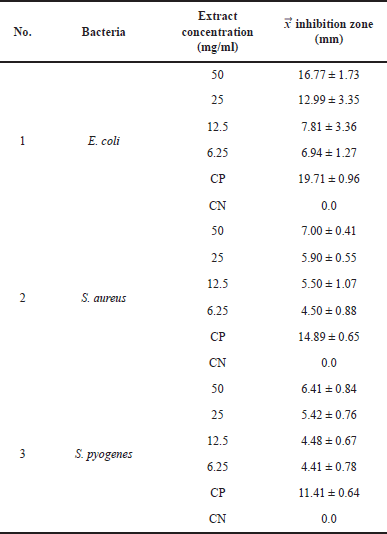 | Table 3. Results of the inhibition zone. [Click here to view] |
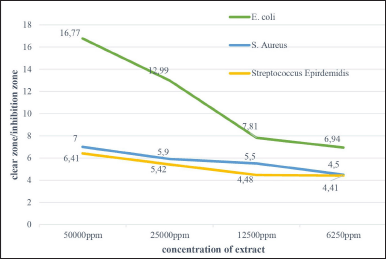 | Figure 5. Antibacterial activity of extract ethyl acetate Aspergillus sp. against pathogenic bacteria. [Click here to view] |
CONCLUSION
The fungus Aspergillus sp. (MIC 6B1) is the dominant type of fungal symbiont associated with the sponge Rhabdastrella sp., which has antibacterial activity and has the potential as a source of chemical compounds for isolation.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelfattah MS, Elmallah MIY, Hawas UW, El-Kassem LA, El-Desoky AH. Isolation and characterization of marine-derived actinomycetes with cytotoxic activity from the Red Sea coast. Asian Pac J Trop Biomed, 2016; 6:651–7.
Affi Kakou B, Benie A, N’Guessan AH, Fernique KK, Guessennd NK, Bekro YA. Phytochemical analysis, antibacterial activity of hydromethanol extracts from stems of Ximenia americana, Côte d’Ivoire species on methicillin-resistant Staphylococcus aureus. Int J Biol Chem Sci, 2016; 14:3429–40.
Al-Saleem MSM, Hassan WHB, El Sayed ZI, Abdel-Aal MM, Abdel-Mageed WM, Abdelsalam E, Sahar A. Metabolic profiling and in vitro assessment of the biological activities of the ethyl acetate extract of Penicillium chrysogenum “endozoic of Cliona sp. Marine sponge” from the Red Sea (Egypt). Mar Drugs, 2022; 20:326.
Asif M. Biologically active compounds from natural and marine natural organisms with antituberculosis, antimalarial, leishmaniasis, trypanosomiasis, anthelmintic, antibacterial, antifungal, antiprotozoal, and antiviral activities. TANG [Humanitas Medicine], 2016; 6:1–19.
Beesoo R, Bhagooli R, Neergheen-Bhujun VS, Li WW, Kagansky A, Bahorun T. Antibacterial and antibiotic potentiating activities of tropical marine sponge extracts. Comp Biochem Physiol C Toxicol Pharmacol, 2017; 196:81–90.
Bouarab CL, Foruquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, Pascal D, Bordes C. Antibacterial properties of polyhnols: charactrization and QSAR (quantitative structure-activity relationship) models. Front Microbiol, 2019; 10:1–22.
Brinkman CM, Marker A, Kurtb?k DI. An overveiw on marin sponge-symbiotic bacteria as unexhausted sourcs for natural product discovery. Diversity, 2017; 9(4):40.
Calcinai B, Bastari A, Bavestrello G, Bertolino M, Horcajadas SB, Pansini M, Makapedua DM, Cerrano C. Demosponge diversity from North Sulawesi, with the description of six new species. ZooKeys, 2017; 680:105–50.
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep, 2019; 36:122–73.
Casertano M, Marialuisa M, Concetta I. The ascidian-derived metabolites with antimicrobial properties. Antibiotic, 2020; 9(8):1–30; doi: 10.3390/antibiotics9080510
Chu R, Li S, Zhu L, Yin Z, Hu D, Liu C, Mo F. A review on co-cultivation of microalgae with filamentous fungi: efficient harvesting, wastewater treatment and biofuel production. Renewable Sustainable Energy Rev, 2021; 139:110689.
Gaino EB, Betti F, Bertolino M, Scoccia F, Bavestrello G. Ultrastructural evidence of a fungus-sponge association in the Ligurian Sea: a case study of Clathrina coriacea (Porifera: Calcarea). Ital J Zool, 2014; 81:501–7.
Gao W, He Y, Li F, Chai C, Zhang J, Guo J, Chen C, Wang J, Zhu H, Hu Z, Zhang Y. Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorg Chem, 2019; 83:98–104.
Handayani D, Aminah I. Antibacterial and cytotoxic activities of ethyl acetate extract of symbiotic fungi from West Sumatra marine sponge Acanthrongylophora ingens. J Appl Pharma Sci, 2017; 7:237–40.
Handayani D, Artasasta MA. Antibacterial and cytotoxic activities screening of symbiotic fungi extract isolated from marine sponge Neopetrosia chaliniformis AR-01. J Appl Pharm Sci, 2017; 7:66–69.
Hidayat, Afif R, Isnawati I. Isolation and characterization of cellulolytic fungi in germetodege: fermented feed made from a mixture of water hyacinth, rice bran, and corn cobs. LenteraBio Berkala Ilmiah Biol, 2021; 10:176–87.
Hikmawan BD, Wahyuono S, dan Setyowati EP. Marine sponge compounds with antiplasmodial properties: focus on in vitro study against Plasmodium falciparum. J Appl Pharm Sci, 2020; 8:001–11.
Holler G, Wright A, Matthée GM, Draeger S, Aust HJ, Barbara S. Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol Res, 2000; 104:1354–65.
Hong LL, Ding YF, Zhang W, Lin HW. Chemical and biological diversity of new natural products from marine sponges: a review (2009–2018). Mar Life Sci Technol, 2022; 4:356–372.
Huang W, Wang Y, Tian W, Cui X, Tu P, Li J, Shi S. Flavonoid antimicrobial agents derived from medicinal plants. Antibiotics, 2022; 10(11):1380.
Ibrahim AH, Attia EZ, Hofny HA, Alsenani F, Zayed A, Rateb ME. Metabolic profiling and biological potential of the marine sponge associated Nocardiopsis sp. UR67 along with docking studies. Nat Prod Res, 2022; 0:1–7.
Ibrahim AH, Uttu AJ, Sallau MS, Iyun ORA. Gas chromatography–mass spectrometry (GC–MS) analysis of ethyl acetate root bark extract of Strychnos innocua (Delile). Beni Suef Univ J Basic Appl Sci, 2021; 10:1–8.
Kanjana M, Kanimozhi G, Udayakumar R, Panneerselvam A. Biomedical and pharmaceutical sciences GC-MS analysis of bioactive compounds of endophytic fungi Chaetomium globosum, Cladosporium tenuissimum and Penicillium janthinellum. J Biomed Pharm Sci, 2019; 2:1–10.
Kaur D, Shalu M, Chinchu GS, Azitha BN, Ashwin K, Mukta SJ, Samir KB. Structure based drug discovery for designing leads for the non-toxic metabolic targets in multi drug resistant mycobacterium tuberculosis. J Transl Med, 2017; 15(1):1–16; doi: 10.1186/s12967-017-1363-9
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5:479–90.
Kumar GS, Noorjahan G, Reddy, Syed KM, Ashwini T, Chary V. Extraction, phytochemical studies and in vitro screening of the leaves and flowers of crossandra infundibuliformis against Mycobacterium tuberculosis. Asian J Res Pharm Sci, 2018; 8:247–52.
Lai KH, Huang ZH, El-Shazly M, Peng BR, Wei WC, dan Su JH. Isomalabaricane triterpenes from the marine sponge Rhabdastrella sp. Mar Drugs, 2021; 19:4–11.
Larasati SJH, Sabdono A, Sibero MT. Identifikasi molekuler kapang asosiasi spons menggunakan metode dna barcoding. J Mar Res, 2021; 10:48–54.
Liu Y, Cui Y, Lu L, Gong Y, Han W, Piao G. Natural indole-containing alkaloids and their antibacterial activities. Arch Pharm, 2020; 353:1–10.
Mahfur M, Setyowati EP, Wahyuono S, Purwantini I. Sponge hyrtios reticulatus: phytochemicals and bioactivities. Res J Pharm Tech, 2022a; 15:1–7.
Mahfur M, Wahyuono S, Purwantini I, Setyowati EP. In vitro antiplasmodial activities of the fractions of hyrtios reticulatus sponge extract. J Appl Pharm Sci, 2022b; 12:114–120.
Manalundong YC, Uy MM. The cytotoxic activities of Philippine marine sponges Stelodoryx Procera Topsent 1904 and Rhabdastrella sp. against colorectal cancer cell line Hct116. Int J Pharm Sci Res, 2021; 12:4708–13.
Manuel QRJ, Pinilla L, Montoya-Castrillón M, Johana Serna-Vasco K, Osorio-Echavarria J, Maria Cardona-Bermúdez L. Isolation and characterization of filamentous fungi from wood and soil samples of “La Lorena”, Sonsón, Antioquia (Colombia) natural reserve. DYNA, 2021; 88:219.
Mioso R, Marante FJT, Bezerra RDS, Borges FVP, Santos BVDO, dan De Laguna IHB. Cytotoxic compounds derived from marine sponges. A review (2010-2012). Molecules, 2017; 22:208.
Nguyen MTHD, Thomas T. Diversity, host-specificity and stability of sponge-associated fungal communities of co-occurring sponges. Peer J, 2018; 2018:1–26.
Novanna M, Ethiraj KR, Kannadasan S. An overview of synthesis of indole alkaloids and biological activities of secondary metabolites isolated from hyrtios species. Mini Rev Med Chem Mini Rev Med Chem, 2018; 19:194–205.
Pallela R, Ehrlich H. Marine sponges: chemicobiological and biomedical applications. Springer, New Delhi, India, pp 1–381, 2016.
Panggabean JA, Adiguna SP, Murniasih T, Rahmawati SI, Bayu A, Putra MY. Structure–activity relationship of cytotoxic natural products from Indonesian marine sponges. Rev Bras Farmacogn, 2022; 32:12–38.
Pejin B, Karaman M. Antitumor natural products of marine-derived fungi. Fungal metabolites. Springer, Cham, Switzerland, pp 1–28, 2017.
Raut LS, Rakh RR, Hamde VS. In vitro biocontrol scenarios of Bacillus amyloliquefaciens sub sp. Amyloliquefaciens strain rls19 in response to Alternaria macrospora, an alternaria leaf spot phytopathogen of BT cotton. J Appl Biol Biotechnol, 2021; 9:75–82.
Rendowaty A, Djamaan A, Handayani D. Optimal cultivation time and antibacterial activity of the ethyl acetate extract of the symbiont fungus Aspergillus unguins (WR8) with Haliclona fascigera. J Sains Farm Klin, 2017; 4:49.
Risikat O, Iyun A, Uttu AJ, Ibrahim AH. GC-MS analysis of methanol extract of Strychnos Innocua ( Delile ) Root Bark. Adv J Chem-Section A, 2022; 5:104–17.
Rustamova N, Gao Y, Zhang Y, Yili A. Biological activity of endophytic fungi from the roots of the medicinal plant Vernonia anthelmintica. Microorganisms, 2020; 8:1–15.
Sallau M, Uttu A, Ibrahim H, Idris A, Dama H. Isolation of a major antimicrobial compound from stem bark of Glossonema boveanum (Decne). Br Biotechnol J, 2016; 16:1–10.
Sallau MS, Uttu AJ, Risikat O, Iyun A, Ibrahim H. Strychnos innocua (Delile): phytochemical and antimicrobial evaluations of its root bark extracts. Adv J Chem, Section B, 2022; 4:17–28.
Samirana PO, Murti YB, Jenie RI, Setyowati EP. Marine sponge-derived fungi: fermentation and cytotoxic activity. J Appl Pharm Sci, 2021; 11:21–39.
Setyowati EP, Pratiwi SUT, Hertiani T, dan Samara PO. Bioactivity of fungi Trichoderma reesei associated with sponges Stylissa flabelliformis collected from National Park West Bali, Indonesia. J Biol Sci, 2017; 17:362–8.
Setyowati EP, Pratiwi SUT, Purwantiningsih, dan Samirana PO. Antimicrobial activity and identification of fungus associated Stylissa flabelliformis sponges collected from Menjangan Island West Bali National Park, Indonesia. Indonesian J Pharm, 2018; 29:66–7.
Shady NH, Fouad MA, Kamel MS, Schirmeister T, Abdelmohsen UR. Natural product repertoire of the genus Amphimedon. Mar Drugs, 2019; 17:19.
Tanod WA, Muliadin, Adel YS, Dewanto DK. Potential marine-derived fungi isolated from sponge in produce new and beneficial compounds. Kauderni, 2020; 2:52–66.
Thacker RW, Freeman CJ. Sponge-microbe symbioses. recent advances and new directions. Adv Mar Biol, 2012; 62:57–111.
Trang DT, Hang DTT, Dung DT, Cuc NT, Yen PH, Huong PTT, Mai NT, Nhiem NX, Tai BH, Van Kiem P. Rhabdastrenones A-D from the sponge Rhabdastrella globostellata. RSC Adv, 2022; 12:10646–52.
Uttu AJ, Sallau MS, Ibrahim AH, Dambatta B, Idris AY. Phytochemical and antimicrobial screening of stem bark extracts from Glossonema boveanum (Decne). J Pharm Phytochem, 2015; 4:86–8.
Uttu AJ, Sallau MS, Risikat O, Iyun A. Recent sdvances in isolation and antimicrobial efficacy of selected strychnos species?: a mini review. J Chem Rev, 2021; 4:1–10.
Valentin BB, Vinod V, Beulah MC. Biopotential of secondary metabolites isolated from marine sponge Dendrilla nigra. Asian Pac J Trop Dis, 2011; 1:299–303.
Xie Y, Yang W, Tang F, Chen X, dan Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem, 2014; 22:132–49.
Yan Y, Li X, Zhang C, Lv L, Gao B, dan Li M. Research progress on antibacterial activities and mechanism of natral alkaloids: a review. Antibiotics, 2021; 10(3):318.
Zhang H, Zhou H, Bai J, Li Y, Yang J, Ma Q, Qu Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf A, 2019; 571:9–16.
Zhang H, Zou J, Yan X, Chen J, Cao X, Wu J, Liu Y, Wang T. Marine-derived macrolides 1990–2020: AN overview of chemical and biological diversity. Mar Drugs, 2021; 19:180.