INTRODUCTION
The failure to conceive children after recurrent trials is experienced as a major social and psychological issue by couples worldwide (Cousineau and Domar, 2007). Infertility on account of the malefactor is reported to be responsible for nearly a third of infertility cases. Infertility rates differ concerning regional components, with the highest rate reported in Africa (Agarwal et al., 2015). Despite the limited knowledge of the reasons behind male infertility, declining sperm count has still considered the most common cause. Other potential risk factors for male infertility were categorized as biological, physiological, genetic, lifestyle, environmental, and sociodemographic reasonings (Okonofua et al., 2022).
Medications’ side effects on the endocrine system leading to hormonal disturbances and decreased sperm count could induce infertility even in males with healthy reproductive organs leading to temporary or, in some cases, permanent failure to conceive children (Mishail et al., 2009).
Metronidazole (MTZ) is a prescribed nitroimidazole antimicrobial for treating microbial infections of organs. It is also used to treat trichomoniasis, a sexually transmitted disease caused by a parasite (Margarita et al., 2022). The medicine MTZ is confirmed to have a negative effect on the spermatogenesis process. Male gonadotropins and testosterone levels would decrease after treatment with the drug (Sohrabi et al., 2007).
Oxidative stress caused by boosting in the reactive oxygen species (ROS) generation or a decreased level of available antioxidants has been considered a causative agent of male infertility. The increase in ROS leads to generating poor sperm quality. Cytoplasmic droplets caused by spermiogenesis abnormalities have been reported to be the primary source of ROS (Gomez et al., 1996; Mannucci et al., 2022). Therefore, the effects exerted by oxidative stress on male reproductive health cannot be neglected.
The reliance on alternative herbal medicines, based on their claimed biological activities or in response to traditional recipes, is considered a common practice among individuals. Recently, published reports have declared the potential activity of herbal remedies against several illnesses, including hyperglycemia, hyperlipidemia, wounds, cytotoxicity, obesity, and dysmenorrhea (Al-Halaseh et al., 2022a, 2022b; Al-Samydai et al., 2022; Awwad et al., 2023; Kattuoa et al., 2022).
Regarding infertility, plant extracts have been proven to enhance semen parameters and overall male fertility profile (Park et al., 2017). Several in vivo and in vitro studies have demonstrated the benefit of herbal remedies in improving fertilization, such as Ginkgo biloba, Glycyrrhiza uralensis, Zingiber officinale, and Silybum marianum (Abedi et al., 2016; Ahmed et al., 2016; Khaki et al., 2009; Wang et al., 2016).
Moringa is a medicinal plant genus that contains flavonoids, quercetin, saponins, triterpenoids/steroids, cyanogens, alkaloids, vitamins, carotene, proteins, and other phenolics compounds in abundance (El-Haddad et al., 2019; Widiastini et al., 2022). There are 46 identified secondary metabolites in Moringa species known for their potent antioxidant properties. Their mechanism of action includes preventing new free radicals, inhibiting chain reactions, protecting cells in the body from free radical attacks, and preventing oxidative damage to most biomolecules (Sreelatha and Padma, 2011). Moringa oleifera was discovered to establish and preserve critical sperm functions, avoid excess sperm-produced superoxide, and conserve the acrosome reaction as well as DNA integrity (Moichela et al., 2021).
Moringa peregrina is a Jordanian species that has colonized areas of high aridity and edaphic conditions in Jordan’s southern regions, especially Wadi Araba (Alrawashdeh et al., 2016). The phenolic and flavonoid content of M. peregrina leaf extract can be connected to its antioxidant activity, in addition to terpenes and other compounds found in the plant’s aerial section (Dehshahri et al., 2012; Hasan et al., 2022).
In this study, we are considering investigating the male infertility prevention effect of the water extract of M. peregrine leaves in an in vivo study, considering its well-known antioxidant activity. In addition, this study aims to justify the self-prescription of Moringa herbal tea by folk as an infertility prophylactic agent used by men.
MATERIAL AND METHODS
Plant material
Fresh M. peregrina leaves were collected from South Jordan. The leaves were washed, dried at shades, and then crushed into reduced size. Identification and authentication of the plant were made, and a voucher sample was deposited in the herbarium. Aqueous and methanolic extracts were prepared separately by maceration, then concentrated under reduced pressure. The aqueous was lyophilized using a Benchtop Manifold Freeze Dryer from Millrock Technology®, UK. On the other hand, the methanol extract was concentrated under reduced pressure using the Rotary-Evaportaor machine.
The extraction yield % was estimated using Equation 1:
Yield of extract (%w/w) = the dried extract weight/the dried leaves weight × 100%. (1)
Phytochemical analysis
Antioxidant activity
Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay according to the specification published by Proestos et al. (2013) with some modifications. The radical scavenging activity was computed using Equation (2) for each sample after measuring the absorbance at λmax = 517 nm.
where is the absorbance of the control sample and is the absorbance of the sample.
The effective concentration required to scavenge 50% of the free radicals (IC50) was calculated and used to compare the antioxidant activity of each extract to each other and to the ascorbic acid standard solution prepared at various concentrations.
Determination of total flavonoids and total phenol content
Total flavonoids were determined using a colorimetric approach based on the production of a complex flavonoid–aluminum complex, as reported by Agbo et al. (2015). The total flavonoid content was calculated as quercetin equivalent.
The Folin–Ciocalteu technique was used to determine total phenol content following the specification reported by Margraf et al. (2015). The total flavonoid content was calculated as gallic acid equivalent.
Animal model
Male albino mice, with an average weight of 28 ± 3.21 g, were obtained from the Animal House, Faculty of Pharmacy, Mutah University. Male mice were moved from the animal house to the animal laboratory a week before the experiment to be acclimatized.
Ethical approval for the study
All procedures were performed in accordance with international regulations for the care and use of laboratory animals. Ethical approval on the study was obtained by the Institutional Review Board at Mutah University, Karak, Jordan, Approval no. 6/2021/2022.
Induction of male infertility
Infertility was gradually induced in male mice after successive daily intraperitoneal doses of MTZ solution at a concentration of 40 mg/kg. The protocol was adopted and modified after several pilot studies based on previous work by Al-Alami et al. (2017). The experimental animals were distributed into four groups of eight mice each as follows: the negative control group (no medication was given), the positive control group (given MTZ), the low-dose treatment group (MTZ doses in combination with the herbal aqueous extract (500 mg/kg), and the high-dose treatment group (MTZ doses in combination the herbal aqueous extract at a concentration of (1,000 mg/kg). The medications were given daily via the intraperitoneal route for 2 weeks. The prophylactic herbal treatments were given 8 hours prior to the MTZ doses. No anesthesia was needed during this experiment. The experimental animals were confirmed to develop infertility when semen analysis showed at least one abnormal result, including azoospermia, inactivity, teratozoospermia, and motility.
Blood samples collection
Blood samples were collected from the heart of the experimental animals under mild anesthesia using diethyl ether. The mouse was constrained on the anatomical plate, a cotton pad filled with the anesthetic agent covering the nostrils. Blood samples were collected in gel tubes and centrifuged for 5 minutes (3,500 rpm). The supernatant was collected and kept at −20°C for further biochemical analysis.
Hormonal analysis
Serum analysis for hormonal profile was undertaken in MedLabs® AlKarak branch. The collected serum was analyzed for the concentration of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone.
Semen collection and analysis
Directly after collecting the blood samples, the vasa deferentia and cauda epididymis were dissected using a sterile surgical blade. The sperm was stripped from the deferens and the epididymis was cut in a test tube containing 1 ml of normal saline. The sperm suspension samples were transferred to the laboratory for further analysis. The freshly extracted samples were analyzed via Spermolyzer®, computed-assisted semen analysis. Physical properties, including volume, pH, color, odor, and liquefaction time, were recorded. In addition, concentration, total sperm count, vitality, and morphology were investigated.
Sperms morphology was considered normal according to the morphology index and teratogenicity index compared to the negative control group. These are concluded after examining the head abnormalities, neck and midpiece abnormalities, excess residual cytoplasm, and tail abnormalities. The visualized sperm were compared to schematic drawings of abnormal sperms by expert laboratorians.
Statistical analysis
Data analysis was performed using Microsoft 13 Excel sheet to calculate the average ± SD for the numerical data. p-values were computed using data analysis and paired two samples for the mean. Significant values were considered at p < 0.05.
RESULTS
Extraction yield
The extraction yields for the aqueous and methanol extracts of M. peregrina leaves were calculated (3.65% ± 0.002% and 4.08% ± 0.025%) % w/w dry weight ± SD.
Phytochemical analysis
Antioxidant effect (DPPH assay) for M. peregrina leaf extracts
Free radical inhibition activity was used to calculate the antioxidant efficiency of serial concentrations of plant extracts and the standard ascorbic acid using the scavenging DPPH assay methods. Figure 1 demonstrates the percentage inhibition of free radical activity for serial concentrations of plant extracts and ascorbic acid.
Quantification of total flavonoid and total phenolic in M. peregrina leaf extracts
According to the findings, comparable levels of total flavonoids and total phenols were found in both the aqueous and methanolic extracts. The average total phenols content for the aqueous and methanolic extracts were found to be 170.64 ± 0.94 and 185.78 ± 4.12 μg /mg plant extract ± SD, respectively. The average total flavonoids content for the aqueous and methanolic extracts were found to be 60.22 ± 1.3 and 57.03 ± 2 μg/mg plant extract ± SD, respectively.
In vivo animal study
Serum sex hormones analysis
The aqueous plant extract showed no effect on the levels of both FSH and LH hormones. The total testosterone levels showed to be lowered using the prepared plant extract with a nonsignificant effect. The concentrations of FSH and LH in all the tested groups were undetectable (?0.3 IU/l). The concentration of total testosterone dropped after inducing toxicity with MTZ to be 1.249 ± 0.376 compared to 4.563 ± 3.46 ng/ml in untreated groups. Treatment with the herbal remedy failed to significantly reverse the effect of MTZ on the total testosterone; the total concentration after treatment with low and high doses was found to be 0.772 ± 0.443 and 0.27 ± 0.006 ng/ml.
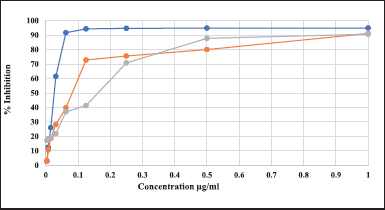 | Figure 1. The % inhibition of free radical activity for serial concentrations of plant extracts and ascorbic acid, using the scavenging DPPH assay methods. The percentage inhibition of ascorbic acid is represented in blue, the aqueous extract in orange, and for the methanol extract in grey color. [Click here to view] |
Semen analysis
No variations were detected in the physical properties of the tested semen samples in all experimental groups. The pH was measured to be six in both the negative and positive control groups, while it was measured to be slightly higher (pH = 7) in the groups treated with low- and high-dose herbal extracts. There was no variation in color and odor to be recorded.
Semen analysis for sperm count, motility, and vitality for all experimental animal groups is shown in Table 1. Results showed that sperm count and motility did not improve when using the plant extract as a prophylactic agent in combination with MTZ. On the other hand, sperm vitality showed an improvement when treated with plant extract as a prophylactic agent in combination with MTZ.
The progressive and the nonprogressive motility patterns in the sperms were color-coded by the Spermolyzer®and then analyzed. Images showing different types of motility were taken, and reports were generated by expert laboratorians.
A dramatic drop in the percentage of viable sperms exhibiting progressive speed patterns was recorded after treatment with MTZ. On the other hand, conjugated prophylactic herbal doses enhanced the motility patterns and increased the percentage of progressive motile viable sperms compared to those treated only with MTZ. Overall, herbal treatment improves not only total motility but also the pattern of motility and the spermatozoa speed track. The percentage of sperms that exhibits different speed rates in the semen sample was analyzed, as shown in Figure 2. The graphs indicate an increase in the percentage of sperms that exhibit higher speed after treatment with the herbal remedy (low and high concentration).
According to the vitality parameter, live and dead sperms were color-coded by the Spermolyzer®, and the generated reports were read by expert laboratorians. The percentage of viable sperms was computed via the computer-assisted system. The curled tail sperms were considered to live, while sperms with straight tails were considered dead. Viable sperms have semipermeable cell membranes which allow the passage of surrounding fluid across the membrane. The accumulated fluid should result in swollen spermatozoa. Therefore, swollen cytoplasm causes the tail to curl. On the other side, dead sperms have damaged membranes that allow free passing of the fluid in and out of the cytoplasm with no accumulation; accordingly, the tail remains straight. Sperms vitality was enhanced after treating the experimental animals with the herbal remedy compared to those treated only with MTZ (Table 1).
Regarding teratogenicity, the percentage of normal to terato sperms was reported by the computer-assisted system and expert laboratorians. Sperms were considered normal when no defect in the head, tail, neck, midpiece, and the excess residual cytoplasm were reported. Teratogenicity is confirmed when the mean of the terato sperms exceeds the reference value of morphological specialties. The scan system reported a tapered and big head as the most observed defect of the examined sperms in the MTZ-treated groups. Expert laboratorians described other defects after closely examining the slides, such as a detached head and abnormal cytoplasmic droplets. The morphology of the scanned sperms showed more abnormalities in the MTZ-treated groups than that of the negative control. A slight reduction in the number of teratogenic sperms after treatment with the herbal remedy was reported by at least two expert laboratorians with no statistical data. Clarifying images are shown in Figure 3.
DISCUSSION
Findings suggest that the M. peregrina leaves aqueous extract as a potential remedy for the reduction of reproductive toxicity in the murine sperm model that was induced by MTZ medication. In addition, improvement in the physical characteristics of the semen but not the hormonal values are interesting findings to investigate. In the interpretation of the obtained results, we compared them with the previously published reports. Previous reports support our findings, where low FSH and LH values do not necessarily negatively affect the fertility window (Ma et al., 2022). Some other reports confirm the negative correlations between FSH value and the morphology of the sperm (Meeker et al., 2007). Although the role of testosterone in male puberty and maturation is not doubtful, some controversial reports support our findings and conclude that decreased serum testosterone levels may not account for sperm motility. There is a lack of correlation between testosterone serum level and sperm’s physical characteristics, in particular motility.
There was good agreement with the result reported by Al-Owaisi et al. (2014), as the antioxidant effects of the aqueous and methanolic extracts had similar IC50. The prepared crude extracts showed to contain a variety of plant phytochemical components, revealed by phytochemical analysis, including phenols and flavonoids, among many others. The methanolic extract was found to contain the highest phenolic content. The total flavonoid content of the two extracts was comparable and relatively equal. Therefore, the antioxidant activity of M. peregrina leaf extract can be linked to its phenolic and flavonoid content, which agrees with several reports on the Moringaceae family (Dehshahri et al., 2012; Senthilkumar et al., 2018).
Various phytocompounds have been previously detected in the study plant extract by our research group (Hasan et al., 2022), demonstrating significant pharmacological actions, including protection effects for male fertility. Of special interest in this study are the flavonoid compounds, including spiraeoside, known to act by regulating abnormal sex hormones, in addition to decreasing oxidative stress and improving fertility and apoptosis (Karna et al., 2019). Rutin is also known to possess a protective role in the context of male reproduction, but further studies remain to be elucidated (Kolarevic et al., 2019; Sun et al., 2017). Chlorogenic acid was also found to increase the sex hormone binding globulin, which plays an important role in male infertile (Esther et al., 2017). Succinic acid was shown to restore reproductive ability in 85.6% of cows (Mikhailova et al., 2021). Benzoic acid was also found to affect male infertility by altering DNA integrity in germ cells (Rabiu et al., 2021). Similarly, 2,5-dihydroxybenzoic acid was found to lower seminal plasma lipid peroxidation levels but increase sperm nuclear DNA fragmentation (Camargo et al., 2014). In addition, 4-hydroxybenzoic acid was found to play an important role in the treatment of infertility among bovines via mitochondria-directed antioxidant effect when it was studied on bovine sperm function and embryo production (Santos et al., 2022). p-Coumaric acid was found to have a protective effect on sperm parameters and apoptosis. It can also protect from ethanol-induced testicular dysfunction and improve fertility (Nishi et al., 2018). Caffeic acid was found to have an inhibitory effect on testicular damage induced by Cd, where this effect is shared by the most possible pathway, scavenging ROS extensively (El-Refaei and Abdallah, 2021).
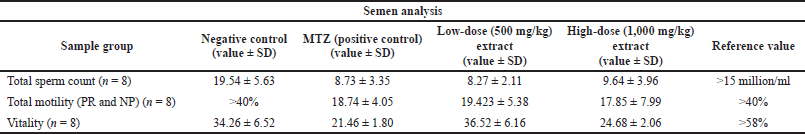 | Table 1. Semen analysis for sperm count, motility, and vitality for all experimental animal groups. [Click here to view] |
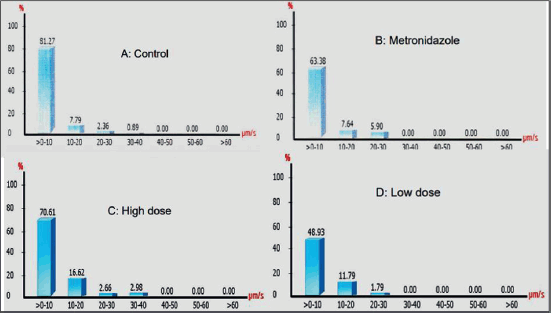 | Figure 2. Sperms speed tracking. (A) control; (B) MTZ-treated group; (C) high-dose extract-treated group (1,000 mg/kg); and (D) low-dose extract-treated group (500 mg/kg). [Click here to view] |
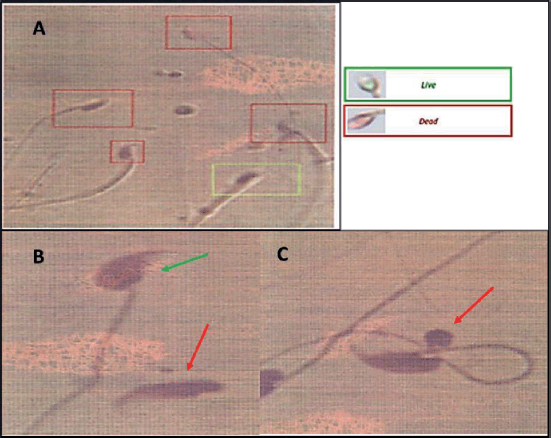 | Figure 3. Sperms viability and morphology: (A) live sperm is surrounded by a green rectangle, while dead sperm are surrounded by red rectangles. (B) a green arrow points to a normal sperm and a red arrow points to a sperm with a detached head. (C) a red arrow points to cytoplasmic droplet abnormality. [Click here to view] |
Therefore, the findings of the present study would be of special interest among researchers in the failure of herbal medicine, infertility, and pharmaceutical nutrition that are seeking the power of natural medicine and the discovery of new treatments, which would be utilized to improve human health.
CONCLUSION
The proposed activity M. peregrina was largely referring to the antioxidant properties of the plant extract, which contains numerous valuable constituents such as polyphenols and flavonoids. Many detected compounds were previously reported to have protective and treatment roles on male infertility. These findings were important when treating male infertility as directed toward semen’s physical properties rather than hormonal imbalances. It would also suggest the investigation of the combined effect of the study plant extract with other conventional medications that are widely used to treat male infertility, with the aim of getting the benefit of potentially additive effects. This approach of alternative treatments is well recognized among traditional and herbal medicine healers.
ACKNOWLEDGMENT
The authors are thankful to the Faculty of Postgraduate Studies and the Faculty of Pharmacy at Al-Ahliyya Amman University for the grant support to fund this project.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that no conflicts of interest are associated with this work.
ETHICAL APPROVAL
All procedures were performed in accordance with international regulations for the care and use of laboratory animals. Ethical approval on the study was obtained by the Institutional Review Board at Mutah University, Karak, Jordan, Approval no. 6/2021/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abedi H, Jahromi HK, Hashemi SMA, Jashni HK, Jahromi ZK, Pourahmadi M. The effect of silymarin on spermatogenesis process in rats. Int J Med Health Res, 2016; 5(6):146–50.
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol, 2015; 13(1):1–9; https://doi.org/10.1186/s12958-015-0032-1.
Agbo MO, Uzor PF, Nneji UNA, Odurukwe CUE, Ogbatue UB, Mbaoji EC. Antioxidant, total phenolic and flavonoid content of selected Nigerian medicinal plants. Dhaka Univ J Pharm Sci, 2015; 14(1):35–41; https://doi.org/10.3329/dujps.v14i1.23733
Ahmed AI, Lasheen NN, El-Zawahry KM. Ginkgo biloba ameliorates subfertility induced by testicular ischemia/reperfusion injury in adult wistar rats: a possible new mitochondrial mechanism. Oxid Med Cell Longev, 2016; 2016; https://doi.org/10.1155/2016/6959274
Al-Alami ZM, Shraideh ZA, Taha MO. Rosmarinic acid reverses the effects of metronidazole-induced infertility in male albino rats. Reprod Fertil Dev REP, 2017; 29(10):1910–20; https://doi.org/10.1071/RD16174
Al-Halaseh LK, Al-Jawabri NA, Al-Btoush H, Al-Suhaimat R, Majali S, Hajleh MN, Al-Samydai A, Ahmed MA. In vivo investigation of the potential hypoglycemic activity of Pennisetum setaceum: justification of the traditional use among Jordanians. Res J Pharm Technol, 2022a; 15(7):3185–9.
Al-Halaseh LK, Hajleh MN, Al-Samydai AM. Weight reduction in response to unauthorized treatment in a poorly controlled obese woman. Biomedicine, 2022b; 42(2):410–2.
Al-Owaisi M, Al-Hadiwi N, Khan SA. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) fiori leaves. Asian Pac J Trop Biomed, 2014; 4(12):964–70.
Alrawashdeh NQ, AlRawashdeh IM, AlZghoul TM. Amino acids and mineral composition analysis of Moringa peregrina forssk (fiori) in Jordan. ARPN J Agric Biol Sci, 2016; 11(5):175–9.
Al-Samydai A, Qaraleh MA, Alshaer W, Al-Halaseh LK, Issa R, Alshaikh F, Abu-Rumman A, Al-Ali H, Al-Dujaili EA. Preparation, characterization, wound healing, and cytotoxicity assay of PEGylated nanophytosomes loaded with 6-gingerol. Nutrients, 2022; 14(23):5170.
Awwad S, Abu-Zaiton A, Issa R, Said R, Sundookah A, Habash M, Mohammad B, Abu-Samak M. The effect of excessive coffee consumption, in relation to diterpenes levels of medium-roasted coffee, on non-high-density lipoprotein cholesterol level in healthy men. Pharmacia, 2023; 70(1):49–59.
Camargo M, Intasqui P, Bruna de Lima C, Montani DA, Nichi M, Pilau EJ, Gozzo FC, Lo Turco EG, Bertolla RP. Maldi-tof fingerprinting of seminal plasma lipids in the study of human male infertility. Lipids, 2014; 49(9):943–56; https://doi.org/10.1007/s11745-014-3922-7
Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol, 2007; 21(2):293–308.
Dehshahri SH, Wink M, Afsharypuor S, Asghari G, Mohagheghzadeh A. Antioxidant activity of methanolic leaf extract of Moringa peregrina (Forssk.) fiori. J Pharm Sci, 2012; 7(2):111.
El-Haddad AE, El-Deeb EM, Koheil MA, El-Khalik SMA, El-Hefnawy HM. Nitrogenous phytoconstituents of genus Moringa: spectrophotometrical and pharmacological characteristics. Med Chem Res, 2019; 28(10):1591–600; https://doi.org/10.1007/s00044-019-02403-8
El-Refaei MF, Abdallah EA. Protective effects of caffeic acid phenethyl ester on cadmium-induced testicular injury: a crucial role of antioxidant enzymes in male mice infertility. Heliyon, 2021; 7(5):e06965; https://doi.org/10.1016/j.heliyon.2021.e06965
Esther MYJ, Subramaniyan V, Kumar AP, Subramanian M, Palani M. Molecular docking, ADMET analysis and dynamics approach to potent natural inhibitors against sex hormone binding globulin in male infertility. Pharmacogn J, 2017; 9(6s); doi: 10.5530/pj.2017.6s.155
Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl, 1996; 17(3):276–87; https://doi.org/10.1002/j.1939-4640.1996.tb01783.x
Hasan A, Issa R, Al-Halaseh L, Abbas MA, Al-Jawabri N, Al-Suhaimat R. Investigation of the nephroprotective activity of Moringa peregrina leaves aqueous extract in mice. Pharmacia, 2022; 69(4):1095–102.
Karna KK, Choi BR, You JH, Shin YS, Cui WS, Lee SW, Kim JH, Kim CY, Kim HK, Park JK. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement Altern Med, 2019; 19(1):1–13; https://doi.org/10.1186/s12906-019-2736-9
Kattuoa M, Issa R, Beitawi S. Commonly used herbal remedies for the treatment of primary dysmenorrhea and heavy menstrual bleeding by herbalists in Amman, Jordan: a cross-sectional survey. Jordan J Pharm Sci, 2020; 13(4):467–83.
Khaki A, Fathiazad F, Nouri M, Khaki AA, Ozanci CC, Ghafari-Novin M, Hamadeh M. The effects of ginger on spermatogenesis and sperm parameters of rat. Int J Reprod Bio Med, 2009; 7:7–12; http://ijrm.ssu.ac.ir/article-1-135-en.html
Kolarevic A, Pavlovic A, Djordjevic A, Lazarevic J, Savic S, Kocic G, Anderluh M, Smelcerovic A. Rutin as deoxyribonuclease I inhibitor. Chem Biodivers, 2019; 16(5):e1900069; https://doi.org/10.1002/cbdv.201900069
Ma Y, Awe O, Radovick S, Yang X, Divall S, Wolfe A, Wu S. Lower FSH with normal fertility in male mice lacking gonadotroph kisspeptin receptor. Front Physiol, 2022; 26(13):868593; doi: 10.3389/fphys.2022.868593.
Mannucci A, Argento FR, Fini E, Coccia ME, Taddei N, Becatti M, Fiorillo C. The impact of oxidative stress in male infertility. Front Mol Biosci, 2022; 8:1344.
Margarita V, Cao LC, Bailey NP, Ngoc THT, Ngo TMC, Nu PAT, Diaz N, Dessì D, Hirt RP, Fiori PL, Rappelli P. Effect of the symbiosis with Mycoplasma hominis and candidatus Mycoplasma girerdii on Trichomonas vaginalis metronidazole susceptibility. Antibiotics, 2022; 11(6):812; https://doi.org/10.3390/antibiotics11060812
Margraf T, Karnopp AR, Rosso ND, Granato D. Comparison between folin-ciocalteu and Prussian blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J Food Sci Technol, 2015; 80(11):C2397–403; https://doi.org/10.1111/1750-3841.13077
Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl, 2007; 28(3):397–406; https://doi.org/10.2164/jandrol.106.001545
Mikhailova I, Leshchenko T, Evlevsky A, Ortyakova I. The causes of alimentary infertility of highly productive cows and prevention measures. In: BIO Web of Conferences, EDP Sciences, 2021, Vol. 37, p 00081. https://doi.org/10.1051/bioconf/20213700081
Mishail A, Marshall S, Schulsinger D, Sheynkin Y. Impact of a second semen analysis on a treatment decision making in the infertile man with varicocele. Fertil Steril, 2009; 91(5):1809–11; https://doi.org/10.1016/j.fertnstert.2008.01.100
Moichela FT, Adefolaju GA, Henkel RR, Opuwari CS. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in human spermatozoa in vitro. Andrologia, 2021; 53(1):e13903; https://doi.org/10.1111/and.13903
Nishi K, Ramakrishnan S, Gunasekaran VP, Parkash K, Ramakrishnan A, Vijayakumar N, Ganeshan M. Protective effects of p coumaric acid on ethanol induced male reproductive toxicity. Life Sci, 2018; 209:1–8; https://doi.org/10.1016/j.lfs.2018.07.045
Okonofua FE, Ntoimo LF, Omonkhua A, Ayodeji O, Olafusi C, Unuabonah E, Ohenhen V. Causes and risk factors for male infertility: a scoping review of published studies. Int J Gen Med, 2022; 15:5985–97.
Park HJ, Koo YK, Park MJ, Hwang YK, Hwang SY, Park NC. Restoration of spermatogenesis using a new combined herbal formula of epimedium koreanum nakai and Angelica gigas nakai in a luteinizing hormone-releasing hormone agonist-induced rat model of male infertility. World J Mens Health, 2017; 35(3):163–9; https://doi.org/10.5534/wjmh.17011
Proestos C, Lytoudi K, Mavromelanidou OK, Zoumpoulakis P, Sinanoglou VJ. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants, 2013; 2:11–22.
Rabiu S, Abubakar MG, Sahabi DM, Makusidi MA, Dandare A, Bello JH. Benzoic acid based beverages: health implications. Asian Food Sci J, 2021; 20(4):93–105.
Santos JC, Marques CC, Baptista MC, Pimenta J, Teixeira J, Montezinho L, Cagide F, Borges F, Oliveira PJ, Pereira RMLN. Effect of a novel hydroxybenzoic acid based mitochondria directed antioxidant molecule on bovine sperm function and embryo production. Animals, 2022; 12(7):804; https://doi.org/10.3390/ani12070804
Senthilkumar A, Karuvantevida N, Rastrelli L, Kurup SS, Cheruth AJ. Traditional uses, pharmacological efficacy, and phytochemistry of Moringa peregrina (Forssk.) fiori.—a review. Front Pharmacol, 2018; 9:465. https://doi.org/10.3389/fphar.2018.00465.
Sohrabi D, Alipour M, Melati A. Effect of metronidazole on spermatogenesis, plasma gonadotrophins and testosterone in male rats. Iran J Pharm Res, 2007; 6(4):279–83. ISSN: 1735-0328 (Print)1726-6890 (Online)
Sreelatha S, Padma PR. Modulatory effects of Moringa oleifera extracts against hydrogen peroxide-induced cytotoxicity and oxidative damage. Hum Exp Toxicol, 2011; 30(9):1359–68; https://doi.org/10.1177/0960327110391385
Sun J, Wang H, Liu B, Shi W, Shi J, Zhang Z, Xing J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed Pharmacother, 2017; 88:500–6; https://doi.org/10.1016/j.biopha.2017.01.066
Wang C, Jin Y, Jin Y. Promoting effect of licorice extract on spermatogonial proliferation and spermatocytes differentiation of neonatal mice in vitro. Cell Dev Biol Anim, 2016; 52:149–55; https://doi.org/10.1007/s11626-015-9966-z
Widiastini LP, Karuniadi IAM, Karmaya INM, Widhiantara IG. The potential of a balinese traditional medicine kelor leaves (Moringa oleifera) for male infertility treatment: a mini. Indian J Forensic Med Toxicol, 2022; 16(1):735.