INTRODUCTION
Coffee is an important traded agricultural commodity and has entered the daily routine of many people around the world for its unique sensory properties (Lopes et al., 2021). The species of the genus Coffea (Rubiaceae) have been extensively used as medicines for centuries owing to the presence of various bioactive substances. Among various Coffea species, commercially and economically significant species are C. arabica L. (Arabica coffee) and C. canephora Pierre ex A. Froehner (Robusta coffee). The third important commercialized coffee species is C. liberica Hiern (Angeloni et al., 2021; Craig et al., 2018). Coffee is chiefly cultivated in equatorial or subtropical regions. In particular, C. arabica cultivars are cultivated in the high mountains above 1,000 m with temperatures between 5°C and 15°C, whereas C. canephora species are cultivated in the low plains (Folmer, 2017).
In Korea, coffee is one of the most consumed beverages next to the water. The coffee industry is rapidly increasing, and as of 2018, the number of coffee shops in Korea reached 66,000, and it is known that the sales amount reached around 4.8 trillion Korean won (Kim, 2019). The average coffee consumption per adult is 353 cups per year, which is about 2.7 times higher than the world’s average consumption (Korea Coffee Association, 2022; Song, 2020). In the rapidly changing coffee market environment, the coffee industry is continuously developing new high-quality products to satisfy the intense competition in the coffee market and the changing needs of consumers. Therefore, the necessity for improving the flavor and fragrance of coffee beans has been increased to produce high-quality coffee (Angeloni et al., 2021).
The complexity of the aroma of coffee is directly associated with its volatile composition. In general, coffee beans produce different aromas under identical roasting or brewing conditions due to coffee bean cultivars, geographical origins, and postharvest treatments (Lopez-Galilea et al., 2006; Pereira et al., 2019; Rao et al., 2020). Roasting and brewing coffee beans produce the characteristic aroma of the coffee, and these methods play a major role in the price and quality of coffee (Lopes et al., 2021). Further, coffee is known to contain >1,000 chemical components that contribute to the flavor and fragrance of coffee. These chemical components affect the sensorial perceptions of the oral and nasal mucosa (Angeloni et al., 2021; Kreuml et al., 2013).
In coffee beans, the roasting process is highly responsible for the development of flavor. The development of the aroma of coffee is a complex and time-temperature-dependent process (Farah et al., 2006). Numerous chemical reactions and modifications occur during the coffee roasting process (Giacalone et al., 2019; Yang et al., 2016). Maillard reaction and Strecker reaction and protein degradation are important chemical modifications in roasted coffee beans (Kreuml et al., 2013). The gas chromatography and mass spectrometry (GC-MS) technique is an excellent approach for detecting complex mixtures of aroma components in different cultivars of coffee (Lopes et al., 2021). The GC-MS-based analyzes allow the identification of different chemical families in roasted coffee bean cultivars, mainly pyrazines, pyridines, pyrroles, furans, and others (Ongo et al., 2020; Marek et al., 2020; Ryan et al., 2004). There are numerous coffee cultivars in the domestic market, but the appearance of different cultivars of roasted coffee beans is similar. Hence, the determination of the aromatic profile of roasted coffee beans is an appropriate way to clarify the cultivars of coffee. With this background, this study aimed to compare the aromatic profiles of coffee beans of Brazilian and Ethiopian varieties according to different roasting times by solid-phase microextraction (SPME)/GC-MS analysis. In addition, the aromatic profiles of three popular coffee bean cultivars in Korea, such as Colombia Supremo, Ethiopia 210, and Indonesia Mandheling, were studied.
MATERIALS AND METHODS
Collection of plant samples
Green coffee beans of different cultivars such as Brazilian, Ethiopian, Colombia Supremo, Ethiopia 210, and Indonesia Mandheling coffee beans were purchased in the local markets in Korea. Ten batches of each cultivar were purchased from the market. In these, 20 green coffee beans, identical in size, were randomly selected for each roasting condition. The green coffee beans were pale green with a milky smell. The coffee roasting process was carried out in triplicate.
Coffee roasting
The roasting process for the Brazilian and Ethiopian coffee beans was set at 210°C with different roasting times such as 11, 12, 13, and 14 minutes. In the case of Colombia Supremo, Ethiopia 210, and Indonesia Mandheling cultivars, coffee beans were roasted at 210°C for 13 minutes. The coffee beans were roasted using a fluidized bed roaster. Each coffee bean cultivar was roasted separately and powdered. The ground coffee beans were kept at −20°C prior to SPME-GC-MS analysis.
Color determination
The pulverized roasted bean samples were measured using a spectrophotometer (CM-3600A, Konica Minolta, Japan) and were expressed as Hunter’s values, L* (lightness), a* (redness), and b* (yellowness). The color determination was carried out according to the procedure of Dong et al. (2017).
Determination of aromatic profile by SPME/GC-MS analysis
Aromatic components of roasted coffee cultivars were analyzed using SPME-GC-MS based on the method of Caporaso et al. (2018). Ground roasted coffee samples, exactly 100 mg, were placed in 5 ml vials. These samples were allowed to equilibrate for 10 minutes at the constant temperature of 40°C, followed by 20 minutes of fiber exposure using a 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fiber (Supelco, Bellefonte, PA, USA). Volatile components were desorbed by inserting the fiber for 10 minutes into the injection port of the gas chromatograph (GC), kept at 250°C. A Bruker CP-3800 model GC with Varian 1200 Mass spectrometry was used to analyze volatile components. GC/MS column used was VF-5MS (low polarity; 30 m * 0.25 mm * 0.25 μm), and helium was used as a carrier gas at a 1.2 ml/minute flow rate. For GC conditions, the injection volume was 1 μl, the split ratio was 10:1, the inlet temperature was 250°C, and the oven temperature was programmed from 50°C to 250°C at 3°C/minute. The ion source (detector) and interface temperatures were 270°C and 250°C, respectively. For MS analysis, the spectrum was taken at 70 eV with a mass scan range of 50–400 m/z. The detection of volatile components in different coffee bean cultivars was done by mass spectra data (NIST library version 3.0). The results of volatile compositions were expressed as the relative percentage of each compound peak area to the total GC-MS peak area. Each analysis was carried out in triplicate.
RESULTS
Color parameters, such as L* (lightness), a* (redness), and b* (yellowness), were determined to observe the effect of different roasting times on coffee beans (Table 1). The results indicated that the roasting time significantly affected the color parameters of coffee beans. The L*, a*, and b* values of Ethiopian coffee beans (40.86 ± 0.02–36.53 ± 0.04, 6.53 ± 0.04–2.12 ± 0.05, 7.03 ± 0.06–1.13 ± 0.07, respectively) were markedly decreased when increasing roasting time from 11 to 14 minutes. In contrast, the L*, a*, and b* values of Brazilian coffee beans increased during 12 minutes of roasting time and decreased afterward.
The SPME/GC-MS analyses revealed the identification of 234 aromatic components in Brazilian and Ethiopian coffee beans in relation to different roasting times (Table 2). In particular, 2-furanmethanol was the predominant component in all the roasted coffee beans (13.65%–19.30%), followed by pyridine (3.69%–18.7%), 2-furanmethanol, acetate (2.89%–12.00%), 5-methyl-2-furancarboxaldehyde (2.30%–11.60%), methylpyrazine (4.53%–9.42%), and ethylpyrazine (2.06%–3.73%).
It was observed that roasting times significantly affect the aromatic profiles of Brazilian and Ethiopian coffee beans. The concentration of pyridine and 2-furanmethanol, acetate was markedly decreased when increasing roasting times from 11 to 14 minutes in both Brazilian (18.70%–4.01% and 12.00%–2.89%, respectively) and Ethiopian coffee beans (15.30%–3.69% and 10.70%–3.38%, respectively). On the other hand, the concentration of 5-methyl-2-furancarboxaldehyde was increased when increasing roasting time (2.30%–9.40% in Brazilian beans and 3.80%–11.60% in Ethiopian beans). Moreover, some aromatic components were detected during 11 minutes of roasting time, and these components were not detected with subsequent increments of roasting time. For example, trimethylamine, 1,2,3,6-tetrahydro-1-methyl- pyridine, ethane-1,1-diol dipropanoate, 2,6-dimethyl-3-pyridinamine, 3-heptyn-1-ol, and hexadecamethyl-heptasiloxane were detected only during 11 minutes of roasting time in Brazilian coffee beans. In the case of Ethiopian coffee beans, diethylpentamide, β-pinene, 4-methylphenylhydrazine, 1,2,3,6-tetrahydro-1-methyl- pyridine, 1-methyl-1H-pyrrole, and vinylfuran were detected only during 11 minutes of roasting time.
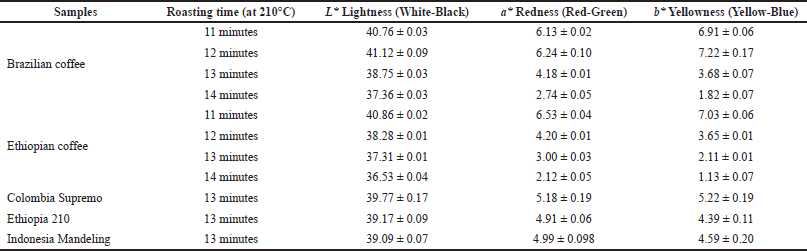 | Table 1. Color analysis of different roasted coffee cultivars. [Click here to view] |
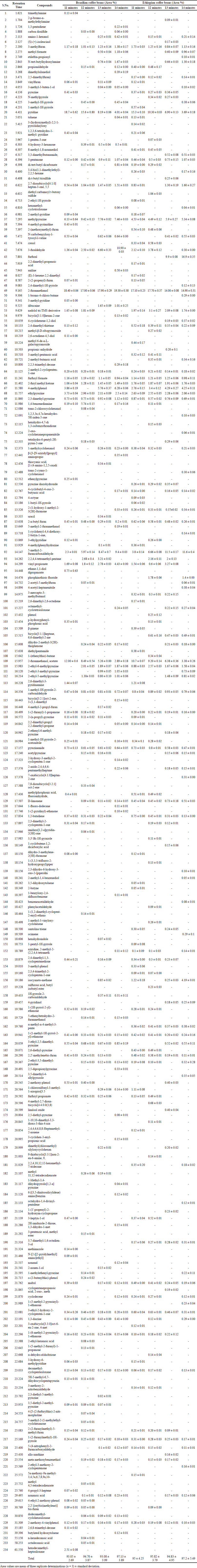 | Table 2. Aromatic profile of roasted coffee beans of Brazilian and Ethiopian varieties according to different roasting times at 210°C. [Click here to view] |
Conversely, some aromatic components were detected only during the highest roasting time (14 minutes) for coffee to caramelize. For example, 5-methyl-2-furanmethanol, 2-(1-hydroxy-1-methyl-2-3(2H)-furanone, 4-cyclobutyl-4-oxo-2-butynoic acid, 2,2,3-trimethyl-decane, octamethyl-cyclotetrasiloxane, 2-azido-2,4,4,6,6-pentamethylheptane, and butylated hydroxytoluene were detected in Brazilian coffee beans. In contrast, 2-ethyl-3-methoxy-2-cyclopentenone, 1-methylethenyl-pyrazine, 3,5-dimethyl-4-allylpyrazole, ocimene, 7-oxabicyclo[4.1.0]heptan-2-one, trans-2-cyano-1-cyclohexanol, 1-bromo-4-chloro-butane, and 3-methyl-1H-pyrrole were detected in Ethiopian coffee beans. In the case of Colombia Supremo, Ethiopia 210, and Indonesia Mandheling coffee beans, 105 aromatic components were identified (Table 3). The SPME/GC-MS results revealed that Colombia Supremo, Ethiopia 210, and Indonesia Mandheling cultivars registered almost identical aromatic profiles with similar major components. Of 105 aromatic components, 60 were detected in these three coffee cultivars of roasted coffee beans. However, there was a significant variation in the composition of minutes or components. In particular, 16 components were identified only in Ethiopia 210, and five components in each Colombia Supremo and Indonesia Mandheling.
Similar to Brazilian and Ethiopian roasted coffee beans, 2-furanmethanol was the most abundant component in Colombia Supremo (15.70%), Ethiopia 210 (14.73%), and Indonesia Mandheling (13.65%) coffee beans roasted at 210°C for 13 minutes. In addition, 5-methyl-2-furancarboxaldehyde (10.89%), furfural (9.16%), methylpyrazine (7.55%), 2-furanmethanol, acetate (6.23%), 4-methylphenol (6.08%), and pyridine (4.43%) were major components in the Colombia Supremo cultivar. 5-Methyl-2-furancarboxaldehyde (10.87%), furfural (10.08%), methylpyrazine (8.09%), pyridine (5.50%), 2,4-methylphenol (5.27%), and 2-furanmethanol, acetate (4.77%) were major components in the Ethiopia 210 cultivar. In the case of Indonesia Mandheling cultivar, 5-methyl-2-furancarboxaldehyde, (10.08%), methylpyrazine, (9.12%), 4-methylphenol (7.18%), furfural (6.69%), 2-furanmethanol, acetate (6.40%), pyridine (5.55%), and 2,2,4,4-tetramethylpentane (4.76%) were major components in this cultivar.
DISCUSSION
In the coffee roasting process, the type of roaster, temperature, and roasting time play a crucial role in the taste of brewed coffee (Angeloni et al., 2018). Further, there is a correlation between coffee quality and chemical constituents (Gancarz et al., 2021). The Brazilian and Ethiopian coffee beans were roasted at 210°C for different roasting times, such as 11, 12, 13, and 14 minutes. Further, Colombia Supremo, Ethiopia 210, and Indonesia Mandheling coffee beans were roasted at 210°C for 13 minutes before SPME/GC-MS analysis. The roasting time markedly affected the color parameters of coffee beans. The darker shade of coffee beans is specified by the lower values of lightness (L*) and higher values of redness (a*) and yellowness (b*) (Kulapichitr et al., 2022). A DVB/Carboxen/PDMS SPME fiber was used to extract volatile components from roasted coffee beans. In previous studies, this type of SMPE fiber was reported to be the most efficient one for analyzing coffee bean aroma (Risticevic et al., 2008; Caporaso et al., 2018).
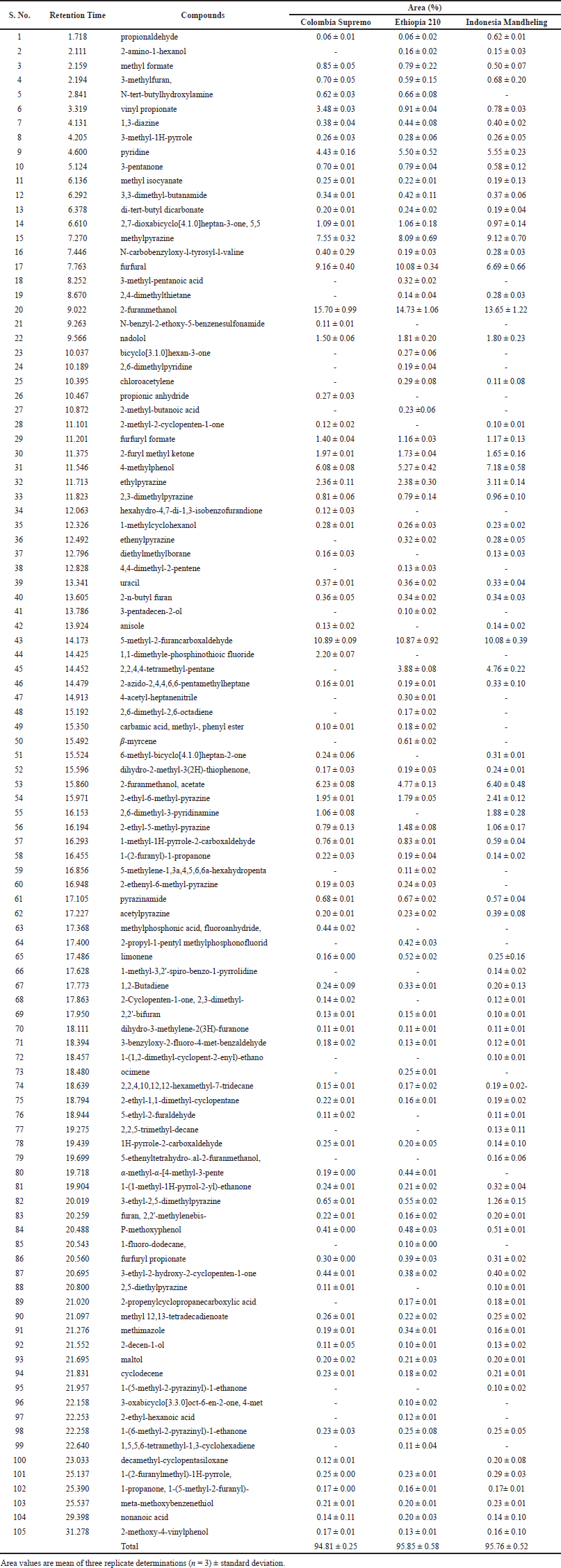 | Table 3. Aromatic profile of different coffee cultivars. [Click here to view] |
Previous studies stated that the roasting temperature (160°C–200°C) and roasting time (4–12 minutes) play a major role in preparing specialty coffee (Fassio et al., 2017; Tolessa et al., 2016; Tugnolo et al., 2019). Different coffee flavors are developed during roasting by nonenzymatic browning reactions, including the Maillard reaction and caramelization (Münchow et al., 2020). There was a highly positive correlation among pyrazine compounds due to the Maillard reaction during the roasting process. Amino acids and reducing sugars were important flavor precursors in the Maillard reaction (Caporaso et al., 2018). In the roasting process, 2,3-butanedione has been exhibited to be stable at high temperatures. Baggenstoss et al. (2008) reported that concentrations of 2,3-butanedione and pentanedione were found to be higher during fast roasts when compared to slower roasts. It is understood that the time-temperature relationship of the roasting process highly influences the aromatic profiles of coffee. Further, regulation of roasting time and temperature is required to obtain a distinctive flavor profile.
Previously, Caporaso et al. (2018) determined the volatile composition of different batches of roasted coffees of Arabica and Robusta using SPME/GC-MS. In their study, Arabica and Robusta species (25 batches) were collected from 13 countries. The coffee beans were roasted at 210°C for 3 minutes. A significant variation of 50 volatile components was observed in roasted coffee within batches. The authors found that 2-furanmethanol, acetic acid, and 2-methyl pyrazine were the major components. Further, 2-furanmethanol acetic acid was highly distributed in Arabica coffee beans compared to Robusta coffee beans. Therefore, Brazilian coffee, Ethiopian coffee, Colombia Supremo, Ethiopia 210, and Indonesia Mandheling cultivars may be derived from Arabica species because 2-furanmethanol was the most abundant component in these cultivars. Zou et al. (2022) compared the volatile composition of regular and decaffeinated coffee by HS-SPME-GC × GC-ToFMS, and the authors found that the regular coffee chiefly contained pyrazine-derived components, whereas the decaffeinated coffee contained mainly furan-derived components.
A recent study identified 390 aromatic components from 17 chemical families in a single-dose espresso capsule obtained from eight commercial coffee samples. In these, 100 components were detected for the first time in roasted coffee or brews (Lopes et al., 2021). The detected chemical components in the study were also identified in various coffee beans (Akiyama et al., 2008; Mahmud et al., 2020; Toledo et al., 2016). Chemical families such as acids, alcohols, aldehydes, and esters are generally linked with the production of industrial coffee during fermentation (Ruta and Farcasanu, 2021). Furans, ketones, pyrazines, pyridines, and pyrroles are mainly associated with roasting processes. In addition, green coffee beans contain certain types of terpene components (Akiyama et al., 2008; Gonzalez-Rios et al., 2007). These volatile components of coffee are mainly responsible for its final flavor and aroma.
Previous studies reported that the time-temperature profile of the roasting process showed a significant effect on coffee’s aromatic composition (Baggenstoss et al., 2008; Franca et al., 2009). In this study, the coffee beans were roasted at 210°C for 11–14 minutes. The roasting of coffee beans highly influences the flavor and aroma of coffee. In addition, the roasting time of coffee beans significantly affects the aromatic profile of coffee cultivars. The flavor and aroma of coffee beans mainly depend on the roasting time and temperature (Kreuml et al., 2013). In particular, the acid types of components in coffee beans were degraded during roasting, thereby forming caffeic acid, lactones, and various phenolic components via the Maillard and Strecker reactions. These chemical changes lead to the development of bitterness, astringency, and aroma of coffee (Pereira et al., 2021).
It is well known that the quality of coffee is determined based on criteria, including size, color, and shape of beans, cupping, and number of defects. In general, roasted beans are susceptible to various physicochemical changes that may significantly influence the sensory characteristics of coffee beverages. Flavor and fragrance play a key role in the sensory analyses of coffee. Ribeiro et al. (2009) investigated the correlation between aromatic components from Brazilian Arabica roasted coffees and sensory properties. The authors found that 3-methypropanal, 2-methylfuran, furfural, furfuryl formate, 2-furanmethanol acetate, and other components were possible markers for the overall quality of the coffee. The aroma components, including pyrroles, pyridines, and pyrazines, are responsible for the aroma characteristics of coffee, such as nutty, roasted, and toasted notes. In these, pyridines and related compounds are associated with the bitterness of coffee (Seninde and Chambers, 2020). Zakidou et al. (2021) reported that 2-furanmethanol, acetate exhibited a fruity and sweet aroma and fruity flavor in coffee beans. According to the sensory quality of coffee, the Robusta variety (Coffea canephora) has woody and earthy flavors, whereas the Arabica variety (Coffea arabica) has fine acidity, better flavor, and more intense aroma (Dippong et al., 2022; Kreuml et al., 2013).
Previous studies found that Arabica varieties possess better sensory characteristics than the Robusta varieties (Olechno et al., 2021; Tungnolo et al., 2019). However, the detection of sensory properties of roasted coffee beans is more complicated due to the initial contents of aromatic precursors in the green beans (Caporaso et al., 2018). It was reported that aromatic components with low odor thresholds significantly affected the coffee flavor. Further, some components, even at very low concentrations, highly influence the sensory properties of roasted coffee (Dong et al., 2019). Bhumiratana et al. (2011) reported that light roasting of coffee beans results in less sour and sweeter flavor than that of medium or dark roasted ones (typical characteristics of coffee). Therefore, sensory differences are possibly due to changes in the concentrations of aromatic constituents during excessive roasting. The qualitative and quantitative changes in the aromatic constituents are the main determinants of coffee cup quality.
CONCLUSION
The data of the present study suggest that the roasting time significantly affected the color parameters and aromatic compositions of coffee beans. The results revealed that 2-furanmethanol was a predominant component in all the roasted coffee beans of Colombia Supremo, Ethiopia 210, and Indonesia Mandheling. Further, 5-methyl-2-furancarboxaldehyde, 2-furanmethanol acetate, methylpyrazine, pyridine, 4-methylphenol, and ethylpyrazine were important components. The variations in the chemical composition of the studied cultivars may be due to the geographical region of the coffee cultivation and chemical reactions during the roasting process. These identified aromatic components, according to different roasting times, can be significant markers for predicting the aromatic quality of particular cultivars. Further studies are warranted about the influence of geographical origin and different roasting temperatures on the aromatic profile of coffee bean cultivars.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akiyama M, Murakami K, Hirano Y, Ikeda M, Iwatsuki K, Wada A, Tokuno K, Onishi M, Iwabuchi H. Characterization of headspace aroma compounds of freshly brewed arabica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J Food Sci, 2008; 73:C335–46.
Angeloni S, Navarini L, Sagratini G, Torregiani E, Vittori S, Caprioli G. Development of an extraction method for the quantification of lignans in espresso coffee by using HPLC-MS/MS triple quadrupole. J Mass Spectrom, 2018; 53:842–8.
Angeloni S, Mustafa AM, Abouelenein D, Alessandroni L, Acquaticci L, Nzekoue FK, Petrelli R, Sagratini G, Vittori S, Torregiani E. Characterization of the aroma profile and main key odorants of espresso coffee. Molecules, 2021; 26:3856.
Baggenstoss J, Poisson L, Kaegi R, Perren R, Escher F. Coffee roasting and aroma formation: application of different time− temperature conditions. J Agric Food Chem, 2008; 56:5836–46.
Bhumiratana N, Adhikari K, Chambers E. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT-Food Sci Technol, 2011; 44:2185–92.
Caporaso N, Whitworth MB, Cui C, Fisk ID. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res Int, 2018; 108:628–40.
Craig AP, Botelho BG, Oliveira LS, Franca AS. Mid infrared spectroscopy and chemometrics as tools for the classification of roasted coffees by cup quality. Food Chem, 2018; 245:1052–61.
Dippong T, Dan M, Kovacs MH, Kovacs ED, Levei EA, Cadar O. Analysis of volatile compounds, composition, and thermal behavior of coffee beans according to variety and roasting intensity. Foods, 2022; 11:3146.
Dong W, Hu R, Chu Z, Zhao J, Tan L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem, 2017; 234:121–30.
Dong W, Hu R, Long Y, Li H, Zhang Y, Zhu K, Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem, 2019; 272:723–31.
Farah A, Monteiro MC, Calado V, Franca AS, Trugo LC. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem, 2006; 95:373–80.
Fassio LO, Malta MR, Liska GR, Alvarenga ST, Sousa MMM, Farias TRT, Pereira RGFA. Sensory profile and chemical composition of specialty coffees from matas de minas Gerais, Brazil. J Agric Sci, 2017; 9(9):78.
Folmer B. The Craft and Science of Coffee, 1st edition, Elsevier, London, UK, 2017.
Franca AS, Oliveira LS, Oliveira RC, Agresti PCM, Augusti R. A preliminary evaluation of the effect of processing temperature on coffee roasting degree assessment. J Food Eng, 2009; 92:345–52.
Gancarz M, Dobrza?ski B, Malaga-Tobo?a U, Tabor S, Combrzy?ski M, ?wik?a D, Strobel WR, Oniszczuk A, Karami H, Darvishi Y, ?ytek A, Rusinek R. Impact of coffee bean roasting on the content of pyridines determined by analysis of volatile organic compounds. Molecules, 2022; 27:1559.
Giacalone D, Degn T, Yang N, Liu C, Fisk I. Munchow, M. Common roasting defects in coffee: Aroma composition, sensory characterization and consumer perception. Food Qual Prefer, 2019; 71:463–74.
Gonzalez-Rios O, Suarez-Quiroz ML, Boulanger R, Barel M, Guyot B, Guiraud JP, Schorr-Galindo S. Impact of “ecological” post-harvest processing on coffee aroma: II. roasted coffee. J Food Compos Anal, 2007; 20:297–307.
Kim, T. Analysis of coffee shop status and market conditions. KB Res Rep 2019-29, 2019; 23.
Korea Coffee Association. Korea coffee industry status survey. Korea Coffee Assoc, 2022; 36–49.
Kreuml MT, Majchrzak D, Ploederl B, Koenig J. Changes in sensory quality characteristics of coffee during storage. Food Sci Nutr, 2013; 1:267–72.
Kulapichitr F, Borompichaichartkul C, Fang M, Suppavorasatit I, Cadwallader KR. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-Lab color of Thai Arabica green coffee beans. Food Chem, 2022; 366:30504.
Lopes GR, Petronilho S, Ferreira AS, Pinto M, Passos CP, Coelho E, Rodrigues C, Figueira C, Rocha SM, Coimbra MA. Insights on single-Dose espresso coffee capsules’ volatile profile: from ground powder volatiles to prediction of espresso brew aroma properties. Foods, 2021; 10:2508.
Lopez-Galilea I, Fournier N, Cid C, Guichard E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J Agric Food Chem, 2006; 54:8560–6.
Mahmud M, Shellie R, Keast R. Unravelling the relationship between aroma compounds and consumer acceptance: coffee as an example. Compr Rev Food Sci Food Saf, 2020; 19:2380–420.
Marek G, Dobrzanski B, Oniszczuk T, Combrzynski M, Cwikla D, Rusinek R. Detection and differentiation of volatile compound profiles in roasted coffee Arabica beans from different countries using an electronic nose and GC-MS. Sensors, 2020; 20:2124.
Münchow M, Alstrup J, Steen I, Giacalone D. Roasting conditions and coffee flavor: A multi-study empirical investigation. Beverages, 2020; 6(2):29.
Olechno E, Puscion-Jakubik A, Socha K, Zujko ME. Coffee brews: are they a source of macroelements in human nutrition? Foods, 2021; 10:1328.
Ongo E, Montevecchi G, Antonelli A, Sberveglieri V, Sevilla F. Metabolomics fingerprint of philippine coffee by SPME-GCMS for geographical and varietal classification. Food Res Int, 2020; 134:109227.
Pereira G, Neto D, Magalhaes A, Vasquez Z, Medeiros A, Vandenberghe L, Soccol C. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem, 2019; 272:441–452.
Pereira LL, Júnior DB, Sousa LHBP, Gomes W, Dos S, Cardoso WSC, Guarçoni RC, Ten Caten CS. Relationship Between Coffee Processing and Fermentation. In: Pereira LL and Moreira TR. (eds.). Quality determinants in coffee production, Springer’s Food Engineering Series, Springer, Cham, Switzerland, pp 255–301, 2021
Rao NZ, Fuller M, Grim MD. Physiochemical characteristics of hot and cold brew coffee chemistry: the effects of roast Level and Brewing Temperature on Compound Extraction. Foods, 2020; 9:902.
Ribeiro JS, Augusto F, Salva TJ, Thomaziello RA, Ferreira MM. Prediction of sensory properties of Brazilian Arabica roasted coffees by headspace solid phase microextraction-gas chromatography and partial least squares. Anal Chim Acta, 2009; 634:172–179.
Risticevic S, Carasek E, Pawliszyn J. Headspace solid-phase microextraction–gas chromatographic–time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal Chim Acta, 2008; 617:72–84.
Ruta LL, Farcasanu IC. Coffee and Yeasts: From Flavor to Biotechnology. Fermentation, 2021; 7(1):9.
Ryan D, Shellie R, Tranchida P, Casilli A, Mondello L, Marriott P. Analysis of roasted coffee bean volatiles by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr, A 2004; 1054:57–65.
Seninde DR, Chambers E. Coffee flavour: A review. Beverages, 2020; 6:44.
Song, M. History of the development of the Korean coffee industry. Food Sci Ind, 2020; 53:397–409.
Toledo P, Pezza L, Pezza H, Toci A. Relationship between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr Rev Food Sci Food Saf, 2016; 15:705–719.
Tolessa K, Rademaker M, De Baets B, Boeckx P. Prediction of specialty coffee cup quality based on near infrared spectra of green coffee beans. Talanta, 2016; 150:367–74.
Tugnolo A, Beghi R, Giovenzana V, Guidetti R. Characterization of green, roasted beans, and ground coffee using near infrared spectroscopy: a comparison of two devices. J Near Infrared Spectrosc, 2019; 27:93–104.
Yang N, Liu C, Liu X, Degn TK, Munchow M, Fisk I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016; 211:206–214.
Zakidou P, Plati F, Matsakidou A, Varka EM, Blekas G, Paraskevopoulou A. Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules, 2021; 26:5609.
Zou Y, Gaida M, Franchina FA, Stefanuto PH, Focant JF. Distinguishing between decaffeinated and regular coffee by HS-SPME-GCxGC-TOFMS, chemometrics, and machine learning. Molecules, 2022; 27:1806.