HIGHLIGHTS
• LPS disrupts antioxidant homeostasis and induced oxidative stress in the brain of mice.
• Fructooligosaccharides (FOS) supplementation ameliorated oxidative stress in LPS-induced mice.
• FOS regulates body and brain weight.
• FOS shows anti-oxidative and healing properties.
INTRODUCTION
Neurotoxicity happens when exposure to natural or artificial substances (neurotoxins) disrupts the normal functioning of the central and peripheral neural systems. It is one of the main contributors to neurodegenerative disorders like schizophrenia and Parkinson’s disease and manifested in various signs of psychiatric illnesses, including oxidative stress and inflammation [1]. For the central nervous system (CNS) to function normally, brain oxidative homeostasis is necessary. The antioxidant response system’s ability to scavenge excess free radicals including superoxide (O2•−), hydroxyl (OH•), and peroxyl radical (ROO•) is reduced, causes organ dysfunction [2]. The brain is susceptible to severe oxidative harm because it consumes a lot of oxygen and has minimal antioxidant action [3]. Gram-negative bacteria (like Escherichia coli) produce the endotoxin lipopolysaccharide (LPS) in their cell walls, which is a well-known neuro and immunotoxic agent [4]. LPS disturbs the barrier between brain tissues and circulating blood and indirectly affects nerve cells [5]. LPS is considered a psychotic agent that can activate the neuroimmune response via toll-like receptors (TLRs) present in several neuronal cell types, including microglia, endothelial cells, and astrocytes, and generate free radicals, inflammatory cytokines, and other mediators to cause inflammation and oxidative stress [6]. The LPS-induced brain oxidative stress is not well explored; therefore, a comprehensive study is necessary to understand the mechanism.
The prebiotics are nondigestible substances, such as fibers and carbohydrates that encourage the growth of beneficial microbe that are already present in the gut, which, in turn, may positively impact the microbiota-gut-brain axis [7]. The FOS are used as prebiotics, which are thought to be advantageous for the host’s health because they encourage the healthy gut microbiota (GM) [8]. Many food, including wheat, chicory roots, asparagus, and garlic, contain FOS in various amounts. The fructose units in FOS are joined with β (2 ? 1) glycosidic connections along with a terminal glucose unit [9]. Small intestine glycosidase does not hydrolyze FOS; therefore, it passes through to the colon where it is extensively fermented by GM. Their ensuing creation of organic acids might boost the host’s defense against harmful bacteria [10]. Few studies have suggested that the supplementation of prebiotics might enhance psychiatric health by acting as an antioxidant. In this study, we hypothesized that FOS supplementation inhibits oxidative stress to improve mental health. The present study is a pioneer attempt to investigate how FOS may potentially counteract or mitigate the oxidative stress caused by LPS in the brain of mice.
MATERIALS AND METHODS
Animals and study design
The 8 weeks old Swiss albino female mice (body weight: 22 ± 3 g) were purchased from the Indian Institute of Toxicology Research, Lucknow, India, and kept in polypropylene cages with ad-libitum food and water with 12/12 light-dark cycles in a suitable environment (temperature, 23°C ± 2°C and humidity, 55% ± 5%). Mice were split into six groups at random after 2 weeks of acclimatization.
I. Control: given 0.9% NaCl (saline) for 5 days.
II. LPS: exposed to LPS 1 mg/kg bw (E. coli O26:B6, L-2654; Sigma-Aldrich, USA) for 5 days and reared up to 28 days.
III. LPS + FOSLOW: given LPS 1 mg/kg bw for 5 days, followed by FOS 2 g/kg bw (F8052, Sigma-Aldrich, USA) for 28 days.
IV. LPS + FOSHIGH: given LPS 1 mg/kg bw for 5 days, subsequently supplemented FOS 4 g/kg bw for 28 days.
V. FOSLOW: given FOS 2 g/kg bw for 28 days.
VI. FOSHIGH: given FOS 4 g/kg bw for 28 days.
Exposure to LPS was intraperitoneal (ip), while FOS was through oral gavaging. The administered FOS dose (w/v) is comparable to the oral dosage of FOS given to rats [11]. The FOS high dose (4 g/kg bw) used in this study is approved as safe with no adverse effects in rats [12]. Body weight was assessed every day. Pentobarbital (ip, 100 mg/kg bw) was used to sacrifice the animals after the experiment.
The animals were maintained and handled according to the guidelines of the Committee for Control and Supervision of Experimental Animals, Ministry of Environment, Forest and Climate Change, Government of India. The experimental protocols were approved (approval number: IAEC/AU/2019(1)/01) by the Institutional Animal Ethics Committee of the University of Allahabad, India.
Assay of brain oxidative stress
Protein estimation
Using the Bradford procedure [13] and bovine serum albumin (1 mg/ml) as the reference, the total protein contents of the samples were calculated. 900 μl of Bradford reagent was combined with 100 μl of standard solution or an unknown protein sample. The UV-visible spectroscopy measured the absorbance [optical density (OD)] of the sample at 595 nm.
Antioxidant defense parameters
PPB, a potassium phosphate buffer with a pH of 7.4, was used to homogenize the brain tissue. The supernatants from the centrifugation of 2 ml of tissue homogenate at 10,000 g for 15 minutes were used to assess the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR).
Superoxide dismutase
According to Beauchamp and Fridovich’s approach [14], the activity of SOD was measured. In simple terms, 900 μl of the reaction mixture [0.05 M PPB, 0.1 M methionine, 0.1 M ethylenediaminetetraacetic acid, 0.45 M nitrobluetetrazolium chloride (NBT), 0.01 M riboflavin] was combined with 100 μl of tissue supernatant, which was then incubated for an hour under lights. The UV-visible spectroscopy measured the OD of the purple-colored complex at 560 nm against the blank. One unit of SOD is defined as the amount of enzyme that reduces NBT (Code: MB107, HIMEDIA, Mumbai, INDIA) by 50% and is expressed in units per mg of protein.
Catalase
The approach of Cohen et al. [15] and Aebi [16] was used to assess CAT activity. In brief, 500 μl of supernatant was combined with 5 μl of ethanol and placed in the ice box for 30 minutes. Then, 450 μl of this aliquot was combined with 50 μl of Tritan-X-100. 100 μl of this sample was obtained, and 1.4 ml of 13 mM H2O2 was added. The sample’s OD was estimated using the absorption coefficient of H2O2, which is 43.6 M−1cm−1, and measured spectrophotometrically at 240 nm. One unit of CAT was defined as the amount of enzyme required to degrade 1 μM of H2O2 in 1 minute.
Glutathione reductase
The GR activity was estimated by Massey and William’s method [17]. 900 μl of the reaction mixture [50 mM PPB, 120-mM oxidized glutathione (GSSG), and 4.5-mM nicotinamide adenine dinucleotide phosphate (NADPH)] was mixed with 100 μl of tissue supernatant. OD was recorded at 340 nm against blank. Enzyme activity was calculated using NADPH’s extinction coefficient, which was 6.22 mM−1cm−1.
Estimation of total glutathione (T-GSH), GSSG, and reduced glutathione (R-GSH)
With slight modifications, the methods of Tietze [18] and Griffith [19] were used to estimate the T-GSH, R-GSH, and GSSG levels. Briefly, 1 ml of tissue supernatant was added to an equivalent quantity of the ice-cold trichloroacetic acid (5% v/v) and centrifuged at 1,000 rpm for 20 minutes. For the T-GSH assay, 900 μl of a reaction mixture comprising 6 mM 5,5′-Dithiobis (2-nitrobenzoic acid), 0.3 mM NADPH, 50 mM PPB, and 25 units/ml of GR were combined with 100 μl of cell supernatant. T-GSH was determined by OD at 412 nm using R-GSH as standard. For the GSSG assay, endogenous GSH is fully derivatized by adding 2 μl of 4-vinyl pyridine in 100-μl supernatant and kept at 25°C. The assay was performed similarly to that of the T-GSH assay. According to Olsvik et al. [20], the quantity of R-GSH (T-GSH-GSSG) and the oxidative stress index (OSI = 100× (2 × GSSG) / T-GSH) were determined.
Measurement of malondialdehyde (MDA)
The modified Ohkawa et al. [21] method was used to determine the lipid peroxides in the tissue homogenate. In brief, 1,800 μl of the reaction mixture comprising 10% w/v sodium dodecyl sulfate, 20% v/v acetic acid, 1% w/v thiobarbituric acid (TBA), 1% w/v butylatedhydroxytoluene was added to 200 μl of brain tissue supernatant and properly mixed. Then, for around 60 minutes, the reactants were heated at 95°C in the water bath. The tubes were brought to 25°C temperature and separation of the organic layer was facilitated by 10 minutes of centrifugation at 1,000 rpm and OD was recorded at 532 nm. The 1,1,3,3-tetraethoxypropane, used as the standard, was used to determine the production of TBA reactive substance (TBARS). The concentration of TBARS was calculated using the molar extinction value of 1.56 × 105 M−1cm−1. In terms of nmol TBARS formed/mg protein was used to express the outcome.
Statistical analysis
The data was analyzed by GraphPad Prism5 software and reported as mean ± SD. Moreover, one-way analysis of variance (ANOVA) and Tukey’s post hoc test were used for the measurement of statistical differences at *p < 0.05, **p < 0.01, and ***p < 0.001.
RESULTS
FOS supplementation prevents the body and brain weight decreased by LPS exposure
Post hoc analysis revealed a significant effect was observed on body weight [F (5, 166) = 20.49, p < 0.001], and brain weight [F (5, 35) = 20.87, p < 0.001]. When compared to the control group, the body weight of the LPS (p < 0.001) and LPS + FOSLOW (p < 0.01) treated mice was considerably lower. Other groups revealed no substantial differences in body weight from that of the control. Further, compared to LPS-exposed mice, the body weight was increased in LPS + FOSLOW (p < 0.01), LPS + FOSHIGH, FOSLOW, and FOSHIGH (p < 0.001 for all) group. There was a significant decrease in brain weight in LPS-induced mice (p < 0.001) whereas increased in only FOSHIGH dose (4 g/kg bw, p < 0.05) treated mice in comparison with control. Furthermore, results manifested that the FOS supplementation significantly enhanced brain weight in LPS + FOSLOW (p < 0.01), LPS + FOSHIGH (p < 0.01), FOSLOW (p < 0.01), and FOSHIGH (p < 0.001) exposed mice compared with the LPS treatment (Fig. 1).
Analysis of LPS and FOS exposure on oxidative stress of brain
Estimation of SOD, CAT, and GR in the brain
In the treatment group, post hoc analysis revealed a significant shift in the levels of SOD [F (5, 35) = 19.97, p < 0.001], CAT [F (5, 35 = 16.32, p < 0.001], and GR [F (5, 35) = 12.31, p < 0.001] in the brain tissue. The LPS significantly decreased activities of SOD, CAT, and GR (52%, 40%, and 34%, p < 0.001), and only the FOSHIGH group significantly increased activities of SOD and CAT (24% and 25% respectively, p < 0.05) compared to control mice. Furthermore, between experimental groups, the SOD activity was substantially increased in LPS + FOSLOW (71%, p < 0.01), LPS + FOSHIGH (100%, p < 0.001), FOSLOW (78%, p < 0.01), and FOSHIGH (157%, p < 0.001) compared to the LPS treated mice. After FOS treatment, the CAT activity was significantly increased in LPS + FOSLOW (53%, p < 0.01), LPS + FOSHIGH (92%, p < 0.001), FOSLOW (53%, p < 0.01), and FOSHIGH (107%, p < 0.001) groups comparison to LPS exposed mice. GR activity significantly elevated in LPS + FOSHIGH (45%, p < 0.01), FOSLOW (40%, p < 0.01), and FOSHIGH (72%, p < 0.001) comparison to LPS treatment (Fig. 2).
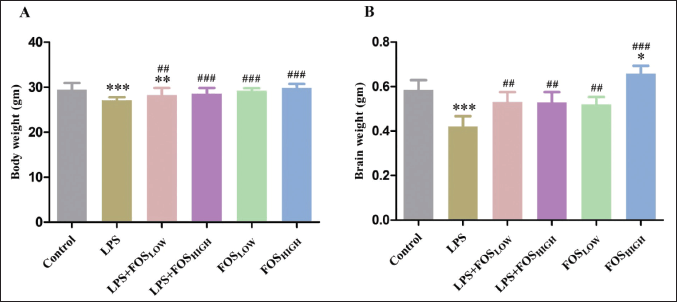 | Figure 1. Graph representing the (A) body weight and (B) brain weight of mice. Data were analyzed by one-way ANOVA, expressed as mean ± SD. Significant differences from the control group at *p < 0.05, **p < 0.01, and ***p < 0.001 and from the LPS-exposed group at ##p < 0.01 and ###p < 0.001. [Click here to view] |
Glutathione (T-GSH, R-GSH, and GSSG) activity
Figure 3 represents the effects of LPS and FOS on GSH (T-GSH and R-GSH), and GSSG status in brain homogenates of mice. A post hoc analysis indicated that the treated mice had a substantial impact on T-GSH [F (5, 35) = 43.57, p < 0.001], R-GSH [F (5, 35) = 54.87, p < 0.001], and GSSG [F (5, 35) = 92.45, p < 0.001]. The activity of T-GSH and R-GSH was significantly decreased in the LPS (50% and 62%, respectively, p < 0.001) and LPS + FOSLOW (14% and 15%, respectively, p < 0.05) compared to the control group. Compared to LPS treatment, T-GSH and R-GSH significantly elevated in both the FOS co-treated groups (LPS + FOSLOW: 71% and 121%, p < 0.001; LPS + FOSHIGH: 79% and 133%, p < 0.001) as well as that only received FOS administration (FOSLOW: 76% and 127%, p < 0.001; FOSHIGH: 80% and 137%, p < 0.001) mice. The activity of GSSG was significantly increased in the LPS (49%, p < 0.001) and LPS + FOSLOW (8%, p < 0.05) exposed mice. While the supplementation of FOS treatment significantly decreased the activity of GSSG in all four groups [LPS + FOSLOW (28%), LPS + FOSHIGH (33%), FOSLOW (31%), and FOSHIGH (37%), p < 0.001 for all] compared with LPS treatment.
Oxidative stress index
A Tukey’s test analysis found that there was a substantial difference in OSI [F (5, 35) = 239.1, p < 0.001] among the groups. The OSI was significantly elevated in LPS (195%, p < 0.001) and LPS + FOSLOW (25%, p < 0.05) groups compared to the control mice. However, supplementation with FOS showed a significant reduction of OSI in LPS + FOSLOW (58%), LPS + FOSHIGH (62%), FOSLOW (61%), and FOSHIGH (65%) (p < 0.001 for all) in comparison with the LPS treated mice (Fig. 3).
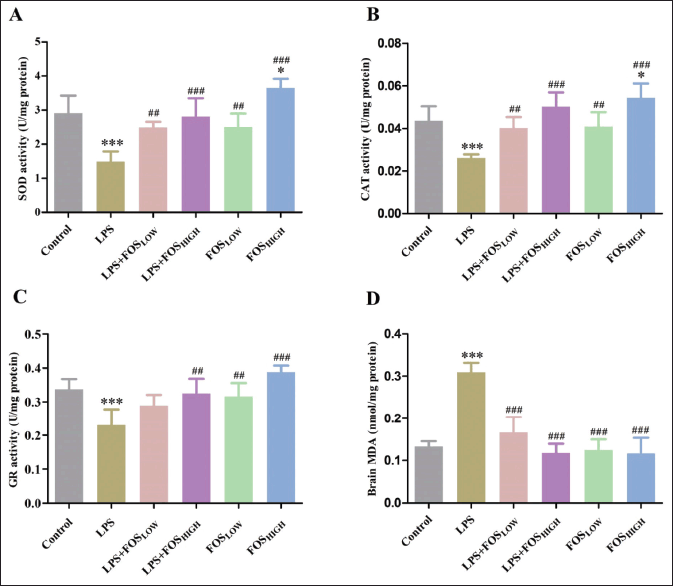 | Figure 2. Graph displaying the level of SOD, CAT, GR, and MDA in the brain as measured (A), (B), (C), and (D), respectively. Data were analyzed by one-way ANOVA, expressed as mean ± SD. Significant differences from the control group at *p < 0.05 and ***p < 0.001 and from the LPS-exposed group at ##p < 0.01 and ###p < 0.001. [Click here to view] |
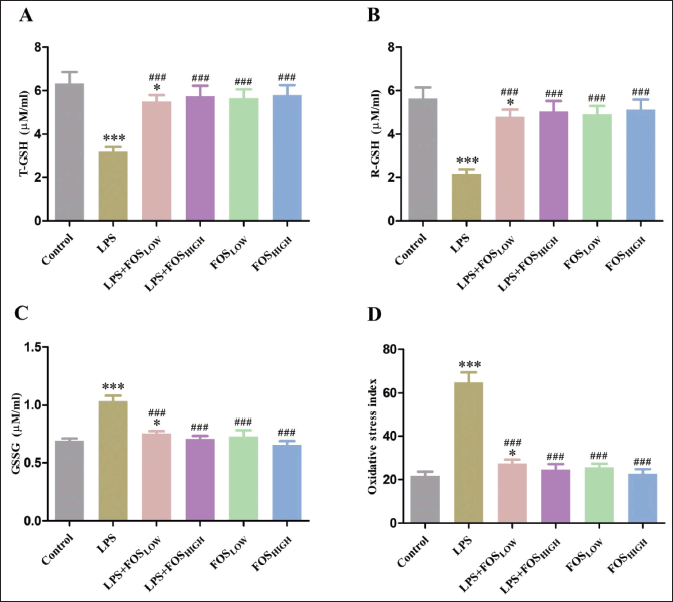 | Figure 3. Graph showing the level of (A) T-GSH, (B) R-GSH, (C) GSSG, and (D) OSI in the brain. Data were analyzed by one-way ANOVA, expressed as mean ± SD. Significant differences from the control group at *p < 0.05 and ***p < 0.001 and from the LPS-exposed group at ###p < 0.001. [Click here to view] |
Determination of MDA level
In post hoc analysis, treated mice demonstrated a significant change in the MDA level [F (5, 35) = 44.53, p < 0.001] (Fig. 2). LPS exposure significantly increased MDA production (130%, p < 0.001) in mice brain when compared with control/vehicle group. In comparison, supplementation with FOS significantly decreased MDA levels in all four groups [LPS + FOSLOW (47%), LPS + FOSHIGH (64%), FOSLOW (60%), and FOSHIGH (64%), p < 0.001 for all] compared with LPS treatment.
DISCUSSION
The current work focused on the prebiotic FOS-mediated amelioration of the oxidative stress in mice’s brains caused by LPS. The outcomes of the research revealed that the FOS modulated the LPS-induced psychiatric illness and neurotoxicity in a dose-dependent way, with high doses of FOS (4 g/kg bw) being more efficacious than low doses (2 g/kg bw). The LPS is a bacterial endotoxin found in the outer body wall of gram-negative bacteria that causes neurotoxicity and psychiatric illness via inflammation and oxidative stress. LPS is also a well-known immune stressor that brings oxidative stress and elevation of the pro-inflammatory cytokines e.g., IL-6, IL-1β, and TNF-α [22] after binding with the TLR-4. The increased inflammatory cytokines and oxidative stress mediators like reactive oxygen species (ROS) collectively resulted in neuroinflammation and further neurodegeneration.
It is possible that LPS-induced neuroinflammation and symptoms of sickness could be associated with decreased body and brain weight [23]. Few studies have already shown a connection between oxidative stress-induced progressive lipid, protein, and DNA damage and weight loss in both the body and the brain [24]. Likewise, this study also showed a decrease in protein content and inflammation which could be corroborated with decreased body and brain weight in LPS-exposed mice. LPS triggers an immediate inflammatory response that results in disproportionately high concentrations of chemically reactive molecules in the brain, such as ROS and peroxides [25]. The LPS exposure activates TLR-4, is primarily exposed to microglia in the brain, and is the key mediator of neuroinflammation [26]. This study presents systemic inflammation caused by LPS treatment that changes the oxidative status of the brain. The decrease of SOD, CAT, GR, T-GSH, and R-GSH, as well as an increase in MDA and GSSG in the brain, are indications of an increase in free radical production. Many studies have shown that LPS alters the cellular anti-oxidant defense system’s equilibrium and exerts a serious negative impact on the brain by increasing superoxide and H2O2 formation and the breakdown of lipids and proteins, as reported [27]. Impairment of this endogenous anti-oxidant enzyme, such as CAT, SOD, GR, and other components, including glutathione, makes it impossible for the enzymes to counteract the generation of ROS following LPS induction. In addition, the reduced strength of the anti-oxidant defense mechanism and high peroxidation activity irreversibly impaired the neuronal cell biochemistry which is a key marker of neuronal aging and death.
The prebiotics FOSs are not digestible carbohydrates and induce beneficial bacterial growth in the gastrointestinal (GI) tract and modulate inflammation [28]. In addition to regulating the GI tract, prebiotics are essential to protect brain cells. Furthermore, the short-chain fatty acid (SCFA), including butyrate, are manufactured by gut microbes using the FOS to generate bioactive metabolites that interact with the CNS and control the inflammatory response of microglia [29]. The previous research showed that the GM influences tryptophan metabolism and controls serotonin signaling by producing neurotransmitters including norepinephrine, dopamine, and gamma amino butyric acid [30]. The balance level of abovementioned neurotransmitters is a prerequisite for the smooth functioning of the neuronal cell mechanism. Consequently, nutritional components with immunoregulatory qualities, such as probiotics and prebiotics, may enhance the host’s health [31].
In the present study, supplementing FOS to LPS-exposed mice restored body and brain weight which might be due to reducing stress levels. In addition, our study showed that FOS supplementation significantly increased SOD, CAT, GR, R-GSH, and T-GSH and reduced GSSG and MDA activity in LPS-exposed mice brains. It is noteworthy that prebiotics prevents mitochondrial ROS production in the brain region [32]. Some reports suggested that SCFA is a potent anti-oxidant, reducing oxidative stress against LPS exposure [33]. In an in vitro study, the SCFA inhibits ROS production and MDA level, enhancing the SOD activity against the LPS [34]. A recent study also suggested that FOS supplementation is positively attributed to anti-oxidative properties, thus enhancing gut immune function and microbial diversity, which may benefit mental health [35]. Few prebiotic oligosaccharides have beneficial effects to combat oxidative stress by directly or indirectly neutralizing free radicals and conferring neuroprotective and neuromodulating benefits [36].
CONCLUSION
The findings of this study proved that FOS is a powerful modulator of the psychiatric condition induced by the LPS. The FOS led the anti-oxidative properties and maintains the anti-oxidant level. Thus, this study established the beneficial role of FOS in brain biochemistry, indicating new avenues in the field of neuropsychopharmacology. However, we hope that our findings regarding the antioxidant activity of FOS would help in future research.
ACKNOWLEDGMENTS
The author is thankful to University Grants Commission for financial support and the Department of Zoology for a research facility in this study.
LIST OF ABBREVIATIONS
% = Percent; μl = Microliter; °C = Celsius; CAT = Catalase; FOS = Fructooligosaccharides; GR = Glutathione reductase; GSSG = Oxidized glutathione; LPS = Lipopolysaccharide; mM = Milli Mole; NBT = Nitrobluetetrazolium chloride; nm = Nanometer; OSI = Oxidative stress index; R-GSH = Reduced glutathione; SCFA = Short chain fatty acid; SOD = Superoxide dismutase; T-GSH = Total glutathione.
AUTHOR CONTRIBUTION
Shreya: Investigation; Analysis; Writing-original draft
Banalata Mohanty: Conceptualisation of Study; Correction and preparation of the final draft.
CONFLICTS OF INTEREST
The authors declared that no conflicts of interest with the current study.
ETHICAL APPROVALS
The experimental protocols (IAEC/AU/2019(1)/01) were approved by the Institutional Animal Ethics Committee (IAEC) of the University of Allahabad, India.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ayeni EA, Aldossary AM, Ayejoto DA, Gbadegesin LA, Alshehri AA, Alfassam HA, et al. Neurodegenerative diseases: implications of environmental and climatic influences on neurotransmitters and neuronal hormones activities. Int J Environ Res Public Health. 2022;19(19):12495. doi: https://doi.org/10.3390/ijerph191912495
2. Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012;1822(5):675–84. doi: https://doi.org/10.1016/j.bbadis.2011.10.017
3. Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25(7):1270–6. doi: https://doi.org/10.1016/j.drudis.2020.05.001
4. Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, De Oliveira ACP. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci. 2019;20(9):2293. doi: https://doi.org/10.3390/ijms20092293
5. Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):1–15. doi: https://doi.org/10.3233/JAD-161141
6. Cuesta CM, Guerri C, Ureña J, Pascual M. Role of microbiota-derived extracellular vesicles in gut-brain communication. Int J Mol Sci. 2021;22(8):4235. doi: https://doi.org/10.3390/ijms22084235
7. Scott KP, Grimaldi R, Cunningham M, Sarbini SR, Wijeyesekera A, Tang MLK, et al. Developments in understanding and applying prebiotics in research and practice—an ISAPP conference paper. J Appl Microbiol. 2020;128(4):934–49. doi: https://doi.org/10.1111/jam.14424
8. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi: https://doi.org/10.3390/foods8030092
9. Martins GN, Ureta MM, Tymczyszyn EE, Castilho PC, Gomez-Zavaglia A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front Nutr. 2019;6:78. doi: https://doi.org/10.3389/fnut.2019.00078
10. Ashaolu TJ. Immune boosting functional foods and their mechanisms: a critical evaluation of probiotics and prebiotics. Biomed Pharmacother. 2020;130:110625. doi: https://doi.org/10.1016/j.biopha.2020.110625
11. Kang S, Johnston TV, Ku S, Ji GE. Acute and sub-chronic (28-day) oral toxicity profiles of newly synthesized prebiotic butyl-fructooligosaccharide in ICR mouse and Wistar rat models. Toxicol Res. 2020;9(4):484–92. doi: https://doi.org/ 10.1093/toxres/tfaa055
12. Phipps KR, Baldwin N, Lynch B, Stannard DR, Šoltesová A, Gilby B, et al. Preclinical safety evaluation of the human-identical milk oligosaccharide lacto-N-tetraose. Regul Toxicol Pharmacol. 2018;99:260–73. doi: https://doi.org/10.1016/j.yrtph.2018.09.018
13. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54. doi: https://doi.org/10.1016/0003-2697(76)90527-3
14. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–87. doi: https://doi.org/10.1016/0003-2697(71)90370-8
15. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34(1):30–8. doi: https://doi.org/10.1016/0003-2697(70)90083-7
16. Aebi H. Catalase. Methods of enzymatic analysis. New York, NY: Academic Press; 1974. pp 673–84.
17. Massey V, Williams Jr CH. On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965;240(11):4470–80. doi: https://doi.org/10.1016/S0021-9258(18)97085-7
18. Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–22. doi: https://doi.org/10.1016/0003-2697(69)90064-5
19. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–12. doi: https://doi.org/10.1016/0003-2697(80)90139-6
20. Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Baeverfjord G, et al. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmosalar) exposed to hyperoxic water during smoltification. Comp Biochem Physiol C-Toxicol Pharmacol. 2005;141(3):314–23. doi: https://doi.org/10.1016/j.cbpc.2005.07.009
21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. doi: https://doi.org/10.1016/0003-2697(79)90738-3
22. Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. 2019;16:180. doi: https://doi.org/10.1186/s12974-019-1564-7
23. Yeh CH, Hsieh LP, Lin MC, Wei TS, Lin HC, Chang CC, et al. Dexmedetomidine reduces lipopolysaccharide induced neuroinflammation, sickness behavior, and anhedonia. PLoS One. 2018;13(1):e0191070. doi: https://doi.org/10.1371/journal.pone.0191070
24. Sharma N, Nehru B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: role of oxidative stress and neuroinflammation. Neurochem Int. 2015;87:92–105. doi: https://doi.org/10.1016/j.neuint.2015.06.004
25. Wang H, Meng GL, Zhang CT, Wang H, Hu M, Long Y, et al. Mogrol attenuates lipopolysaccharide (LPS)-induced memory impairment and neuroinflammatory responses in mice. J Asian Nat Prod Res. 2020;22(9):864–78. doi: https://doi.org/10.1080/10286020.2019.1642878
26. Wolf SA, Boddeke HWGM, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–43. doi: https://doi.org/10.1146/annurev-physiol-022516-034406
27. Prakash R, Sandhya E, Ramya N, Dhivya R, Priyadarshini M, SakthiPriya B. Neuroprotective activity of ethanolic extract of Tinospora cordifolia on LPS induced neuroinflammation. Transl Biomed. 2017;8(4):135. doi: https://doi.org/10.21767/2172-0479.100135
28. Mahalak KK, Firrman J, Narrowe AB, Hu W, Jones SM, Bittinger K, et al. Fructooligosaccharides (FOS) differentially modifies the in vitro gut microbiota in an age-dependent manner. Front Nutr. 2023;9:1058910. doi: https://doi.org/10.3389/fnut.2022.1058910
29. Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141(5):874–80. doi: https://doi.org/10.1038/sj.bjp.0705682
30. Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–72. doi: https://doi.org/10.1089/jmf.2014.7000
31. Paiva IHR, Duarte-Silva E, Peixoto CA. The role of prebiotics in cognition, anxiety, and depression. Eur Neuropsychopharmacol. 2020;34:1–18. doi: https://doi.org/10.1016/j.euroneuro.2020.03.006
32. Sarkar SR, Mazumder PM, Banerjee S. Oligosaccharide and flavanoid mediated prebiotic interventions to treat gut dysbiosis associated cognitive decline. J Neuroimmune Pharmacol. 2022;17(1–2):94–110. doi: https://doi.org/10.1007/s11481-021-10041-4
33. González-Bosch C, Boorman E, Zunszain PA, Mann GE. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021;47:102165. doi: https://doi.org/10.1016/j.redox.2021.102165
34. Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, et al. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp Clin Endocrinol Diabetes. 2017;125(2):98–105. doi: https://doi.org/10.1055/s-0042-121493
35. Costa GT, Vasconcelos QDJS, Aragão GF. Fructooligosaccharides on inflammation, immunomodulation, oxidative stress, and gut immune response: a systematic review. Nutr Rev. 2022;80(4):709–22. doi: https://doi.org/10.1093/nutrit/nuab115
36. Divyashri G, Sadanandan B, Chidambara Murthy KN, Shetty K, Mamta K. Neuroprotective potential of non-digestible oligosaccharides: an overview of experimental evidence. Front Pharmacol. 2021;12:712531. doi: https://doi.org/10.3389/fphar.2021.712531