INTRODUCTION
The cost of therapy is a primary concern among patients in lower-and-middle-income countries (LMICs). Lack of awareness, resources, low socioeconomic status, poor hygiene, and sanitation, contribute to increased transmission of infection in countries like India (Atal and Mathur, 2020). Management of multidrug-resistant (MDR) infections is associated with a higher cost requiring second-line agents, additional investigations, and increased length of stay (LoS). The indirect costs include productivity losses due to increased morbidity and mortality (Prestinaci et al., 2015). Methicillin-resistance Staphylococcus aureus (MRSA) has been associated with high mortality rates every year globally, and MDR Gram-negative bacteria (GNB) have made the treatment challenging (Founou et al., 2017). World Health Organization (WHO) reported crude excess mortality as 23.6% due to hospital-acquired infections (HAIs) from Asian countries, with LoS ranging between 5 and 29.5 days (WHO, 2011). Centers for Disease Control and Prevention (CDC) reports that surgical site infections account for 20% of HAIs, thus, extending the LoS by 9.7 days, and attributing to 75% of the death with an estimated cost of $3.3 billion per year in the United States (US) (NHSN, 2022). The total cost burden of antimicrobial resistance (AMR) due to MDR pathogens was reported as $0.5 billion and $2.8 billion in LMICs and high-income countries, respectively. Since the efficacy of the antibiotics is decreased because of the increasing trends in AMR, physicians are forced to prescribe last-resort antibiotics such as carbapenems, tigecycline, and polymyxins, which are not readily available in LMICs, and incur a higher cost in addition to their side effects. In India, where there is insufficient enforcement of regulatory policies on prescribing, the high prevalence of over-the-counter (OTC) and lengthy courses of antibiotics in humans, animals, and agriculture use, and accessible travel routes have led to the emergence and dissemination of AMR (Dadgostar, 2019). Centre for Disease Dynamics, Economics & Policy records a sharp 30% increase in per capita use of antibiotics in India between 2010 and 2020 (Kaul, 2021). Healthcare costs also increase in patients with susceptible infections or no infection. Patients are routinely given costlier diagnostic tests, such as C-reactive protein, interleukin-6, presence of resistant strains, or prescribed with empirical antibiotics that are costlier than the first-line antibiotics (Jit et al., 2020). Health research funding and insurance schemes are disappointing and inadequate. Drug therapy costs are generally not met under the insurance and are borne mainly by the patient as an out-of-pocket expenditure (Chandra et al., 2021). More than 50% is spent on medicines, which is complicated by the accessibility of numerous branded and generic forms of drugs. The current study aimed to assess the factors affecting the direct cost incurred by the patients. The study also assessed the antimicrobial susceptibility profile of bacterial isolates, their consequences, and the management of infection.
MATERIALS AND METHODS
A prospective, observational study was initiated after the approval of the Institutional Ethics Committee (IEC-477/2019) and with prior registration under the Clinical Trial Registry of India (CTRI/2019/08/020938). In view of the corona virus-2019 (COVID-19) pandemic, a special consideration from IEC was obtained to follow the patients retrospectively who were admitted from March 2020 to December 2020 waiving off their informed consents. Patients above 18 years of age, admitted with clinical infections prescribed with antibiotics were included. Patients solely infected with Mycobacterium tuberculosis and those who were not willing to give consent were excluded from the study. The demographic characteristics and the resistance profile of the bacterial isolates were documented using a predesigned case record form. Biological samples with culture-proven infection for the first time were included in the study. We did not take into account repeated samples with identical isolates from the same patients at various times. However, the same isolates from other biological samples collected at the same time or at a later date were taken into account. Matrix assisted laser desorption ionization time-of-flight was used for microbial identification and Vitek-mass spectrometry was used to detect the antibacterial susceptibility of organisms identified from the biological specimens. The antibiotics tested against bacterial isolates were ceftriaxone (30 μg), ceftazidime (30 μg), ampicillin (10 μg), amoxicillin-clavulanic acid (20/10 μg), trimethoprim-sulphamethoxazole (TMP-SMX) (1.25/23.75 μg), gentamycin (10 μg), amikacin (10 μg), ciprofloxacin (5 μg), nalidixic acid (30 μg), norfloxacin (30 μg), imipenem (10 μg), meropenem (10 μg), azithromycin (15 μg), erythromycin (15 μg), chloramphenicol (30 μg), clindamycin (2 μg), vancomycin (30 μg), tetracycline (30 μg), and nitrofurantoin (30 μg). The results were interpreted according to Clinical and Laboratory Standard Institute (CLSI, 2020) guidelines.
The strains of bacterial isolates were classified as susceptible (S), MDR, extensive/pandrug-resistant (XDR/PDR) based on the definition provided by European centre for disease prevention and control and CDC (Magiorakos et al., 2012). The data regarding the direct cost were collected from the hospital finance department. Antibiotic administration costs were calculated and collected for individual patients. The cost data were represented as US dollar ($). Variables such as community-acquired infections (CAIs), HAIs, mono-infections, and polymicrobial infections were defined as per the scientific consensus.
Based on the organ systems, the clinical diagnoses were grouped into various categories. For instance, cardiac-related events are grouped as “Group A”, pulmonary diseases are grouped as “Group B”, and so on (Supplementary File: Annexure 1).
Statistical analysis
For the aim of using data analytics (machine learning) techniques on the gathered data, a sample size was determined that employed six cases for each variable. In our investigation, we employed 165 variables which yielded 1,000 samples, and examined for the variables associated with cost. Descriptive analysis was used to describe the patient demography, bacterial isolates’ susceptibility profile, and cost. The skewed data were transformed to log data, for statistical analysis. The cost data were represented as median (range). Multiple regression with the backward selection method was used to identify the impact of various attributes on the cost. The multicollinearity statistics between the independent variables were determined by the tolerance (T) values, and variance inflation factor (VIF). If the VIF values were <0.1, and T greater than 5, respectively, then multicollinearity between the independent variables was suspected. The correlation between clinically significant isolates and cost was tested using Pearson’s correlation coefficient method (two-tailed). A p ≤ 0.05 was considered to be significant throughout the study. All statistical analyses were carried out using the SPSS 26.0 package (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY).
RESULTS
Demographic characteristics
A total of 1,000 patients were included in the study and taken up for analysis. The mean age of the patient was found to be 57.07 ± 15.27 and males were more predominant (n = 710) than females (n = 290). The culture test showed GNBs (n = 1,200) higher than the Gram-positive bacteria (GPBs) (n = 329). Escherichia coli (507) was more prevalent among the GNBs, followed by Klebsiella spp. (381), Acinetobacter spp. (n = 142), Pseudomonas spp. (n = 111), and Enterobacter spp. (n = 59). MRSA (n = 139), Enterococcus spp. (n = 97), and closely followed by Streptococcus spp. (n = 93) were highly prevalent among the GPBs. The demographic characteristics are shown in Table 1.
Susceptibility profile
The study found more MDR and XDR/PDR strains of Escherichia coli followed by Klebsiella spp., Acinetobacter spp. among the GNBs. MDR strains of Enterococcus spp., MRSA were found high among the GPBs (Fig. 1). The details of the susceptibility profile of individual isolates are included in the (supplementary file: Figs. 1–6).
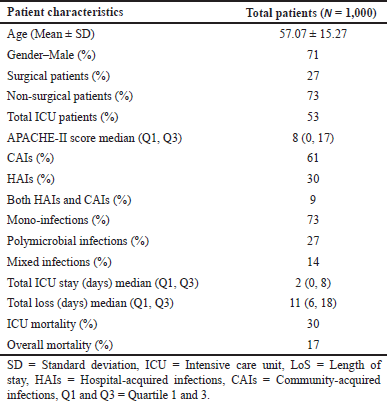 | Table 1. Demographic features of the sample population. [Click here to view] |
Antibiotic administration
In less than 50% (n = 478) of the patients, empirical antibiotics were administered. Among these patients, one to six classes of antibiotics were used. The commonly used antibiotic classes including both oral and parenteral formulations during empirical therapy were penicillin with or without β-lactamase inhibitors (56%), followed by cephalosporins (46%), lincomycins (18%), and so on (supplementary file: Table 1). Fifty-two percent (n = 522) of the patients received definitive therapy (either continuation of the empirical therapy or change in antibiotics based on the culture sensitivity test). Of the 19 different classes of antibiotics, the number of antibiotic classes used ranged between 1 and 10. The most frequent antibiotic class used were cephalosporins (54%), followed by penicillin with or without β-lactamase inhibitors (50%), carbapenems (29%), lincomycins (20%), and so on (supplementary file: Table 1).
Mortality
In the study, 167 (17%) patients died during the course of treatment. Intensive care unit (ICU) mortality was found to be 30%. Most of the patients who died had mono-infections (68%), followed by polymicrobial infections (32%), and mixed infections (14%).
Resistant strains of E. coli (n = 68) followed by Klebsiella spp. (n = 61), Acinetobacter spp. (n = 38), Enterococcus spp. (n = 26), MRSA (n = 17), and Pseudomonas spp. (n = 12) were identified in patients who died (supplementary file: Table 2a). The percentage of death in patients with both HAIs + CAIs and mono-infections were 25% and 68%, respectively (supplementary file: Table 2b). Clinical conditions, such as Group H (blood-related disorders) (97%) followed by Group V (sepsis and septic shock) (83%), Group B (respiratory disorders) (61%), and Group C (renal disorders) (52%) that were present among the mortal patients (supplementary file: Table 2c).
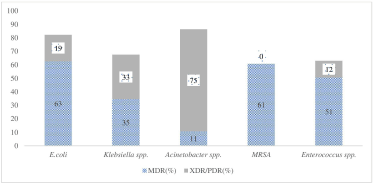 | Figure 1. MDR and PDR strains of different Gram-negative and Gram-positive isolates (%) found in the study. [Click here to view] |
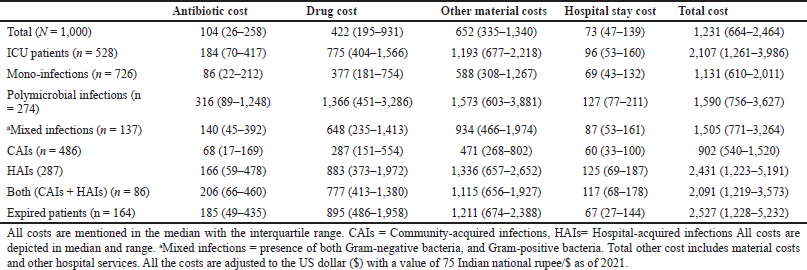 | Table 2. Shows the median costs incurred (in $) due to resistant infections. [Click here to view] |
Cost
The study assessed the direct cost incurred during the treatment for all the included patients. The antibiotic cost constituted 13.2% of the overall cost. The antibiotic costs were higher for patients with polymicrobial infections [$316 ($89–$1,248)], followed by patients admitted to ICU [$184 ($70–$417)], patients with both CAIs and HAIs [$206 ($66–$460)], and with HAIs [$166 ($59–$478)]. The costs for the patients who died during the course of therapy were also found to be high [$185 ($49–$435)]. The cost incurred due to resistant infections has been detailed in Table 2. The median hospital stay costs for the polymicrobial infections were twice the mono-infections, respectively, whereas the hospital stay cost for HAIs was two times the cost of CAIs (Table 2). The median total costs were high for patients who died [$2,527 ($1,228–$5,232)] followed by patients with HAIs [$2,431 ($1,223–$5,191)], patients admitted to ICU [$2,107 ($1,261–$3,986)], and patients with polymicrobial infections [$1,590 ($756–$3,627)]. The overall cost for HAIs was 2.5 times more than the CAIs [$902 (540–1,520)]. Mortality, total LoS, both (HAIs and CAIs), number of classes of empirically used antibiotics per patient, number of diagnoses or clinical findings, number of classes of definitive antibiotics used per patient, HAIs number of days of mechanical ventilation were found highly associated with increasing the overall cost (Table 3a). We tested for different strains of bacteria against cost and found resistant strains (MDR and XDR/PDR) of GNBs, such as Klebsiella spp., E. coli, Acinetobacter spp., Pseudomonas spp., and GPBs Enterococcus spp., had a positive association with the cost as well (Table 3b). However, we found no association between MRSA and direct cost. “Pearson’s correlation coefficient” showed that resistant strains (MDR and XDR/PDR) were highly correlated with increased cost (p = <0.05).
In addition to this, we also tested for the clinical diagnoses against cost and found that Group “V” (sepsis with septic shock, urosepsis, bacteremia), Group “O” (associated with psychological disorders), Group “F” (bone, skeletal muscle, and connective tissue related events), Group “U” (patient with COVID-19), Group “T” (multiple organ failure), Group “D” (neurological, spinal cord events), Group “I” (associated with urinary, prostate-related events), Group “A” (associated with cardiovascular-related events), Group “P” (Viral or Fungal infections), Group “R” (surgical treatment), Group “C” (associated with renal events), and Group “B” (associated with respiratory events), were associated with high cost (Table 4).
A residual scatter plot indicated that the linear model for both the cost and diagnosis fit the data well (supplementary file: Fig. 7a and b).
DISCUSSION
In 2017, WHO published its first-ever list of antibiotic-resistant “priority pathogens” that pose the greatest threat to human health. The most critical group included Acinetobacter spp., Pseudomonas spp., and other Enterobacterales. They can cause severe and often deadly infections such as bloodstream infections and pneumonia (WHO, 2017). Several studies on AMR have been published in India in recent years. In our study, MDR and XDR/PDR isolates of E. coli (63% and 19%), Klebsiella spp. (35% and 33%), and Acinetobacter spp. (11% and 75%) were found among the GNBs, and among the GPBs, MRSA (61%), and MDR and XDR/PDR strains of Enterococci spp. (51% and 12%) were found. A study from Maharashtra, India, reported MDR and XDR isolates of E. coli, Klebsiella spp., and Acinetobacter spp. as 30% and 8%, 38% and 13%, and 45% and 19%, respectively. MRSA and MDR, and XDR/PDR strains of Enterococci spp. were found to be 20%, 29%, and 36%, respectively (Basak et al., 2016). Our study showed more than 85% of E. coli isolates were highly resistant to third-generation cephalosporins (GCs) and fluoroquinolones and carbapenems. A study from Puducherry, India, reported the resistance pattern of MDR E. coli that were resistant to amoxicillin-clavulanic acid (74%), fluoroquinolones (74%), and third-GCs (72%) but were susceptible to amikacin, nitrofurantoin, and carbapenem (Niranjan and Malini, 2014). Our study showed that more than 90% of Acinetobacter spp. were highly resistant to β-lactam/β-lactamases inhibitors, carbapenems, third and fourth-GCs, and fluoroquinolones. A 5-year surveillance study from New Delhi showed an increasing trend of carbapenem-resistant Acinetobacter baumannii (CRAB). The study also showed that Acinetobacter spp. was highly resistant to third and fourth-GCs, fluoroquinolones, and aminoglycosides (Kumari et al., 2019a). Klebsiella spp. were highly resistant to nitrofurantoin (90%), and moderately resistant (>70%) to carbapenems and fourth-GCs. Nonetheless, the isolates were susceptible to colistin. A study from Punjab, India, reported that 8.75% and 52% of Klebsiella spp. were resistant to colistin and carbapenems, respectively, followed by β-lactam/β-lactamase inhibitors (60%) and fourth-GCs (55%) (Sodhi et al., 2020).
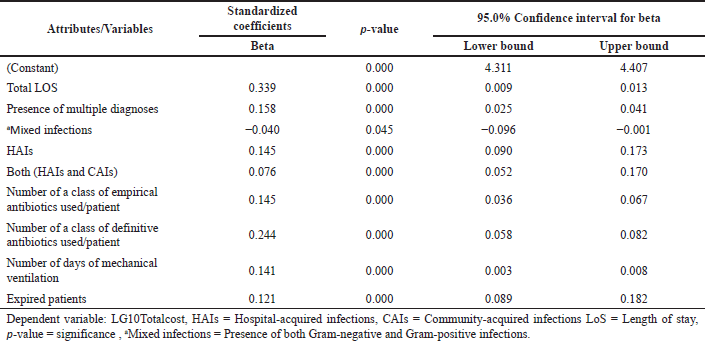 | Table 3a. Association between different attributes and the cost. [Click here to view] |
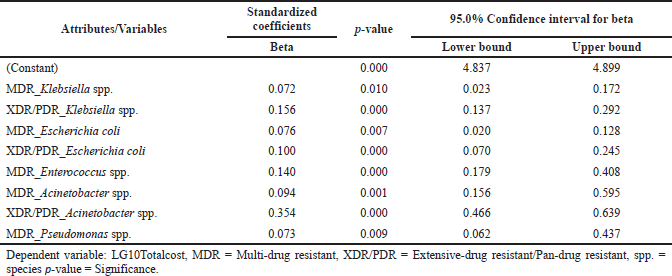 | Table 3b. Association between different bacterial infections and the cost. [Click here to view] |
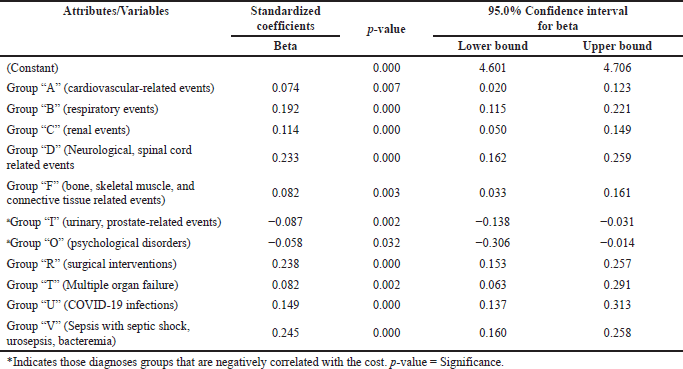 | Table 4. Association between clinical diagnoses and the cost. [Click here to view] |
Our study showed that 75% of Pseudomonas spp. were highly resistant to carbapenems, β-lactam/β-lactamases inhibitors, and tigecycline. A study from New Delhi, India, showed that more than 50% of isolates were resistant to carbapenems, amikacin and over 65% of isolates were resistant to fluoroquinolones and gentamicin (Kumari et al., 2019b).
Our hospital has revised the antibiotic policy and initiated the antimicrobial stewardship program (AMSP) in 2015. However, our study showed a similar or higher bacterial resistant pattern compared with previously reported studies. The challenging issue is that these MDR and XDR/PDR strains of GNBs are developing resistance to last-resort agents at a faster rate than the novel antibiotic development. The high rate of spontaneous mutations and extensively prevalent genetic exchange mechanisms in bacteria are critical contributors to the emergence of this phenomenon. The high antibiotic-resistant shown by CRAB, Klebsiella spp. has led to limited treatment options, and currently, in our settings, few of the patients are treated with polymyxins and tigecycline combinations. Morbidity and mortality due to these infections are on the rise. The cost of treatment is also double that of those with susceptible infections.
Enterococci spp. were completely resistant to third-GCs and TMP-SMX. However, these isolates were completely susceptible to linezolid and clindamycin. Vancomycin-resistant Enterococci (VRE) was found in 13% of the isolates. A study from Lucknow, India reported a prevalence of 7.6% of VRE strains (Tripathi et al., 2016). Our study suggested a gradual increase in the trend of VRE strains over the time.
Our study reported 64% of MRSA strains amongst all S. aureus isolates. Only 3% of MRSA were resistant to linezolid; however, entirely susceptible to glycopeptides such as vancomycin and teicoplanin. Linezolid is an effective antibiotic against MRSA and VRE. A study from Delhi, India, reported the presence of linezolid-resistant MRSA and VRE in donor-recipient patients. The emergence of such resistance is due to the availability of many antibiotics in the oral formulation has made these drugs easily accessible to the general public and are being prescribed as an empirical therapy to the outpatients, with no stringent guidelines on antimicrobial prescribing practices (Rai et al., 2015). Community-acquired methicillin-resistant S. aureus (CA-MRSA) is now being increasingly reported in India (Joshi et al., 2013). Our study reported CA-MRSA and hospital-acquired methicillin-resistant S. aureus (HA-MRSA) as 76% and 24%, respectively. Preeja et al. (2021) study from Mangalore, India, reported the prevalence of MRSA as 25% with CA-MRSA and HA-MRSA at 61% and 39%, respectively (Preeja et al., 2021).
Among dead patients, Group H (blood-related illnesses) (97%) followed by Group V (sepsis and septic shock) (83%), Group B (respiratory disorders) (61%), and Group C (renal disorders) (52%) were the clinical conditions that were present in our study. In the majority of these patients, GNBs were found. A study from Nepal, showed as high as 100% of deaths due to bacterial meningitis followed by sepsis (97% of fatalities), invasive pneumonia (89% of fatalities), and GNB were present in these patients. Older patients who also have comorbid conditions have weakened immune systems and are more susceptible to invasive Gram-negative infections (opportunistic infections) than younger adults (Bhattarai et al., 2021; Lin et al., 2019).
In our study, the most frequently used antibiotic classes were cephalosporins and combinations, penicillins together with β-lactamase inhibitors, carbapenems, lincomycins, glycopeptides, and aminoglycosides. A multicentric study across India has shown the heavy use of different antibiotic classes, such as cephalosporins, penicillin/β-lactamase inhibitors, carbapenems, macrolides, and aminoglycosides, for medical and surgical prophylaxis (Singh et al., 2019). The high prevalence of prescribing broad-spectrum antibiotics is due to the clinician’s perception of these agents, effective against extended-spectrum β-lactamase-producing GNBs. Using these agents as prophylaxis is one of the driving factors for AMR escalation in GNBs and GPBs (Zarb and Goosens, 2011).
Our analysis showed that the median cost for HAIs was 2.5 times higher than CAIs i.e., [$,2431 ($1,223–$5,191)] versus [$901 ($530–$1,513)], respectively. The median total cost for the patient admitted to ICU was also high, [$2,107 ($1,261–$3,986)]. The study showed antibiotics cost constituted only 13.2% of the total cost. ICU mortality rate was found to be 30%, with a median attributable cost of $2,498 ($1,256–$5,328). An ICU-based study by Chacko et al. (2017) from Tamil Nadu, India, has shown that the median attributable cost was $2,773 ($2,151–$3,649) with an ICU mortality of 28% (Chacko et al., 2017). Our study showed the pathogens responsible for HAIs are GNBs, such as E. coli followed by Klebsiella spp., Acinetobacter spp., Pseudomonas spp., Enterobacter spp., and a few GPBs, such as Enterococcus spp., MRSA, S. aureus, and Streptococcus spp., were responsible for HAIs and associated with higher cost. A similar finding was reported from a study from Punjab, India, in which GNBs with few GPBs were associated with HAIs and higher costs. Our study reports the median ICU LoS, and median antibiotic cost in ICU cost for patients with resistant infections as 2 (0.8) days and $184 ($70–$417) (Tiwari and Rohit, 2013). An ICU-based study from Karnataka India, has reported the median LoS as 8.5 days and the median antibiotic cost as $2,343.26 ($126.44–$24,168.32), respectively (Priyendu et al., 2014). Our findings reported that patients with polymicrobial infection have a higher median antibiotic cost [$316 (89–1,248)] and median total cost [$1,590 ($756–$3,627)] compared to mono-infection, indicating that physician consultation fee, antibiotics including surgical prophylaxis, and other drug acquisition costs, room rent, investigation costs, were markedly higher for polymicrobial infection compared to the latter. Drug acquisition cost, especially the antibiotic cost, was the major contributor to the extra cost. The median direct cost in our study was $1,231 which is enormous from an Indian perspective. The average Indian per capita income stands at $1,947 for the financial year 2020–21 which remains as a point of discussion for the increasing need for various insurance policies (The Indian Express, 2021).
The applied multivariate regression model in our study showed that the total LoS (Beta = 0.339) followed by the number of classes of definitive antibiotics (Beta = 0.224), presence of multiple diagnoses (Beta = 0.158), and HAIs (Beta = 0.145) had the maximum positive impact on total cost (p < 0.001), whereas the presence of both HAIs & CAIs (Beta = 0.076) has a minimal positive impact on total cost and the presence of mixed infection (Beta = −0.040; because of negative beta coefficient) has shown to reduce the total cost. A study using multivariable regression analysis has shown a significant increase in healthcare costs due to mortality and LoS associated with MRSA and CRAB with an aggregate estimate of $1.9 billion ($1.3 billion−$2.5 billion) (Nelson et al., 2022). Studies have shown that increased usage of catheters and ventilation has led to high chances of HAIs such as catheter-associated bloodstream infection, catheter-associated urinary tract infections, and ventilator-associated pneumonia, which has led to complicated treatment, thereby, escalating the cost of patients (Bysshe et al., 2017).
Due to the prevailing COVID-19 situation, the in-patient admission was very low ranging between 32% and 75% during the study period. The restricted use of antibiotics during this period could also have influenced the cost of therapy as India is well known for its heavy usage of antibiotics including OTC with an expected increment in cost.
A better understanding of the economic effects of resistant infections may help clinicians, hospital administrators, infection control teams, and policymakers determine how much to invest in medical treatment that can identify and control its spread.
CONCLUSION
Highly resistant strains of GNBs were predominantly high in our study. Acinetobacter spp. and Pseudomonas spp. were highly resistant to last-resort antibiotics such as carbapenems. HAIs are of serious concern in terms of mortality and cost. The mortality and cost for HAIs were twice and 2.5 times higher than the CAIs, respectively. The median antibiotic cost and the total cost for patients admitted to ICU and with polymicrobial infection were found to be high. Increased LoS, the presence of multiple diagnoses along with resistant infections, and increased usage of multiple antibiotics have also increased the cost. The escalating antibiotic cost causes an immense economic burden on patients who bear treatment expenses for resistant infections in India. The current study will help in better understanding the local geographical AMR profile, implementing and strengthening the AMSP to reduce the occurrence of hospital infections, thus, reducing the cost.
ACKNOWLEDGMENTS
We are thankful to ICMR for providing a fellowship (BMI/11(11)/2020) for this study. The authors thank Miss Faiza Iqbal, Ph.D. Scholar from the Department of Paediatrics for helping in understanding the working principles behind the microbiology department. We are thankful to the staffs of the microbiological department, at Kasturba hospital for helping in understanding the basic working principles of microbiological instruments. We thank the medical record department, Kasturba hospital, for providing the neonatal case files for recording the cases. The trial is registered at the Clinical Trial Registry of India CTRI identifier (CTRI/2020/06/025920).
AUTHOR CONTRIBUTIONS
Prashant Chandra: Concept, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review.
S. Elstin Anburaj: Concepts, design, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review.
K. Vijayanarayana: Concepts, design, definition of intellectual content, data analysis, statistical analysis, manuscript editing, and manuscript review.
K. E. Vandana: Definition of intellectual content, clinical studies, experimental studies, data acquisition, manuscript editing, and manuscript review.
Chiranjay Mukhopadhyay: Clinical studies, experimental studies, data acquisition, manuscript editing, and manuscript review.
U. Dinesh Acharya: Manuscript preparation, manuscript editing, and manuscript review.
M. Surulivelrajan: Manuscript preparation, manuscript editing, and manuscript review.
V. Rajesh: Concepts, definition of intellectual content, clinical studies, experimental studies, manuscript editing, manuscript review, and guarantor.
CONFLICT OF INTEREST
There is no conflict of interest. All authors agree to be accountable for all aspects of the work.
ETHICAL APPROVALS
The Institutional Ethical Committee (IEC) approved this observational study that involves human subjects and the patient’s informed consent was obtained in advance. The IEC No. is IEC:477/2019.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Atal S, Mathur ASB. Cost of treating bacterial infections in India: a cost minimization analysis to assess price variations. Biomed Pharmacol J, 2020; 13:765–78.
Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. J Pathog, 2016; 2016; https://doi.org/10.1155/2016/4065603
Bhattarai S, Sharma BK, Subedi N, Ranabhat S, Baral MP. Burden of serious bacterial infections and multidrug-resistant organisms in an adult population of Nepal: a comparative analysis of minimally invasive tissue sampling informed mortality surveillance of community and hospital deaths. Clin Infect Dis Off Publ Infect Dis Soc Am, 2021; 73:S415–21; https://doi.org/10.1093/cid/ciab773
Bysshe T, Gao Y, Heaney-Huls K, Hockenberry J, Hovey L, Laffan AM, Lee S, Murphy DJ, Watts E. Estimating the additional hospital inpatient cost and mortality associated with selected hospital-acquired conditions. Agency Healthc Res Qual, 2017; 75:1–75.
Chacko B, Thomas K, David T, Paul H, Jeyaseelan L, Peter JV. Attributable cost of a nosocomial infection in the intensive care unit: a prospective cohort study. World J Crit Care Med, 2017; 6:79–84; https://doi.org/10.5492/wjccm.v6.i1.79
Chandra P, Unnikrishnan MK, Ke V, Mukhopadhyay C, Dinesh Acharya U, Surulivel Rajan M, Rajesh V. Antimicrobial resistance and the post antibiotic era: better late than never effort. Expert Opin Drug Saf, 2021; 20:1375–90; https://doi.org/10.1080/14740338.2021.1928633 CLSI. M100 performance standard for response: antimicrobial susceptibility testing. 30th edition, 2020. Available via https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist, 2019; 12:3903–10; https://doi.org/10.2147/IDR.S234610
Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One, 2017; 12:e0189621; https://doi.org/10.1371/journal.pone.0189621
Jit M, Ng DHL, Luangasanatip N, Sandmann F, Atkins KE, Robotham JV, Pouwels KB. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: rapid methodological review, conceptual framework and recommendations for future studies. BMC Med, 2020; 18:38; https://doi.org/10.1186/s12916-020-1507-2
Joshi S, Ray P, Manchanda V, Bajaj J, Chitnis DS, Gautam V, Goswami P, Gupta V, Harish BN, Kagal A, Kapil A, Rao R, Rodrigues C, Sardana R, Devi KS, Sharma A, Balaji V. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res, 2013; 137:363–9.
Kaul R. Antibiotic intake in India rises by 30% in a decade, says report - Hindustan Times. 2021. Available via https://www.hindustantimes.com/india-news/antibiotic-intake-in-india-rises-by-30-in-a-decade-says-report-101612377295525.html (Accessed 12 June 2022).
Kumari M, Batra P, Malhotra R, Mathur P. A 5-year surveillance on antimicrobial resistance of Acinetobacter isolates at a level-I trauma centre of India. J Lab Physicians, 2019a; 11:34; https://doi.org/10.4103/JLP.JLP_72_18
Kumari M, Khurana S, Bhardwaj N, Malhotra R, Mathur P. Pathogen burden & associated antibiogram of Pseudomonas spp. in a tertiary care hospital of India. Indian J Med Res, 2019b; 149:295–8; https://doi.org/10.4103/ijmr.IJMR_14_18
Lin E, Lin K, Katz S. Serious and opportunistic infections in elderly patients with inflammatory bowel disease. Gastroenterol Hepatol, 2019; 15:593–605.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis, 2012; 18:268–81; https://doi.org/10.1111/j.1469-0691.2011.03570.x
Nelson RE, Hyun D, Jezek A, Samore MH. Mortality, length of stay, and healthcare costs associated with multidrug-resistant bacterial infections among elderly hospitalized patients in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am, 2022; 74:1070–80; https://doi.org/10.1093/cid/ciab696
NHSN. 2022. Surgical Site Infection 39.
Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res, 2014; 139:945–8.
Preeja PP, Kumar SH, Shetty V. Prevalence and characterization of methicillin-resistant Staphylococcus aureus from community- and hospital-associated infections: a tertiary care center study. Antibiot Basel Switz, 2021; 10:197; https://doi.org/10.3390/antibiotics10020197
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health, 2015; 109:309–18; https://doi.org/10.1179/2047773215Y.0000000030
Priyendu A, Naik NA, Varma M, Vandana KE, Rajesh B. The length of stay and the hospital cost for ICU patients with resistant bacterial infections: a pilot study. BMC Infect Dis, 2014; 14:P38; https://doi.org/10.1186/1471-2334-14-S3-P38
Rai S, Niranjan DK, Kaur T, Singh NP, Hada V, Kaur IR. Detection of the classical G2576U mutation in linezolid resistant Staphylococcus aureus along with isolation of linezolid resistant Enterococcus faecium from a patient on short-term linezolid therapy: first report from India. Indian J Med Microbiol, 2015; 33:21–4; https://doi.org/10.4103/0255-0857.148371
Singh SK, Sengupta S, Antony R, Bhattacharya S, Mukhopadhyay C, Ramasubramanian V, Sharma A, Sahu S, Nirkhiwale S, Gupta S, Rohit A, Sharma S, Raghavan V, Barman P, Sood S, Mamtora D, Rengaswamy S, Arora A, Goossens H, Versporten A. Variations in antibiotic use across India: multi-centre study through global point prevalence survey. J Hosp Infect, 2019; 103:280–3; https://doi.org/10.1016/j.jhin.2019.05.014
Sodhi K, Mittal V, Arya M, Kumar M, Phillips A, Kajla B. Pattern of colistin resistance in Klebsiella isolates in an intensive care unit of a tertiary care hospital in India. J Infect Public Health, 2020; 13:1018–21; https://doi.org/10.1016/j.jiph.2019.10.013
The Indian Express. Bangladesh outpaces India on per capita income. Business News, The Indian Express. 2021. Available via https://indianexpress.com/article/business/economy/bangladesh-outpaces-india-on-per-capita-income/ (Accessed 12 June 2022).
Tiwari P, Rohit M. Assessment of costs associated with hospital-acquired infections in a private tertiary care hospital in India. Value Health Reg Issues, 2013; 2:87–91; https://doi.org/10.1016/j.vhri.2013.03.002
Tripathi A, Shukla SK, Singh A, Prasad KN. Prevalence, outcome and risk factor associated with vancomycin-resistant Enterococcus faecalis and Enterococcus faecium at a tertiary care hospital in Northern India. Indian J Med Microbiol, 2016; 34:38–45; https://doi.org/10.4103/0255-0857.174099
WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. Available via https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (Accessed 12 June 2022).
WHO. Report on the burden of endemic health care-associated infection worldwide. World Health Organization, Geneva, Switzerland, 2011.
Zarb P, Goosens H. European surveillance of antimicrobial consumption (ESAC) value of a point-prevalence survey of antimicrobial use across Europe. 2011. Available via https://www.researchgate.net/publication/297438130_European_Surveillance_of_Antimicrobial_Consumption_ESAC_Value_of_a_Point-Prevalence_Survey_of_Antimicrobial_Use_Across_Europe (Accessed 12 June 2022).
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website