INTRODUCTION
Urolithiasis is a condition in which stones formed in the urinary system. Urinary calculi were formed in humans as a result of calcium oxalate (CaOx) agglomeration in the renal area, and men were more likely to have them than women (Robertson et al., 1978). When related to healthy individuals, sufferers with urinary calculi excrete expressively more oxalate and calcium in the urine. Imbalance among the developers, like less CaOx, uric acid, and urine volume in addition to phosphate, represents the potential factors of lithogenesis. Lithotripsy is a surgical process for the removal of existing stones; however, lithotripsy is a painful procedure and the number of times stones form is higher; hence, research should focus on phyto pharmaceuticals, which show an essential part in preventing the recurrence amount of stone formation (Butterweck and Khan, 2009). Plant extracts exhibit anti-lithogenic effects by altering how calcium and magnesium ions are put together in the urine. Moreover, herbal extracts high in saponins cause disarrangement of mucoprotein suspensions which helps crystallization. (Grases and Costa-Bauzá, 1990).
Since ancient times, India has used a lot of different plants to cure renal stones. One of them is Achyranthes aspera. It spreads like a weed and is widespread across India. It goes to the family Amaranthaceae. It is commonly known as a prickly chaff flower. According to Ayurvedic literature, the roots and leaves of this plant have antilithiatic and diuretic activity (Srivastav et al., 2011). The houseplant is well-known for its immuno-modulatory (Srivastav et al., 2011), anti-inflammatory (Rajeshwari et al., 2020), anticancer (Subbarayan et al., 2012), antinociceptive (Alam et al., 1970), thrombolytic (Ghimire et al., 2017), antidiabetic (Akhtar and Iqbal, 1991) properties. Aerial parts of A. aspera are rich in phytochemicals such as alkaloids, phenols, flavonoids, saponins, tannins, and glycosides (Rajeshwari et al., 2022). To date, no research on the aerial parts of A. aspera as anti-urolithiatic has been reported. The present work is aimed at investigating the In-vitro and In-vivo anti-urolithiatic potency as the saponin rich fraction of the methanolic extract of A. aspera in male Wistar rats.
MATERIAL AND METHODOLOGY
Collection and authentication of plant specimen
Aeriform parts of A. aspera were taken from the Maisammaguda, Hyderabad, and have been approved by Dr. P.V. Prasanna, botanist “G,” Botanical Survey of India, Hyderabad. A voucher specimen was stored in the herbarium for further reference (BSI/DRC/2020-2021/Identification/230).
Achyranthes aspera saponin-rich fraction preparation
From the methanol, extract of A. aspera, a saponin-rich fraction, was taken out as per the method outlined by Latha et al. (2011). Pulverized A. aspera aerial parts were extricated with methanol in a soxhlet extractor and excess solvent was concentrated under vacuum. The dark residue that was left was heated with n-butanol for 2 hours and the parts that could be filtered out were separated. A few milliliters of 2% KOH were added for the separation of the two layers. The n-butanol layer was collected, and the solvent was removed under the vacuum to get the saponin-rich fraction. The yield of the saponin-rich fraction was found to be 1.3%.
Animals
Female Wistar rats (for the acute toxicity study) and male Wistar rats (for the in-vivo anti- urolithiatic activity) weighing around 180–200 g were selected. Animals were placed in cages made of polypropylene at random times with bedding made of rice husks. Animals were housed at a relative humidity of 30%–70% and at a temperature of 24°C ± 2°C. The light–dark cycle was kept at 12:12. All of the animals were served a typical commercial pellet rat diet and were given unlimited access to water. The study’s experimental techniques and methods were examined and approved under 1447/PO/Re/S/11/CPCSEA-58/A by the institutional animal ethics committee before conducting the experimental studies.
Acute toxicity studies
The OECD-423 recommendations were followed for investigating the acute oral toxicity of the saponin-rich portion of A. aspera. Wistar rats who weren’t pregnant were fasted the previous night from food and had allowed contact with water prior their dosing. Before the test extracts were given to them orally, they were weighed. Acute oral toxicity in Wistar rats was studied at ascending doses of 100, 500, and 1,000, in addition to 2,000 mg/kg. Initially, parameters such as behavior, autonomic, neurological symptoms, and mortality were observed after 4 hours of dosage. Body weights were measured 6 hours post-dosing. Body weights were recorded on the 8th and 14th days after drug administration, and behavioral variation, toxic symptoms, or deaths were monitored starting the next day and every day for up to 14 days. The therapeutic dosage was determined to be a cut-off dose of 1/5th and 1/10th of the larger dose since the rats were not dead.
Assessment of anti-urolithiatic activity of the saponin-rich fraction of A. aspera (SAA)
Nucleation assay
The nucleation assay helps in determining the effect of saponin-rich fractions on the formation of CaOx crystals (Patel et al., 2012). Solutions of sodium oxalate (Na2C2O4) (7.5 mM) and calcium chloride (CaCl2) (5 mM) were prepared in buffer (pH 6.5), Tris-HCl (0.5 M) and NaCl (0.15 M). Infusions of Cystone (acquired from Himalaya Herbal Healthcare, India, batch no. 11700204) as well as the saponin fraction were produced in distilled water at concentrations of 100, 200, 400, 600, 800, and 1,000 μg/ ml. One milliliter of every concentration of the Cystone® and the saponin-rich fraction were assorted with 3 ml of CaCl2 stalked by the accumulation of 3 ml of Na2C2O4 solution. The resulting mixture underwent a 30-minute incubation period at 37°C and was cooled to room temperature. Finally, the optical density (OD) of the mixtures at 620 nm was measured using a spectrophotometer. The formula was accustomed to determine the % inhibition of nucleation for the saponin-rich fraction and Cystone (Patel et al., 2012).
% Inhibition = [1 – (ODtest/ODcontrol) × 100] ODtest = OD of the test (saponin-rich fraction/cystone) ODcontrol = OD of the negative control
CaCl2 + Na2C2O4 → CaC2O4 + 2NaCl
Aggregation assay
According to this approach, the impact of the saponin-rich fraction and Cystone on CaOx crystal aggregation was investigated and described through Atmani and Khan (2001) with slight alterations. Sodium oxalate and calcium chloride were combined by a ratio of 50 mmol/l to cCaOx crystals. In a water bath, both solutions were equilibrated at 60°C for 1 hour and incubated overnight at 37°C. By using centrifugation, the crystals were collected. CaOx slurry was prepared at a concentration of 0.8 mg/ml were buffered at pH 6.5 with Tris-HCl (0.05 mol/l) and NaCl (0.15 mol/l). Three milliliters of the CaOx solution were mixed with Cystone and 1 ml from each of the following dilutions of the saponin-rich fraction: 100, 200, 400, 600, 800, and 1,000 μg/ml, respectively. The degree of inhibition of aggregation was computed as indicated similarly to the nucleation test using the final mixtures and OD was measured at 620 nm.
We used a microscope at 1,000× magnification to quantify the quantity, shape, and size of CaOx crystals generated in the presence or absence of extract and Cystone (Atmani and Khan, 2001).
Determination of anti-urolithiatic activity in animal models
We chose thirty healthy male Wistar rats and separated them into five clusters with six in each group. Group I served as the control and administered normal saline. Urolithiasis was induced in animals from Groups II–V by giving them 0.75% ethylene glycol in their drinking water, Group II served as disease control, receiving only ethylene glycol for 28 days, Group III received the standard polyherbal drug Cystone (750 mg/kg), Group IV received 200 mg/kg SAA, and Group V received 400 mg/kg SAA, respectively. All the extracts and the standard regimen were given orally once a day to the respective groups.
Group I = Control (saline treatment)
Group II = Disease Control [ethylene glycol (0.75% v/v)]
Group III = Positive Control [Cystone (750 mg/kg b.w)]
Group IV = 200 mg/kg of pera SAA Group V = 400 mg/kg bw of the SAA
Sampling and analyzing urine
The animals were housed in distinct metabolic crates, and on the 28th day, 24-hour urine trials were taken. Water was available to animals at all times when urine was collected. By adding a drop of hydrochloric acid, urine was made acidic and stored at −20°C and it was analyzed for uric acid, urea, and calcium levels.
Collection and analysis of serum
On the 28th day, a blood sample was taken from each animal via the retro-orbital plexus while it was under anesthesia. Blood was centrifuged for 10 minutes at 10,000 rpm to extract the serum. The biochemical parameters BUN and creatinine were sustained using the appropriate diagnostic supplies.
Histopathology
The abdomens of the rats were cut open, and their kidneys were individually removed in the presence of a large dosage of ether. Separated kidneys had their superfluous tissue removed and stored in a 10% v/v neutral formalin solution. The isolated kidney was divided into two equal halves to identify variations in CaOx deposits and kidney architecture. Single separated kidneys are fixed in paraffin and cut into thin pieces and histopathological examination was done using a rotating vertical microtome and polarised light (magnification 10×). The final histological analysis was performed using eosin and hematoxylin staining. Subsequently, using an optical microscope, these slices were examined and captured on camera. After homogenizing the second half of the kidney using a 5% HCl solution, at 25°C, the homogenate was centrifuged for 10 minutes at 3,000 rpm. To estimate the in-vivo antioxidant properties, the supernatant was employed.
Statistical analysis
Using Graph pad Prism (version 6.3.2), results are presented as mean SEM. Using the statistical implication of one-way analysis of variance, data were evaluated (One way-ANOVA), and then non-parametric methods utilizing the t-tests for independent samples.
RESULTS
Acute oral toxicity studies
Individual therapeutic doses of 1/5th and 1/10th of 2,000 mg/kg were selected based on the results of acute oral toxicity. Specifically, 200 and 400 mg/kg were taken into consideration for animal studies. The respiratory, somatomotor, central, circulatory, and autonomic nervous systems were not adversely affected by either extract with a dose of 2,000 mg/kg. Similar to this, the behavioral pattern is identified as typical throughout the investigation. Not observed any odd changes in the eyes, mucous membranes, or fur, and the animals showed no signs of convulsions or tremors.
In-vitro activity against urolithiasis
Nucleation assay
This spectrometric determination of the turbid solution prepared with the standard and the test samples showed a substantial decrease in the OD. The OD dropped as the concentration of SAA’s increased, showing that this reduced the nucleation of CaOx particles. The SAA inhibited nucleation dose-dependently. The values are compared with the standard Cystone. At higher concentrations, the SAA (80.43%) displayed a lessening in nucleation in contrast to the standard Cystone® (86.96%).
In the control, a significant number of sharp-edged dendrites or rectangular-shaped CaOx monohydrate crystals predominated. The saponin fraction of A. aspera and Cystone has shown a decrease in size and number of CaOx quartzes (Fig. 3).
Aggregation test
The OD of numerous concentrations of the saponin-rich fraction was estimated spectrophotometrically and compared with the standard. The concentrations of a SAA reduced the turbidity of the solution. The percentage inhibition was calculated and represented in Figure 2. In this case, a concentration-dependent rise in the CaOx crystal aggregation’s percent inhibition was observed. A size reduction in the crystals was observed with the increased concentration of SAA from the microscopic study. The extract, with dose-dependent effects, triggered a greater decrease in the size and number of crystals when associated with that of the negative control. At higher concentrations, a flower-like crystal shape was also observed, along with a bipyramidal shape.
In-vivo anti-urolithiatic activity
After the 28-day treatment of ethylene glycol, Group II showed a substantial reduction in urine volume when compared with the control group, and the pH of the urine became acidic. Acidic pH promotes the formation of aggregates in the renal tissue. Treatment with the 400 mg/kg dose of SAA in Group V has shown a marked increase in urine volume (p < 0.001) when associated with the standard group.
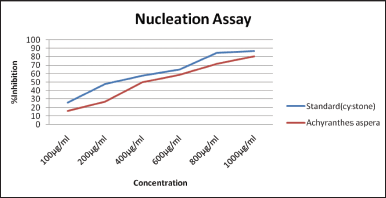 | Figure 1. Effect of the saponin-rich fractions on the nucleation of CaOx. [Click here to view] |
Oral administration of the SAA to rats in Group IV (200 mg/kg body weight) and Group V (400 mg/kg body weight) was discovered to significantly reduce calcium deposits in the kidneys as well as uric acid and urea excretion in the urine. Also, it was shown that the saponin-rich fraction of the A. aspera methanol extract has shown reduced kidney weight and urine creatinine levels while restoring urinary pH levels (Fig. 1). Renal stone-inducing treatment in group II has shown increased levels of urea, uric acid as well as calcium when likened to the control group. This demonstrates ethylene glycol-induced renal hyperoxaluria. However, group V 400 mg/kg bw (p < 0.001) showed a noticeable decrease in levels of uric acid, urea, and calcium in urine when associated with the positive control group II, which is shown in Table 1.
In untreated mice, renal stone formation impairs renal function as seen by indicators of glomerular and tubular damage. It was observed that there was a noticeable advancement of serum creatinine and BUN levels in the lithiatic group; however, these values decreased in a dose-dependent manner. At an advanced dose (400 mg/kg bw), the SAA showed 1.08 ± 0.07 mg/dl of creatinine (p < 0.001) and 46.34 ± 1.51 mg/dl (p < 0.001) of BUN levels, which were shown less among all the treatment groups of SAA next to the standard group, shown in Table 2.
In lithiatic rats (Group II), an important reduction in the levels of catalase enzyme (CAT), superoxide dismutase (SOD), as well as increased malondialdehyde (MDA) were observed associated with the control group and listed in Table 3 due to increased lipid peroxidation indicative of renal damage. When compared to Group II, Wistar rats in Groups IV and V (p < 0.001) showed highly significant differences. Simultaneous treatment with SAA has shown protective action against oxidative variations brought by lithiogenic administration in a dose-dependent method. It was detected that there is a rise in kidney weight in the disease control cluster when associated with the control group (Group-I), which is due to the deposition of CaOx. While treatment with SAA and Cystone (Groups III–V) showed a reduction in kidney weight when compared to disease control, which represents a reduction in CaOx deposition.
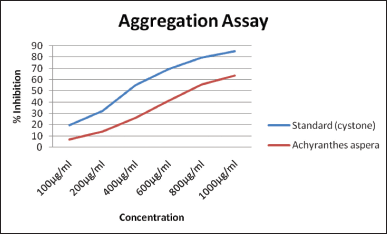 | Figure 2. Effect of the saponin-rich fractions on the aggregation of CaOx. [Click here to view] |
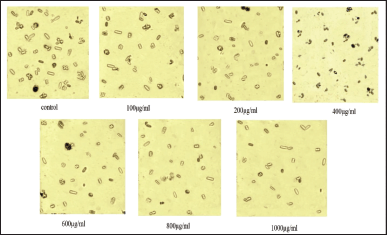 | Figure 3. Effect of saponin fraction of the methanolic extract A. aspera on nucleation assay of CaOx. [Click here to view] |
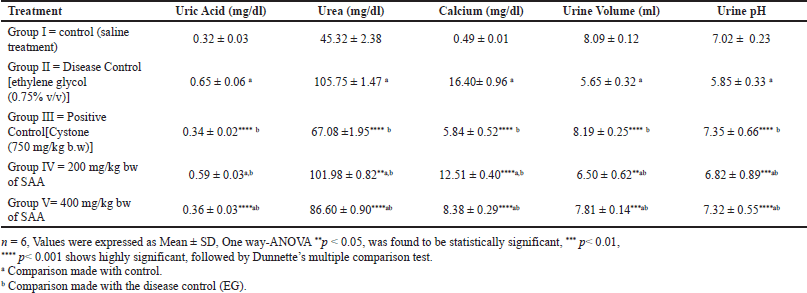 | Table 1. Effect of saponin rich fraction of methanolic extract of A. aspera on urine parameters in ethylene glycol induced urolithiasis (n = 6). [Click here to view] |
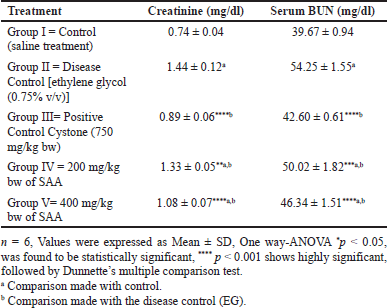 | Table 2. Effect of SAA on serum parameters in ethylene glycol induced urolithiasis (n = 6). [Click here to view] |
Histopathological analysis using phase contrast microscopy exposed no calcium deposits and no further irregularities in the nephron part of the control group (A), whereas the lithiogenic treatment group showed a marked diffused tubular dilatation in the kidney and mild multifocal tubular degeneration, which represents the deposition of CaOxcrystals, as represented in Figure 4. Group II(B) by arrow marks. As associated with the control group, treatment with the SAA and Cystone was shown to decrease renal injury and CaOx crystal formation while promoting the revival of the glomeruli and renal tubules. However, this SAA 400 mg/kg bw treatment group restored kidney architecture with no tubular dilatation and no crystal formation Figure 4. Group V( E).
DISCUSSION
As nucleation is a vital stage for the start of crystals, which subsequently develop and result in aggregates. In-vitro crystallization investigation was conducted in the present work. The saponin-rich fraction from plant extract (SAA), which was identified in the present work, prevented crystallization by preventing the nucleation of CaOx in solution; smaller as well as fewer particles made as the saponin-rich fraction concentration rose. The extract (SAA) includes nucleation-preventing chemicals, according to the results of the nucleation test. Since particles may develop to a size that obstructs the urinary system and causes stone formation, the mechanisms that restrict crystal growth may be the limiting variables in stone formation (Tandon et al., 2010).
There may be components in the chosen plant extract that prevent CaOx crystals from growing. This trait of plants may be essential in preventing kidney stone formation. The stone formation may be significantly influenced by aggregates. Agglomeration, also known as aggregation, is the process that leads to the development of clusters of crystals in urine. (Fleisch, 1978).
After 28 days in the current investigation, a significant rise in urine calcium, urea, and uric acid was observed in the disease control rats, showing that supersaturation of lithogenic-promoting drugs during hyperoxaluria-induced renal injury, which may result in lower urine production as compared to the control group, maybe the source of the renal damage (King, 1967).
In the current research, urolithiatic rats had increased uric acid excretion. It could be a result of uric acid interfering with CaOx solubility, which lessens glycosaminoglycans’ inhibitory function (Selvam et al., 2001). As associated with the control group, treatment with SAA 400 mg/kg decreases the incidence of stone development and decreases uric acid excretion by p < 0.0001.
Due to the obstruction caused by urinary system stones in urolithiasis, GFR is diminished, and as a result, nitrogenous waste products including creatinine and BUN build up in the blood (Grover and Resnick, 1995). In the current investigation, the nephrotoxic effects of ethylene glycol were characterized by significant increases in BUN and serum creatinine. In terms of serum creatinine, treatment with SAA 200 mg/kg resulted in p < 0.05 when associated with the control, whereas SAA 400 mg/kg resulted in p < 0.001 when associated with the control and diseased control, treatment groups showed an enhanced glomerular filtration rate and, thus, decreased the quantity of nitrogenous unwanted products BUN and serum creatinine.
A reduced concentration of SOD and CAT is a sign of constrained antioxidant activity and increased lipid peroxidative action, which may cause damage to the renal tubular cells, increases crystal adherence, and promotes the aggregation of renal stones. This was observed in Group II diseased control, which triggered the CaOx deposition in the renal tissue (Selvam and Bijikurien, 1992). Subsequent treatment with Cystone and a plant extract dosage regimen led to an increase in catalyze and SOD levels when compared to disease control.
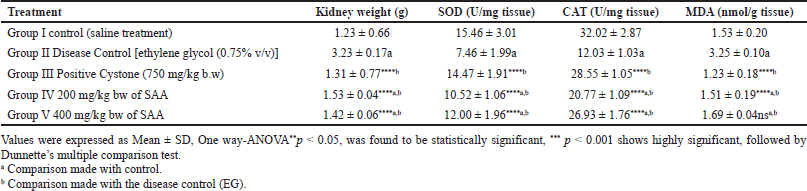 | Table 3. Effect of SAA on kidney parameters in ethylene glycol induced urolithiasis (n = 6). [Click here to view] |
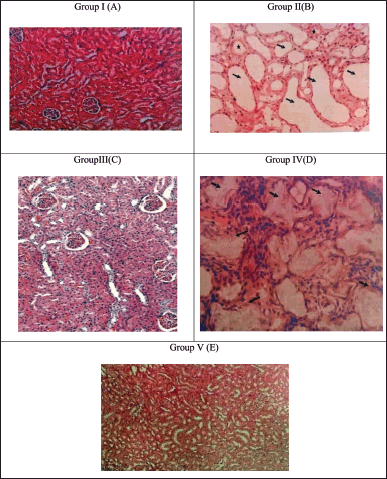 | Figure 4. Microscopic images of 10× 20× magnification of kidney sections (A). Control treatment shows the absence of calcium oxalate deposition (B). Treatment group shows numerous calcium oxalate deposition with tubular dilation. (C) Cystone treatment and SAA treated groups (D) 200 mg/kg bw and (E) 400 mg/kg bw has shown few tabulation and deposition of CaOx indicated by arrow marks. [Click here to view] |
Urinary pH has been linked to crystaluria, with changes in pH being shown to encourage the breakdown of urinary stones. CaOx stone development is encouraged by urine pH levels of 5.0–6.3 (Betanabhatla et al., 2009). The current research shows that the production of CaOx calculi is supported by the urine pH dropping from 7.02 to 5.85. Also, it was shown that restoring the pH of the urine (5.85–7.35) supported the breakdown of CaOx .
CONCLUSION
The outcomes of the current study showed that male Wistar rats with CaOx kidney stones were effectively treated with the saponin fraction of an A. aspera. On renal and urinary parameters, with the development of CaOx kidney stones, the extract demonstrated a dose-dependent therapeutic effect. These results corroborate earlier claims that the extract may be utilized to treat renal and urinary tract issues. Findings from this work rationalize the medicinal use of this extract in urolithiasis.
ACKNOWLEDGMENTS
Authors thankful to the Management and Principal of Mallareddy College of Pharmacy, Secunderabad, India for providing the necessary facilities to carry out the work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study’s experimental techniques and methods were examined and approved under 1447/PO/Re/S/11/CPCSEA-58/A by the institutional animal ethics committee before conducting the experimental studies.
DATA AVAILABILITY
Data is available with the Author and can be submitted upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akhtar MS, Iqbal J. Evaluation of the hypoglycaemic effect of Achyranthes aspera in normal and alloxan-diabetic rabbits. J Ethnopharmacol, 1991; 31(1):49–57. CrossRef
Alam MA, Slahin N, Uddin R, Hasan SMR, Akter R, Kamaluddin M, Faroque A, Ghani A. Analgesic and neuropharmacological investigations of the aerial part of achyranthes aspera linn. J Pharm Sci, 1970; 1(1):44–50. CrossRef
Atmani F, Khan SR. Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. Br J Urol, 2001; 85(6):621–5. CrossRef
Betanabhatla KS, Christina AM, Syama Sundar BS, Selvakumar S, Sundara Saravanan, K. Antilithiatic activity of Hibiscus sabdariffa linn. On ethylene glycolinduced lithiasis in rats. Indian J Nat Prod Resour, 2009; 8(3):3–47.
Butterweck V, Khan S. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med, 2009; 75(10):1095–103. CrossRef
Fleisch H. Inhibitors and promoters of Stone Formation. Kidney Int, 1978; 13(5):361–71. CrossRef
Ghimire K, Banerjee J, Gupta AK, Pokhrel P, Khanal H, Bhateja P. Comparative study on thrombolytic and antihelminthes activities of aqueous extracts of each root, stemand leaves of medicinal plant Achyranthes aspera Linn. IntJ Pharm Sci Rev Res, 2017; 44(2):115–20.
Grases F, Costa-Bauzá A. Study of factors affecting calcium oxalate crystalline aggregation. Br J Urol, 1990; 66(3):240–4. CrossRef
Grover PK, Resnick MI. Evidence for the presence of abnormal proteins in the urine of recurrent stone formers. J Urol, 1995; 153:1716–21. CrossRef
King JS. Etiologic factors involved in urolithiasis: a review of recent research. J Urol, 1967; 97(4):583–91. CrossRef
Latha BP, Vijaya T, Reddy RM, Ismail M, Rao SD. Therapeutic efficacy of Achyranthes aspera Saponin extract in high fat diet induced hyperlipidaemia in male Wistar rats. Afr. J. Biotechnol, 2011; 10(74):17038–42.
Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR. Anti-urolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum schrad. and Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol, 2012; 144(1):160–70. CrossRef
Rajeshwari T, Suresh R, Sudhakar M. A review on phytopharmacological studies of an Indian medicinal weed: Achyranthes Aspera. Int Res J Pharm, 2020; 11(6):1–5. CrossRef
Rajeshwari T, Suresh R, Sudhakar M. Phytochemical investigation and antioxidant potential of two amaranthaceae plants: Achyranthes aspera linn and chenopodium album linn. Int Res J Pharm, 2022; 13(2): 902–11.
Robertson WG, Peacock M, Heyburn PJ, Marshall DH, Clark PB. Risk factors in calcium stone disease of the urinary tract. Br J Urol, 1978; 50(7):449–54. CrossRef
Selvam R, Bijikurien T. Effect of citrate feeding on free radical induced changes in experimental urolithiasis. Ind J Exp Biol, 1992; 30:705–10.
Selvam R, Kalaiselvi P, Govindaraj A, Bala Murugan V, Sathish Kumar AS. Effect of A. lanata leaf extract and Vediuppu Chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res, 2001; 43(1):89–93. CrossRef
Srivastav S, Singh P, Mishra G, Jha KK and Khosa RL. Achyranthes aspera-An important medicinal plant: a review. J Nat Prod Plant Resour, 2011; 1(1):14.
Subbarayan PR, Sarkar M, Nagaraja Rao S, Philip S, Kumar P, Altman N, Reis I, Ahmed M, Ardalan B, Lokeshwar BL. Achyranthes aspera (apamarg) leaf extract inhibits human pancreatic tumor growth in athymic mice by apoptosis. J Ethnopharmacol, 2012; 142(2):523–30. CrossRef
Tandon C, Chaudhary A, Singla SK. In vitro evaluation of Terminalia arjuna on calcium phosphate and calcium oxalate crystallization. Indian J Pharm Sci, 2010; 72(3):340. CrossRef