INTRODUCTION
Unstable molecules, free radicals, are incomplete electrons in their external shell of molecules. This instability is promoted by the reactive free radical that eventually induces the tendency to bind nearby electrons (Elsayed Azab et al., 2019; Gutowski and Kowalczyk, 2013; Liguori et al., 2018). Free radicals, such as superoxide radical (•O2), hydroxyl radical (•OH), nitric oxide (NOX, NO•), and hydrogen peroxide (H2O2), could react with other molecules, causing various damage to cells, tissues, and organs (Wu, 2020). The increasing level of free radicals in the body caused by pollution, cigarette, and poor lifestyle may generate an imbalance of free radicals and antioxidant enzymes response, defined as oxidative stress (OS). OS generates biomolecular damage and causes many diseases, such as heart disease, cancer, neurological disorder, diabetes mellitus (DM), and cardiovascular disease (CVD). (Costantini, 2019; Hajam et al., 2022; Lushchak and Storey, 2021; Silveira et al., 2021). Antioxidants are chemically capable of inhibiting the excessive oxidation process in the body and suppressing OS. These agents are also known as free radical scavengers (Haider et al., 2020). Antioxidants possess many mechanisms against OS, such as stimulating antioxidant enzymes, scavenging the free radical directly, intercalating to DNA to prevent DNA damage, and other mechanisms. One type of antioxidant agent is catechins. Catechins are the primary phytochemical contained in tea (Dias et al., 2018).
The antioxidant activity of white tea (WT) (Dias et al., 2013), green tea (GT) (Zhao et al., 2022), and the potential of Camellia sinensis (C. sinensis) constituents as an antioxidant in lipid system (Gramza and Korczak, 2005) have been discussed. Moreover, the role of GT in auto-photoaging, stress resistance, neuroprotective agent, and autophagy, which involves antioxidant activity, has also been reported (Prasanth et al., 2019). Recent studies have also exposed some of the antioxidant mechanisms of polyphenol compounds in tea (Yan et al., 2020). However, the role of catechins of C. sinensis in modulating antioxidant enzymes still needs to be thoroughly described. This review focuses on catechin’s ability, especially on epigallocatechin gallate (EGCG), to stimulate the release of antioxidant enzymes in cells, animals, clinical study, and molecular mechanisms in antioxidant enzyme modulation based on the selenium level. Furthermore, a review of the case reports on the effect of drinking tea is also provided.
CATECHIN CLASSIFICATION
Catechins are polyhydroxylated polyphenols found in abundant levels in tea leaves (Huang et al., 2019). High-performance liquid chromatography analysis reported several types of catechin compounds (Fig. 1), which includes EGCG, epicatechin gallate (ECG), epigallocatechin (EGC), and epicatechin (EC) (Ahmed et al., 2019; Cao et al., 2020; De Almeida Gonçalves et al., 2015). All catechins and their diastereoisomers contain numerous phenolic hydroxyl groups connected to a benzene ring as their chemical structure. As a proton donor (H+), the hydroxyl group helps stabilize the free radical, and the aromatic ring plays a role in maintaining the proxy stability via electronic resonance. The antioxidant activity of each catechin is different depending on the number of hydroxyl groups attached to the structure. The more the hydroxyl groups attached, the more the protons are available for donation for free radical stabilization (Reddyvari et al., 2017). The antioxidant characteristics of each catechin are presented in Table 1. Based on their ability as antioxidants, catechins were categorized into EGCG > ECG > EGC > EC. Considering the highest antioxidant activity of EGCG, most studies have concentrated on evaluating the potential of this particular catechin (He et al., 2018).
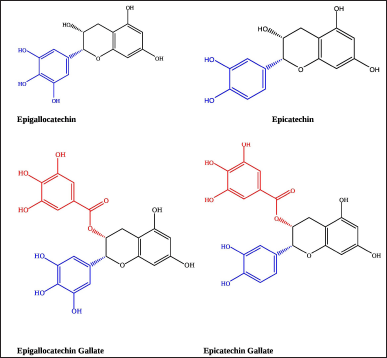 | Figure 1. 2D structure of catechins: (a) EGC; (b) EC; (c) EGCG; (d) ECG. [Click here to view] |
EGCG, the primary polyphenol in C. sinensis leaves, has revealed numerous health-promoting effects, including antioxidants. Many studies have proven the EGCG activity as an antioxidant to suppress OS by forming chelate, donating hydrogen ions (H+), and stimulating endogenous antioxidant enzymes (Abdel-Moneim et al., 2018). EGCG is produced through the esterification of EGC, with moiety gallate linked to the C ring via an ester link. Due to its powerful reduction ability, EGCG possesses a potent antioxidant effect (Huang et al., 2019). EGCG has eight hydroxyl sites that play an important role in forming free radicals, and radicalization reactions will produce antioxidant radicals with minimal reactivity. Proton donors in the ortho-hydroxyl group in ring B will create oxidized EGCG, which is more stable than meta-hydroxyl (Chiodo et al., 2010; Hashim and Fry, 2020; Wagner et al., 2006). EGCG has the most vigorous antioxidant activity among all catechin monomers because having six hydroxyls site in the ortho position (Xie et al., 2020); hence, it has excellent potential as an iron chelation agent and free radical (Bernatoniene and Kopustinskiene, 2018). After contributing a proton, EGCG creates a resonance to stabilize the antioxidant radical, which is more stable than free radicals (Phimphilai et al., 2021). The splitting of covalent bonds in organic molecules forms new free radicals, which initiate a chain reaction (Pan et al., 2019). In the complex formed by the chelation of EGCG, the gallate group works to trap •OH. The binding of •OH by such gallate is believed to have no prooxidant effect on EGCG (Lo´pez-Burillo et al., 2003).
THE EFFECT OF EGCG IN STIMULATING ANTIOXIDANT ACTIVITY IN CELL LINES
Reactive oxidative stress (ROS) elimination occurs physiologically through free radical scavenging and the upregulation of antioxidant enzymes. Several studies have demonstrated that EGCG acts as a potent antioxidant via activating the nuclear factor-erythroid-2 related factor 2 (Nrf2) pathway. Activation of Nrf2 is the primary regulatory route of the antioxidant response to the decrease of OS (Xie et al., 2020), transcription factors that induce antioxidant genes, and detoxification of the exogenous and endogenous OS (Yang et al., 2015). Another study showed an increased role of antioxidant activity in the nuclear maturation process of bovine oocytes. The antioxidant modulation mechanism was assumed to be caused by Nrf2 pathway activation, which increases the action of the messenger ribonucleic acid superoxide dismutase (mRNAs SOD1), catalase (CAT), and glutathione peroxidase-4 (GPx4). EGCG could also surge the Nrf2 translocation to the nucleus and heme oxygenase-1 (HO-1) in human intestinal epithelial cells (HIEC), resulting in elevated levels of GPx4 and Solute Carrier Family 7 Member 11 (SlC7A11) in response to ionizing radiation (IR). This work demonstrated the antioxidant effect of EGCG not only via activating the Nrf2 pathway but also by preventing the production of 8-hydroxy-2-deoxyguanosine (8-Ohdg), which damages intestinal epithelial cells when exposed to infrared light. (Xie et al., 2020)—the antioxidant mechanism via activation of Nrf2 pathway in mouse hippocampal neuronal cell line (HT22 cells) by GCG. GCG has been shown to protect neuronal cells from injury by inhibiting glutamate induction and stimulating N-acetylcysteine (NAC) production. The reduction in glutamate synthesis avoids cellular ROS and Ca2+ storage and modulates Nrf2/HO-1 signaling, releasing NAC, a glutathione (GSH) antioxidant percussor capable of controlling glutamate production lines (Park et al., 2021). GCG is an epi-isomer of EGCG, which is not commonly detected in fresh tea. GCG is only contained in tea products after being processed by drying, lighting, or extreme pH conditions during processing and storage (Wang et al., 2021).
The existence of a mechanism of antioxidant enzyme overexpression via the signalling activation pathway of mitogen-activated protein kinase (MAPK) owing to phosphorylation of mitogen p38 and extracellular-signal-regulated kinase (ERK ½) has been proven. The activation of the MAPK signaling pathway will regulate Nrf2, thus elevating the expression of HO-1, which is responsible for enhancing antioxidant defense by upregulating antioxidant enzymes. HO-1 is one of the detoxifying pathways for phase 2 enzymes (Yang et al., 2015; Zhao et al., 2019). Moreover, EGCG has revealed an antioxidant activity in OS-induced human umbilical vein endothelial cells (HUVECs) and an increase in SOD1, CAT, and GPx activity in Ultraviolet B (UVB)-induced human epidermal keratinocyte cell line (HaCaT cells) (Yang et al., 2015). In HaCaT cells, EGCG was thought to prevent the activation of the caspases-8 and caspases-3 pathways and the production of hyaluronidases (HYALs) limiting the number of radicals generated by UVB and sodium nitroprusside (SNP) exposure (Kim et al., 2018). Table 2 summarizes the effects of EGCG on various OS-induced cell lines.
Another research attempted to investigate the antioxidant capacity of EGCG in preventing cell damage from ROS caused by gamma radiation water radiolysis. Surprisingly, EGCG has successfully intercalated in DNA helixes and combated ROS directly via hydroxyl groups on aromatic rings (Fig. 2). EGCG can indirectly stimulate SOD and glutathione S-transferase (GST) activities. The study also discovered that EGCG has a greater antioxidant capacity than quercetin and vitamin C (Richi et al., 2012). EGCG can defend DNA from free radical attacks and repair DNA damaged by free radicals (Zhao et al., 2016). Other studies have confirmed the potential of EGCG as a chemopreventive drug by trapping ROS directly in cancer cells. EGCG also improved cervical cancer biopsy potential by increasing cellular SOD and GPx activity (Hussain, 2017). SOD and GPx activity increases in human lung adenocarcinoma cell line (A549 cells) treated with EGCG (Cromie and Gao, 2015). However, some of these researches still lack explanation on the precise processes involved in enhancing antioxidant activity and sustaining the activity of antioxidant enzymes. Several studies on the effect of EGCG in stimulating antioxidant activity in cell lines are summarized in Table 2.
THE EFFECTS OF EGCG IN STIMULATING ANTIOXIDANT ACTIVITY IN ANIMALS
Several studies on the effect of EGCG in stimulating antioxidant activity in animals have been summarized in Table 3. EGCG could induce antioxidant enzymes and lower serum malondialdehyde (MDA) levels in mice induced by aluminum oxide nanoparticles (Al2O3-NPs). A dose of 10 mg EGCG increased creatinine, urea, and uric acid (UA) to near-normal levels, implying no oxidative damage in the form of nephrotoxicity and kidney dysfunction. Antioxidant mechanisms may occur, including stabilizing free radicals via H+ donors or induction of antioxidant enzymes by activating phase II enzyme production pathways (EI Fattah et al., 2018). EGCG can bind metals and form stable conjugate bonds with thiol-carrier compounds. As a result of dimethyl hydrazine (DMH) induction, the direct mechanism of EGCG in scavenging OS caused an increase in the activity of GSH, GST, glutathione reductase (GR), SOD, and CAT in the large intestine of rats (Afzal et al., 2021). Another study reported that mice were induced with copper nanoparticles (CNP) and treated with green tea extract (GTE). GTE showed the capability as the hepatoprotective activity of GTE by lowering the activity of serum alanine aminotransferase (ALT) and aspartate transaminase (AST) enzymes, restoring the antioxidant activity of enzymes (SOD and CAT), increasing GSH concentrations, reducing MDA levels, and minimizing DNA fragmentation. The mechanisms underlying its antioxidant activity included directly scavenging free radicals by donating hydrogen, chelating processes that inhibit the Fenton reaction, thereby inhibiting the formation of •OH radicals, and advanced responses that inhibit the occurrence of lipid peroxidation (LPO). EGCG and other catechins can induce mild OS levels to induce intracellular endogenous antioxidant expression (Ibrahim et al., 2015).
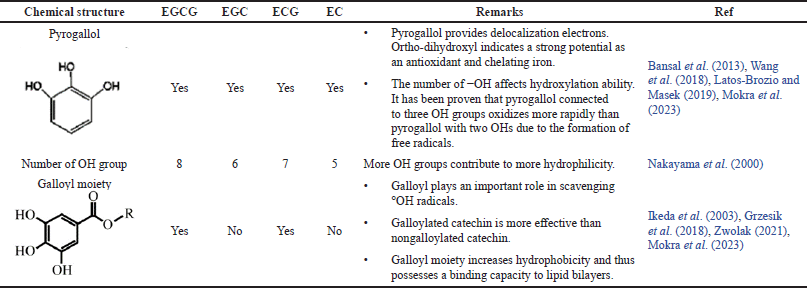 | Table 1. The antioxidant characteristics of catechins based on their chemical structures. [Click here to view] |
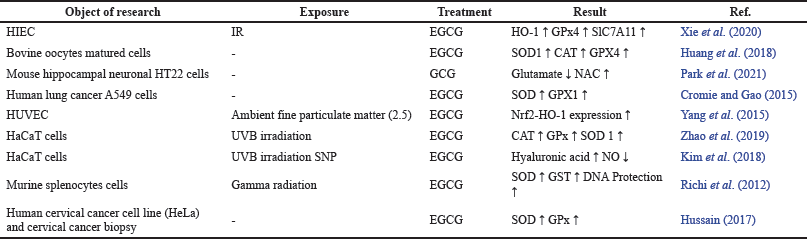 | Table 2. The effects of EGCG on various OS-induced cell lines. [Click here to view] |
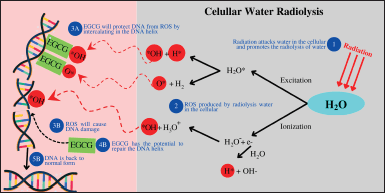 | Figure 2. DNA protection from free radicals by EGCG [The concept was made by combining the idea from Reeves and Kanai (2017) and Yousefi and Moazami (2019)]. [Click here to view] |
Alcohol-induced diabetic rats treated with aqueous GTE exhibited a nonsignificant increase in antioxidant activity (GPx, SOD, GST, and CAT) and GSH concentrations compared to the control group. It concluded that the antioxidant mechanism possibly occurred by involving ROS scavenging (Kodidela et al., 2020). A similar mechanism was shown in the study by Bártíková et al. (2017). This study did not show increased antioxidant enzyme activity and GSH concentrations in obese rats treated with a GTE-enriched diet. It might be owing to the long delay (7 months) in the induction of monosodium glutamate (MSG) and GTE-enriched diet, which caused severe damage to the body’s antioxidant system, making it more challenging to recover (Bártíková et al., 2017).
EGCG could improve structural disorders in the corpus cavernosum of aged rats by elevating of SOD activity of the penile, diminishing MDA levels, increasing its ability to counteract free radicals, and donating hydrogen donors to scavenge free radicals directly (Chen et al., 2016). Supported by another study, catechins could develop germ cells and increase rat sperm viability by stimulating lactate production in Sertoli cells (Opuwari and Monsees, 2020). Pretreatment EGCG prevents kidney disease and mediates sperm dysfunction in fluoride-exposed male mice. In this case, EGCG antioxidant mechanisms increase mitochondrial transmembrane potential, radical scavenging, and Nrf2-antioxidant response element (ARE) pathway. EGCG has a direct and indirect protective role in antioxidants. EGCG can directly prevent membrane permeability changes and stabilize it by OS induction because of its unique chemical structure. EGCG’s chemical characteristics with the gallate moiety esterified (Ring A), the catechol group (Ring B), and the meta-hydroxyl group in Ring C played essential roles in directly scavenging radicals and protecting rat testes from fluoride-induced OS. The direct mechanism of EGCG is reported to be able to reduce the workload of SOD, CAT, and GPx by scavenging free radicals directly. In addition, EGCG can restore the antioxidant enzymes and uphold their activity. The amount of hydroxyl group in the structure of EGCG has electrophilic characteristics and has the potential to modify SH-residue in Keap 1, which causes Nrf2 to accumulate in the nucleus and bind the ARE to upregulate antioxidant enzyme activity (Thangapandiyan and Miltonprabu, 2015).
GTE can restore the antioxidant activity of enzymes (SOD and CAT), raise GSH levels, and limit DNA fragmentation in mice exposed to CNPs. Direct scavenging of free radicals by donating hydrogen and a chelating process, which inhibited the Fenton reaction, is also possible. The cessation of the Fenton reaction causes the inhibition of hydroxyl radicals’ production and prevents the occurrence of LPO. EGCG and other catechins can promote intracellular endogenous antioxidant expression by inducing mild OS (Ibrahim et al., 2015).
EGCG in another potential has been proven by Ibrahim et al. (2019). EGCG acts as a chemopreventive agent, which might be used with an anticancer drug. The study established the efficacy of EGCG as a chemopreventive medication compatible with cisplatin without adverse drug interactions. EGCG in GT has cardioprotective properties, specifically repairing oxidative and cardiac damage caused by cisplatin in cancer treatment by inducing and restoring SOD and GPx. Activation of Nrf2 may boost the expression of genes involved in manufacturing antioxidant enzymes and HO-1 for cell protection and anti-inflammatory signaling pathways (Ibrahim et al., 2019). Long-term administration of WT in trained mice elevated endurance because it prevented hepatic tissue from LPO and enhanced trained mice’s endogenous antioxidant defenses. The blood antioxidant status of antioxidant enzymes (SOD, GPx, GR, and GSH) improved in response to WT-induced exogenous antioxidants. GPx and antioxidant GSH system in the liver demonstrated higher activity than other antioxidant enzymes, with evidence of reduced peroxidative liver damage. The reaction between H2O2 and lipid hydroperoxide (LOOH) will produce MDA as a sign of peroxidative damage (Berilli et al., 2022). Wang et al. (2022) reported a similar mechanism of antioxidant induction in their study of the reduction of aflatoxin B1 (AFB1)-induced ducks. The elevation in Nrf2 and HO-1 expression is caused by EGCG administration. It indicated the consequences of restoring antioxidant enzyme activity and preventing liver damage in ducks (Wang et al., 2022).
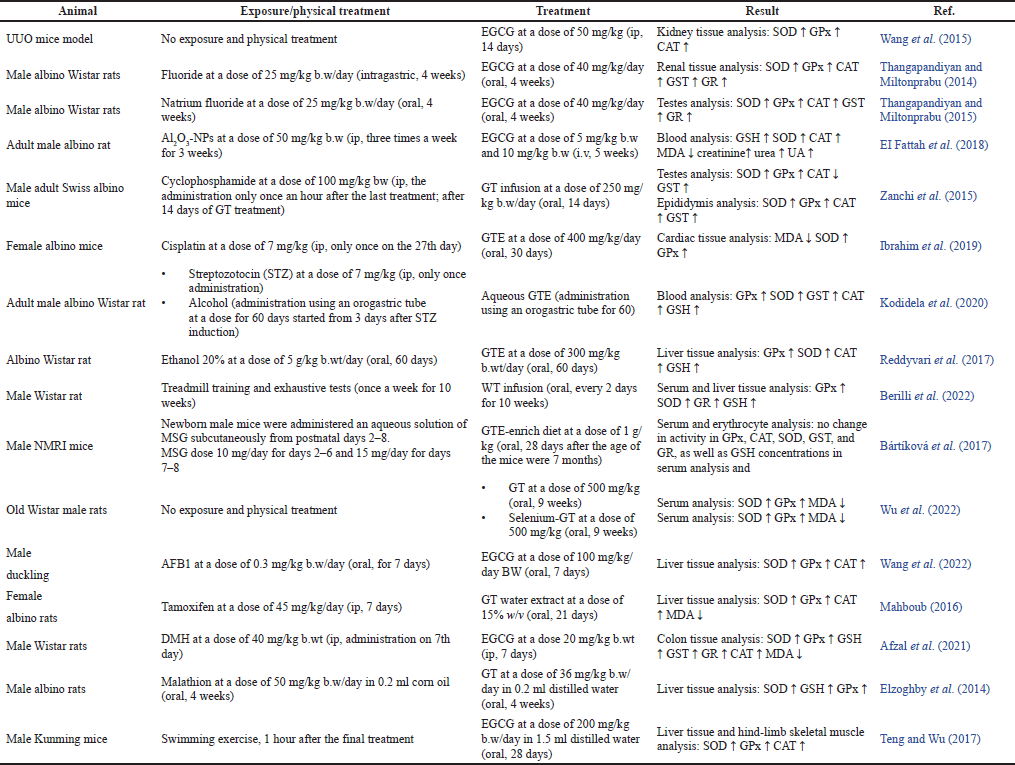 | Table 3. The effects of EGCG in modulating antioxidant activity in animals. [Click here to view] |
The ability of EGCG to increase antioxidant enzyme activity by signaling the Nrf2 pathway was also exhibited in a preclinical investigation using the unilateral ureteral obstruction (UUO) mouse model. EGCG stimulates increasing the nuclear accumulation of Nrf2 and promotes Nrf2-ARE consensus element binding in the kidney UUO to increase antioxidant enzyme activity. Consequently, renal function improved dramatically, and the obstructed kidney gained weight (Wang et al., 2015). Pretreatment EGCG in the rats’ group induced by fluoride upregulated Nrf2 level by HO-1 signaling pathway. EGCG encourage Nrf2 to translocate to the nucleus and bind the ARE, increasing antioxidant activity in the renal tissue of rat (Thangapandiyan and Miltonprabu, 2014).
The catechins in GTE could also restore the activity of SOD, CAT, GPx, and GSH to near-normal levels following alcohol induction (p < 0.05). Antioxidants prevented D-loop mutations in alcohol-induced rat mitochondrial DNA. Mutations in the D-loop, the primary regulatory region for mitochondrial deoxyribonucleic acid (mtDNA) replication and transcription, would influence the stability of mtDNA. It can be hypothesized that the increase in antioxidant activity was due to catechins’ capacity to repair SOD, CAT, GPx, and GSH activity (Reddyvari et al., 2017). Antioxidant enzyme amelioration in prediabetic rats also occurred after treating WT infusion. Catechins of WT were estimated to rebuild the activity of SOD and GPx to near-normal levels. Although the precise process of enhancing the antioxidant activity of enzymes has not been conclusively elucidated yet, it is believed that activation of the Nrf-2 pathway increases the expression of antioxidant enzymes (Silveira et al., 2021). Drinking WT regularly in prediabetic rats led to an increase in CAT levels, efficient ROS clearance, and restoring cerebral cortex antioxidant ability. EGCG, which belongs to the polyphenol group, is believed to have a neuroprotective effect from tea extracts (Nunes et al., 2015). EGCG had antifatigue effects in rats because it could potentially improve the activities of SOD, CAT, and GPx to protect corpuscular membranes against LPO. There was no precise mechanism for upregulating the SOD, CAT, and GPx (Teng and Wu, 2017). In another study, EGCG in GT was predicted to prevent memory deficit and hippocampal oxidative damage. EGCG plays a role in diminishing the ROS and thiobarbituric acid reactive substances (TBARS) in ischemic mice hippocampus and avoids the elevation of excitotoxicity, nerve injury, and degeneration induced by OS in the brain (Martins et al., 2017).
THE EFFECTS OF EGCG IN STIMULATING ANTIOXIDANT ACTIVITY IN HUMANS
Several studies reported the potential of EGCG in stimulating antioxidant activity in humans and the resume is provided in Table 4. Radiation-induced dermatitis in breast cancer patients following mastectomy could be treated with topical EGCG. EGCG suppresses radiation damage via an antioxidant process, including DNA protection. EGCG intercalated into the DNA to bind the free radicals directly and reduce radiation harm. EGCG prevents DNA from free radicals and repairs the DNA damage induced by free radicals (Zhu et al., 2016). Clinical trials of GTE administration on DM 2 patients with comorbid conditions showed the elevation of SOD and GPx activity after 9 months of therapy but declined after the 18th month. Compared to baseline levels, CAT activity increased only in the 18th month. The study also found that GTE had a beneficial effect on LPO markers, such as the lowering of LOOH and MDA, indicating antioxidant activity in the body (Spadiene et al., 2014). MDA exhibited LPO-modified low-density lipoprotein is one of the initiators of atherogenesis (Suraphad et al., 2017). The antioxidant activity of EGCG also occurred in peripheral blood mononuclear cells of patients with multiple sclerosis. EGCG suppresses NOX overactivity by competitively inhibiting the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzyme with NADPH. The presence of neuronal calcium is promoted by NOX activation, a pathophysiological mechanism of neuronal dysfunction (Mossakowski et al., 2015).
Though the increases were insignificant, GT infusion and GTE treatment increased GSH, GPx, and total antioxidant status (TAS) activity in individuals with metabolic system disease. The findings suggest that catechins selectively enhance endogenous antioxidant capacity in patients with metabolic syndrome by modulating endogenous antioxidant indicators. EGCG also exhibited a drop in iron levels in blood plasma, which was thought to be caused by the chelation process of EGCG (Basu et al., 2013). Another research reported the potential of catechins in modulating antioxidant enzymes in chronic cigarette smokers. The rising of GSH, GPx, and SOD activity is due to EGCG’s capacity to bind directly to ROS and accumulate the level of antioxidant enzymes, resulting in enhanced antioxidant activity in the blood (Shakeela Begum et al., 2018). A clinical study of EGCG and ECG found both catechins in the plasma of healthy subjects given a single dose of decaffeinated GT catechins at random. Decaffeinated GT was generally well accepted by the participants. However, EGCG and ECG are free, whereas other catechins were primarily founded as glucuronide and sulfate conjugates (Chow et al., 2005).
CrossFit exercise and GT ingestion built up aerobic capacity and antioxidant activity. The elevation in UA in plasma, an outcome of purine metabolism, is evidence of increased antioxidant activity. Another mechanism was proven by inhibiting ROS attacks on the bilayer membrane through direct contact with catechins. This study also demonstrated catechins’ capacity to balance ROS generation with endogenous antioxidant defenses. The elevation of GSH and SOD levels in the blood, triggered by cellular signaling pathways, indicates a rise in antioxidant activity. Catechins can influence the expression of the first enzyme in the cellular GSH production pathway, namely, glutamyl cysteine synthetase (GCS) (Sadowska-Kr?pa et al., 2019). Supported by other studies, catechins were observed to modulate the expression of γ-GCS as a precursor to increasing the total GSH levels in various organs (Carlsen et al., 2003; Moskaug et al., 2005). A different result was shown in the study by Jówko et al. (2015). GTE supplementation in sprinter athletes after repeated cycles of sprint tests (RST) exhibited a significant elevation in UA and plasma polyphenol levels in total rest. Even higher results were demonstrated in total plasma after 24 hours of recovery. SOD and GPx revealed different effects, with a decrease in activity followed by a decrease in MDA levels 24 hours after recovery. In this investigation, GTE supplementation elevates plasma antioxidant activity, as seen by an increase in UA and MDA levels, suggesting antioxidant activity in the body. Catechins in GT stimulated antioxidant enzymes were thought to function synergistically to restore the body’s equilibrium after RST in sprinter athletes (Jówko et al., 2015). The lowering of TBARS oxidation indicated the presence of antioxidant activity, which suppress LPO, due to the presence of catechin-stimulated SOD activity in GT. The great activity of SOD in reducing LPO caused the level of SOD to decrease after treatment lowering (Maeda-Yamamoto et al., 2018).
CASE REPORTS OF THE CONSUMPTION OF TEA IN HYPERTENSIVE PATIENTS WITH COMPLICATION
Several previous studies have been explored to investigate the influence of tea drink consumption on pharmacological effects in humans—several case studies are provided in Table 5.
Controlling the use of tea drinks is very important in this regard. People with postmenopause, celiac disease, atrophic gastritis, Helicobacter pylori infection, dietary deficits, intestinal resection, and bariatric surgery with gastric bypass cannot consume large doses of tea to avoid iron deficiency. Ingesting in the high level of tea alleviates Ferri’s levels—because of the chelating process by catechin—and the amount of hemoglobin in the anemia patients’ body leads to the exacerbation of anemia. In addition, excessive tea consumption inhibits the body’s absorption of Ferri and other irons. EGCG in high concentration was similarly toxic to hepatocytes (Kucera et al., 2015). The occurrence of hepatotoxicity in black tea is relatively uncommon, but this case demonstrated that large-scale black tea consumption possessed a risk of hepatotoxicity (Hadjipanayis et al., 2019). This is possible because catechins act as pro-oxidants, replenish the potential mitochondrial membrane, and cause hepatotoxicity (Mazzanti et al., 2015).
The case report by Chong et al. (2016) showed the occurrence of hypokalemia in patients with regular GT consumption. Still, the exact cause of hypokalemia has not been explained. Chong et al. (2016) suspected that due to the influence of bendroflumethiazide, the drug could cause electrolyte imbalances, causing a decrease in potassium. However, it turned out that hypokalemia also occurred in female patients (67 years old) who did not take the drug. Hypokalemia may be caused by theophylline, which affects the activity of sodium/potassium ATPase, which causes extracellular hypokalemia (Chong et al., 2016). We hypothesized that there are drug interactions with catechin compounds that cause hypokalemia. Lisinopril is a drug used to lower blood pressure (BP) by inhibiting the angiotensin-converting enzyme (ACE) and preventing the formation of angiotensin II. A decrease in angiotensin II causes a reduction in aldosterone secretion, so the reabsorption of natrium in the collecting duct decreases, and so does potassium excretion. It will cause an increase in potassium in the serum of hypertensive patients. Downregulating sodium levels and elevating potassium levels will reduce BP (Lopez et al., 2022; Messerli et al., 2018; Regulski et al., 2015). GTE with a high EGCG content, when consumed with lisinopril, will cause a decrease in plasma concentration and excretion of lisinopril in the urine. It is because EGCG can inhibit the absorption of lisinopril in the gastrointestinal tract. Lowering lisinopril levels will reduce the efficacy of BP-lowering drugs, such as potassium supplements (Misaka et al., 2021).
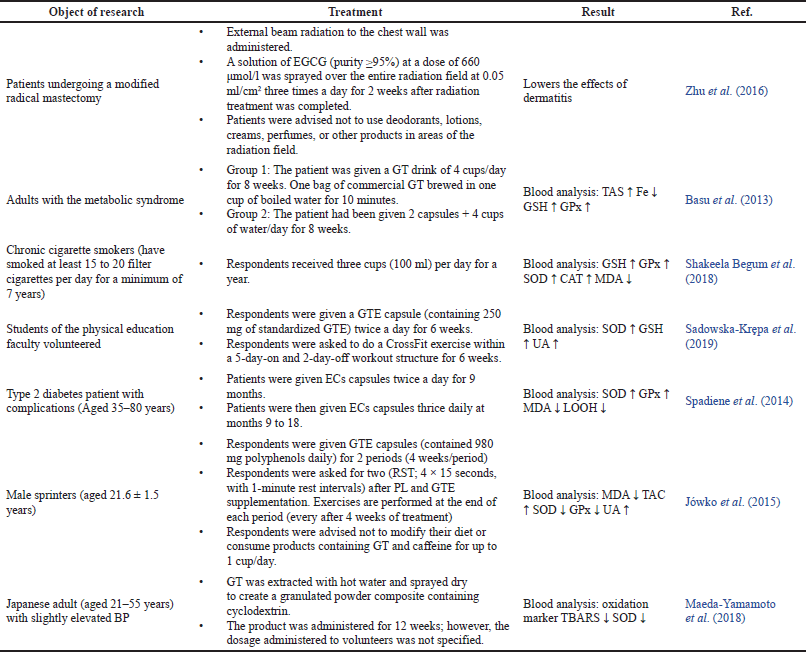 | Table 4. The effects of EGCG in modulating antioxidant activity in humans. [Click here to view] |
Patients with high levels of iron in the body, thalassemia, are advised to drink tea regularly. Tea EGCG will reduce iron levels by chelating iron with 3′, 4′-dihydroxy and galloyl groups in its chemical structure (Fan, 2016; Heikal et al., 2013; Jetsrisuparb et al., 2014). Excess iron in cells produced OS through the Fenton reaction, resulting in oxidative cell damage and organ damage. Metal ions can combine with H2O2 to form hydroxyl radicals, which are highly reactive and toxic (Ibrahim et al., 2015; Phimphilai et al., 2021). Therefore, iron-chelating compounds will suppress iron levels and improve cellular function in thalassemia patients (Phimphilai et al., 2021). Chelating iron can also inhibit the Haber–Weiss reaction, which causes several chronic diseases (Codoñer-Franch et al., 2010; Valko et al., 2007). Another benefit is that tea can also be an alternative hydration therapy in intracranial hypotension (Petramfar et al., 2016).
MOLECULAR MECHANISM IN MODULATING ANTIOXIDANT ENZYMES
Antioxidant enzymes in the molecular mechanism are divided into two types based on selenium levels. Dong et al. (2016) and Wu et al. (2022) discovered that selenium levels influence the antioxidant mechanism in EGCG (Dong et al., 2016; Wu et al., 2022). EGCG, in a selenium-rich condition, activates the thioredoxin (Trx) and GSH pathways as the first line of antioxidant defense. Selenium is a component of Trx reductase, which also contributes to the body’s antioxidant defense mechanism. Without selenium, EGCG will activate the Nrf2 pathway and increase HO-1 and NADPH levels, hence inducing antioxidant enzymes (Fig. 3) (Dong et al., 2016).
Nrf2 transcriptional activation by EGCG occurs via Michael’s reaction between an electrophile of EGCG with the cysteinyl thiol group in Keap 1 (Ma, 2013; Thangapandiyan and Miltonprabu, 2014). Residues C273 and C288 are direct sensors responsible for the induction of the ARE-regulated enzymes when EGCG and free radicals bind the C273 or C288 in the intervening region (IVR) domain (in the Keap 1). The binding will downregulate Keap 1, and Nrf2 will translocate to the nucleus. Nrf will bind with ARE to stimulate gene expression (HO-1 and NADPH quinone dehydrogenase 1 [(human)]) in the nucleus, thereby triggering the release of antioxidant enzymes, such as SOD, GPx, and CAT (Fig. 3) (Guo et al., 2021; Han et al., 2017; Thangapandiyan and Miltonprabu, 2014; Wu et al., 2010).
SOD, GPx, and CAT are the primary antioxidant enzymes found in human plasma (Szczeklik et al., 2016). SOD acts as an antioxidant by neutralizing superoxide (O2*) (Abolfathi et al., 2012; Didangelos et al., 2020) and converting it into H2O2 (Mazur-Bialy et al., 2018; Vrankovi?, 2016). CAT catalyzes the decomposition of H2O2 into water molecules (H2O) and oxygen O2. Research by Ezeja et al. (2022) reported that the elevation of CAT activity after paracetamol administration indicated that CAT could simultaneously detoxify paracetamol. It was reasonable to assume that CAT had nearly the same capabilities as GPx in detoxifying hazardous chemical substances that caused the generation of ROS in the body (Ezeja et al., 2022). GPx detoxifies H2O2 and decomposes organic H2O2 into appropriate alcohols (Vrankovi?, 2016; Zahra et al., 2021). CAT and GPx avoid the formation of hydroxyl radicals (OH*) by preventing the accumulation of H2O2 (Abrahim et al., 2012).
EGCG shows another antioxidant activity in high selenium level conditions. EGCG can trigger the Trx antioxidant defense mechanism and GSH antioxidant mechanism. Activating mechanism of Trx by EGCG is still unclear (Dong et al., 2016). GSH antioxidant mechanism is another way EGCG stimulates the antioxidant activity in the high selenium level. GSH is a nonprotein thiol that helps coordinate the body’s antioxidant defense mechanisms by an electron donor and disulfide bond reducer mechanism (Abolfathi et al., 2012). In the presence of the enzyme GPx, GSH in the GSH antioxidant system is converted into its oxidized state glutathione disulfide (GSSH). GR converts GSSH back to GSH (reduced form) and maintains the average cellular GSH level (Codoñer-Franch et al., 2010; Rakshit et al., 2018; Saravana Kumari and Anuradha, 2016; Valko et al., 2007). GSH at high concentrations can scavenge ROS directly and indirectly (Kodidela et al., 2020). GSH is an indirect mechanism in conjugating GPx and GST (EI Fattah et al., 2018). GSH also aids in detoxifying chemicals via conjugation mechanisms (Heikal et al., 2013; Khan et al., 2014). Apart from being a substrate for GPx, GSH can directly eliminate free oxygen species, such as superoxide anions, and radical alkoxy, such as H2O2, to replace the role of GPx and is primarily responsible for maintaining membrane protein thiols (Fig. 3) (Reddyvari et al., 2017).
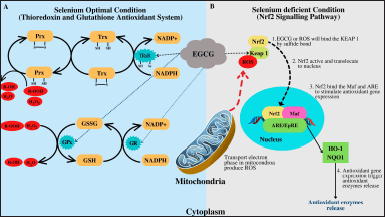 | Figure 3. Cellular antioxidant defense pathways depend on selenium status: (A) Trx mechanism and GSH antioxidant enzymes and (B) Nrf2 pathway illustration [The concept was built by the combination concept of Karlenius and Tonissen (2010), Mattmiller et al. (2013), Dong et al. (2016), Jaganjac et al. (2020)]. [Click here to view] |
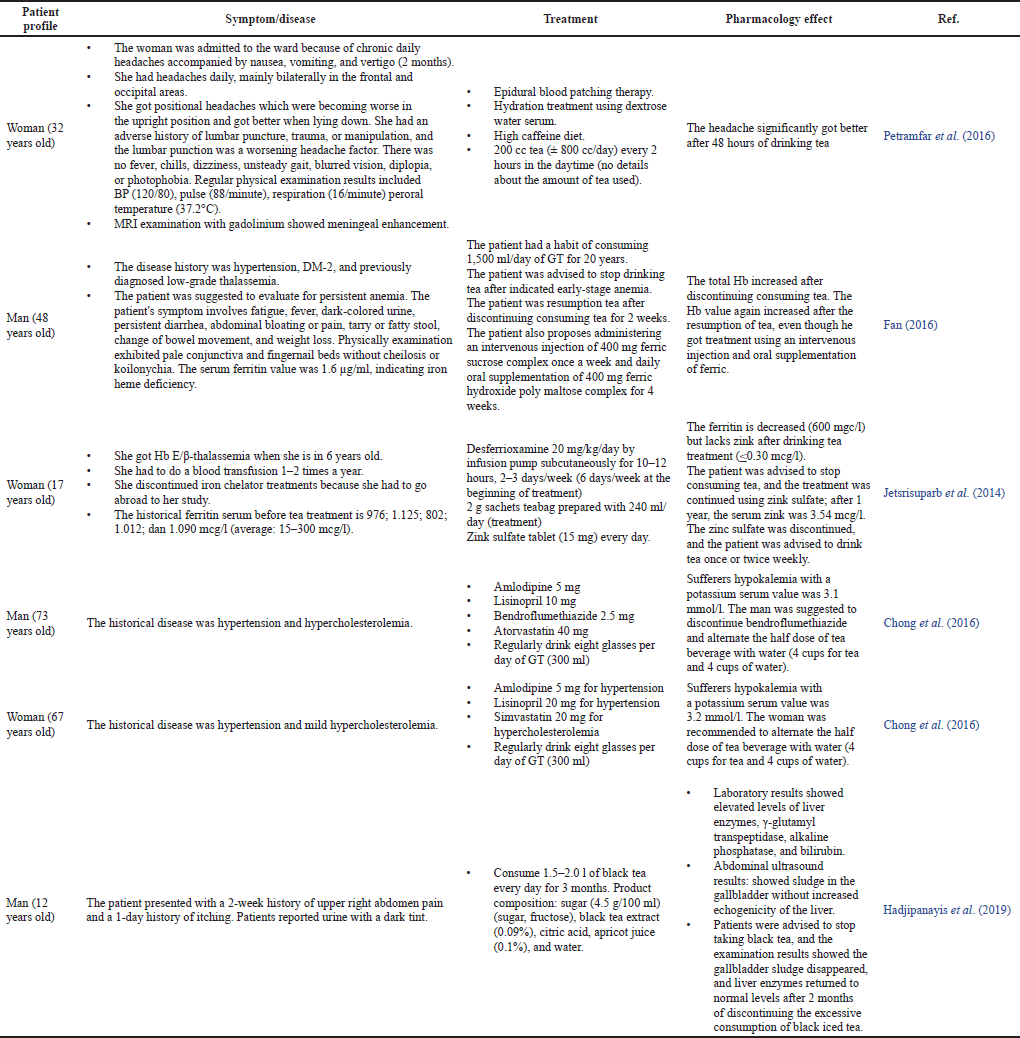 | Table 5. Case reports of the consumption of tea in hypertensive patients with complications. [Click here to view] |
CONCLUSION AND FUTURE ASPECTS
Camellia sinensis catechins have a variety of antioxidant mechanisms that benefit our bodies. EGCG is the most powerful antioxidant activity compared to other catechins due to its unique chemical composition. EGCG involves eight hydroxyl groups and three benzene rings, contributing to its antioxidant properties. Catechins have several antioxidant mechanisms, including chelating iron to prevent the Fenton reaction, scavenging free radicals, inserting into the DNA helix and binding to free radicals directly, and increasing antioxidant enzymes. The review results reveal that catechins can stimulate antioxidant enzymes. Even though many studies have described the various antioxidant mechanisms of enzymes, some of the mechanisms remain unknown. Further study is needed to elucidate the exact functions of catechins that can replenish and accumulate antioxidant enzymes in the body and the distinction between these methods. Studies about the specific mechanisms of EGCG in activating the Trx antioxidant system are also essential.
LIST OF ABBREVIATIONS
AAPH, 2,2-azobis(2-amidinopropane) dihydrochloride; ACE, Angiotensin-converting enzyme; AFB1, aflatoxin B1; Al2O3-NPs, Aluminum oxide nanoparticles; AFB1, Aflatoxin B1; ALT, Alanine Aminotransferase; ARE, Antioxidant response element; AST, Aspartate transaminase; BTE, Black Tea Extract; CAT, Catalase; CNP, Copper nanoparticles; DMH, Dimethyl hydrazine; ECs, Extract Camellia sinensis; EGCG, Epigallocatechin gallate; ERK, Extracellular-signal-regulated kinase; FBTA, Fuzhuan-brick tea; GPx, Glutathione peroxidase; GR, Glutathione reductase; GSH, Glutathione; GSSH, Oxidized glutathione; GST, Glutathione S-transferase; GT, Green tea; GTE, Green tea extract; HO-1, Heme oxygenase-1; i.p, Intraperitoneally administration; IR, Ionizing radiation; i.v, Intravenally administration; IVR domain, intervening region domain; LDL, low-density lipoprotein; LOOH, Lipid hydroperoxides; LPO, Lipid peroxidation; MAPK, Mitogen-activated protein kinase; MDA, Malondialdehyde; MSG, Monosodium glutamate; mtDNA, Mitochondria deoxyribonucleic acid; NAC, N-acetylcysteine; NADPH, Nicotinamide adenine dinucleotide phosphate; NAF, Natrium Flouride; Nrf2, Nuclear factor-erythroid-2 related factor 2; NQO1, NADPH Quinone Dehydrogenase 1 [(Human)]; NOX, Nitric oxide; SNP, Sodium nitroprusside; SOD, Superoxide dismutase; STZ, Streptozotocin; TAS, Total antioxidant status; Trx, Thioredoxin; UA, Uric acid; UUO, Unilateral ureteral obstruction; UVB, Ultraviolet B; WT, White tea; 8-Ohdg, 8-Hydroxy-2-Deoxyguanosine.
AUTHOR CONTRIBUTIONS
Jutti Levita (JL) was responsible for the conception and design of the review and checked, finalized, and revised the manuscript. Lidya Cahyo Bawono searched, collected, and reviewed and wrote the original draft preparation. Miski Aghnia Khairinisa and Supat Jiranusornkul checked the manuscript and collected articles. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
The article processing charge (APC) is funded by Padjadjaran University via the Directorate of Research and Community Engagement in the scheme of Academic-Leadership Grant of Prof. Dr. Jutti Levita.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not include experiments on animal or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
The article remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Moneim A, El-Senousy WM, Abdel-Latif M, Khalil RG. Association between antioxidant enzyme activities and Enterovirus-infected type 1 diabetic children. Med Princ Pract, 2018; 27:86–91; https://doi.org/10.1159/000486718
Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid-Based Complement Altern Med, 2012; 2012; https://doi.org/10.1155/2012/740671
Abrahim NN, Kanthimathi MS, Abdul-Aziz A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med, 2012; 12:1; https://doi.org/10.1186/1472-6882-12-220
Afzal SM, Vafa A, Rashid S, Shree A, Islam J, Ali N, Sultana S. Amelioration of N,N′-dimethylhydrazine induced colon toxicity by epigallocatechin gallate in Wistar rats. Hum Exp Toxicol, 2021; 40:1558–71; https://doi.org/10.1177/09603271211002884
Ahmed OM, Abdul-Hamid MM, El-Bakry AM, Mohamed HM, Abdel Rahman FEZS. Camellia sinensis and epicatechin abate doxorubicin-induced hepatotoxicity in male Wistar rats via their modulatory effects on oxidative stress, inflammation, and apoptosis. J Appl Pharm Sci, 2019; 9:30–44; https://doi.org/10.7324/JAPS.2019.90405
Bansal S, Vyas S, Bhattacharya S, Sharma M. Catechin prodrugs and analogs: a new array of chemical entities with improved pharmacological and pharmacokinetic properties. Nat Prod Rep, 2013; 30:1438; https://doi.org/10.1039/c3np70038k
Bártíková H, Boušová I, Matoušková P, Szotáková B, Skálová L. Effect of green tea extract-enriched diets on insulin and leptin levels, oxidative stress parameters and antioxidant enzymes activities in obese mice. Polish J Food Nutr Sci, 2017; 67:233–40; https://doi.org/10.1515/pjfns-2017-0004
Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res, 2013; 33:180–7; https://doi.org/10.1016/j.nutres.2012.12.010
Berilli P, Fanaro GB, Santos JP, Reyes Reyes FG, Iglesias AH, Reis M, Cazarin CBC, Junior MRM. White tea modulates antioxidant defense of endurance-trained rats. Curr Res Physiol, 2022; 5:256–64; https://doi.org/10.1016/j.crphys.2022.06.002
Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules, 2018; 23:1–11; https://doi.org/10.3390/molecules23040965
Cao SY, Li BY, Gan RY, Mao QQ, Wang YF, Shang A, Meng J, Xu X, Wei X, Li H. The in vivo antioxidant and hepatoprotective actions of selected Chinese teas. Foods, 2020; 9; https://doi.org/10.3390/foods9030262
Carlsen H, Myhrstad MCW, Thoresen M, Moskaug JØ, Blomhoff R. Berry intake increases the activity of the γ-glutamylcysteine synthetase promoter in transgenic reporter mice. J Nutr, 2003; 133:2137–40; https://doi.org/10.1093/jn/133.7.2137
Chen D, Zhang KQ, Li B, Sun DQ, Zhang H, Fu Q. Epigallocatechin-3-gallate ameliorates erectile function in aged rats via regulation of PRMT1/DDAH/ADMA/NOS metabolism pathway. Asian J Androl, 2016; 18:291–7; https://doi.org/10.4103/1008-682X.178486
Chiodo SG, Leopoldini M, Russo N, Toscano M. The inactivation of lipid peroxide radical by quercetin. A theoretical insight. Phys Chem Chem Phys, 2010; 12:7662–70; https://doi.org/10.1039/b924521a
Chong SJK, Howard KA, Knox C. Hypokalaemia and drinking green tea: a literature review and report of 2 cases. BMJ Case Rep, 2016; 2016; https://doi.org/10.1136/bcr-2016-214425
Chow HHS, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res, 2005; 11:4627–33; https://doi.org/10.1158/1078-0432.CCR-04-2549
Codoñer-Franch P, Pons-Morales S, Boix-García L, Valls-Bellés V. Oxidant/antioxidant status in obese children compared to pediatric patients with type 1 diabetes mellitus. Pediatr Diabetes, 2010; 11:251–7; https://doi.org/10.1111/j.1399-5448.2009.00565.x
Costantini D. Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol, 2019; 222:1–9; https://doi.org/10.1242/jeb.194688
Cromie MM, Gao W. Epigallocatechin-3-gallate enhances the therapeutic effects of leptomycin B on human lung cancer A549 cells. Oxid Med Cell Longev, 2015; 2015; https://doi.org/10.1155/2015/217304
De Almeida Gonçalves G, De Sá-Nakanishi AB, Wendt MMN, Comar JF, Bersani Amado CA, Bracht A, Peralta RM. Green tea extract improves the oxidative state of the liver and brain in rats with adjuvant-induced arthritis. Food Funct, 2015; 6:2701–11; https://doi.org/10.1039/c5fo00548e
Dias TR, Carrageta DF, Alves MG, Oliveira PF, Silva BM. White tea. Elsevier Inc., Amsterdam, The Netherlands, vol. 2024, 2018; https://doi.org/10.1016/B978-0-12-812491-8.00058-8
Dias TR, Tomas G, Teixeira NF, Alves MG, Oliveira PF, Silva BM. White tea (Camellia sinensis (L.)): antioxidant properties and beneficial health effects. Int J Food Sci Nutr Diet, 2013; 2:19–26; https://doi.org/http://dx.doi.org/10.19070/2326-3350-130005
Didangelos T, Karlafti E, Kotzakioulafi E, Kontoninas Z, Margaritidis C, Giannoulaki P, Kantartzis K. Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin B12, and carnitine for 12 months in patients with diabetic neuropathy. Nutrients, 2020; 12:1–15; https://doi.org/10.3390/nu12113254
Dong R, Wang D, Wang X, Zhang K, Chen P, Yang CS, Zhang J. Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice. Redox Biol, 2016; 10:221–32; https://doi.org/10.1016/j.redox.2016.10.009
EI Fattah MEA, Abdelgawad MR, EI Boughdady BAE. The protective role of epigallocatechin gallate (EGCG) on oxidative stress in normal and treated rats with aluminum oxide nanoparticles. Int J Adv Biochem Res, 2018; 2:43–52; https://doi.org/10.33545/26174693.2018.v2.i2a.21
Elsayed Azab A, Adwas AA, Elsayed ASI, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng, 2019; 6:43–7; https://doi.org/10.15406/jabb.2019.06.00173
Elzoghby RR, Hamoda AF, Abed-Ftah A, Farouk M. Protective role of vitamin C and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats. Am J Pharmacol Toxicol, 2014; 9:174–85; https://doi.org/10.3844/ajptsp.2014.174-185
Ezeja EP, Onuoha NO, Ufere EA. Effects of green tea (Camellia sinensis) on paracetamol-induced oxidative stress markers in Wistar rats. J Dietitians Assoc Niger, 2022; 12:30–7; https://doi.org/10.4314/jdan.v12i1.5
Fan FS. Iron deficiency anemia due to excessive green tea drinking. Clin Case Rep, 2016; 4:1053–6; https://doi.org/10.1002/ccr3.707
Gramza A, Korczak J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food Sci Technol, 2005; 16:351–8; https://doi.org/10.1016/j.tifs.2005.02.004
Grzesik M, Napar?o K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem, 2018; 241:480–92; https://doi.org/10.1016/j.foodchem.2017.08.117
Guo C, Bi J, Li X, Lyu J, Liu X, Wu X, Liu J. Immunomodulation effects of polyphenols from thinned peach treated by different drying methods on RAW264.7 cells through the NF-κB and Nrf2 pathways. Food Chem, 2021; 340:127931; https://doi.org/10.1016/j.foodchem.2020.127931
Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological dignificance. Acta Biochim Pol, 2013; 60:1–16
Hadjipanayis A, Efstathiou E, Papaevangelou V. Hepatotoxicity in an adolescent with black iced tea overconsumption. Pediatr Gastroenterol Hepatol Nutr, 2019; 22:387–91; https://doi.org/10.5223/pghn.2019.22.4.387
Haider K, Haider MR, Neha K, Yar MS. Free radical scavengers: an overview on heterocyclic advances and medicinal prospects. Eur J Med Chem, 2020; 204:112607; https://doi.org/10.1016/j.ejmech.2020.112607
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, Pramodh S, Alsulimani A, Alkhanani MF, Harakeh S, Hussain A, Haque S, Reshi MS. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells, 2022; 11; https://doi.org/10.3390/cells11030552
Han XD, Zhang Y, Wang KL, Huang YP, Yang ZB, Liu Z. The involvement of Nrf2 in the protective effects of (-)- epigallocatechin-3-gallate (EGCG) on NaASO2-induced hepatotoxicity. Oncotarget, 2017; 8:65302–12.
Hashim M, Fry J. Evaluation of direct and indirect antioxidant properties of selected four natural chemical compounds: quercetin, epigallocatechin-3-gallate, indole-3-carbinol and sulforaphane by DPPH radical scavenging assay. J Biomed Res Environ Sci, 2020; 1:389–92; https://doi.org/10.37871/jbres1170
He J, Xu L, Yang L, Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med Sci Monit, 2018; 24:8198–206; https://doi.org/10.12659/MSM.911175
Heikal TM, Mossa ATH, Rasoul MAA, Marei GIK. The ameliorating effect of green tea extract against cyromazine and chlorpyrifos induced liver toxicity in male rats. Asian J Pharm Clin Res, 2013; 6(1):47–55
Huang ST, Hung YA, Yang MJ, Chen IZ, Yuann JMP, Liang JY. Effects of epigallocatechin gallate on the stability of epicatechin in a photolytic process. Molecules, 2019; 24:1–13; https://doi.org/10.3390/molecules24040787
Huang Z, Pang Y, Hao H, Du W, Zhao X, Zhu H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian Australas J Anim Sci, 2018; 31:1420–30; https://doi.org/10.5713/ajas.17.0880
Hussain S. Comparative efficacy of epigallocatechin-3-gallate against H2O2-induced ROS in cervical cancer biopsies and HeLa cell lines. Wspolczesna Onkol, 2017; 21:209–12; https://doi.org/10.5114/wo.2017.70110
Ibrahim MA, Bakhaat GA, Tammam HG, Mohamed RM, El-Naggar SA. Cardioprotective effect of green tea extract and vitamin E on cisplatin-induced cardiotoxicity in mice: toxicological, histological and immunohistochemical studies. Biomed Pharmacother, 2019; 113:108731; https://doi.org/10.1016/j.biopha.2019.108731
Ibrahim MA, Khalaf AA, Galal MK, Ogaly HA, Hassan AHM. Ameliorative influence of green tea extract on copper nanoparticle-induced hepatotoxicity in rats. Nanoscale Res Lett, 2015; 10; https://doi.org/10.1186/s11671-015-1068-z
Ikeda I, Kobayashi M, Hamada T, Tsuda K, Goto H, Imaizumi K, Nozawa A, Sugimoto A, Kakuda T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J Agric Food Chem, 2003; 51:7303–7; https://doi.org/10.1021/jf034728l
Jaganjac M, Milkovic L, Sunjic SB, Zarkovic N. The NRF2, thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants, 2020; 9:1151; https://doi.org/10.3390/antiox9111151
Jetsrisuparb A, Komwilaisak P, Wiangnon S. Green tea consumption prevented iron overload: a case report of thalassemia intermedia. J Hematol Transfus Med, 2014; 24:389–94.
Jówko E, D?ugo??cka B, Makaruk B, Cie?li?ski I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur J Nutr, 2015; 54:783–91; https://doi.org/10.1007/s00394-014-0757-1
Karlenius TC, Tonissen KF. Thioredoxin and cancer: a role for thioredoxin in all states of tumor oxygenation. Cancers (Basel), 2010; 2:209–32; https://doi.org/10.3390/cancers2020209
Khan G, Haque SE, Anwer T, Ahsan MN, Safhi MM, Alam MF. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol Pharm Drug Res, 2014; 71:861–8; https://doi.org/10.3742/opem.2005.5.2.137
Kim E, Hwang K, Lee J, Han SY, Kim EM, Park J, Cho JY. Skin protective effect of epigallocatechin gallate. Int J Mol Sci, 2018; 19:1–14; https://doi.org/10.3390/ijms19010173
Kodidela S, Shaik FB, Chinta V, Mohammad SA, Pasala C, Mittameedi CM, Maddu N, Wudayagiri R, Nallanchakravarthula V. Possible ameliorative role of green tea on chronic alcohol mediated renal toxicity of STZ -induced diabetic rats. Clin Nutr Exp, 2020; 34:1–25; https://doi.org/10.1016/j.yclnex.2020.09.001
Kucera O, Mezera V, Moravcova A, Endlicher R, Lotkova H, Drahota Z, Cervinkova Z. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid Med Cell Longev, 2015; 2015; https://doi.org/10.1155/2015/476180
Latos-Brozio M, Masek A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem Biodivers, 2019; 16; https://doi.org/10.1002/cbdv.201900426
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress and diseases. Oxid Stress Dis, 2018; 13:757–72; https://doi.org/10.5772/2535
Lopez EO, Parmar M, Pendela VS, Terrell JM. Lisinopril. StatPearls Publishing LLC, Treasure Island, FL, 2022.
Lo´pez-Burillo S, Tan DX, Mayo JC, Sainz RM, Manchester LC, Reiter RJ. Melatonin, xanthurenic acid, resveratrol, EGCG, vitamin C and a-lipoic acid differentially reduce oxidative DNA damage induced by Fenton reagents: a study of their individual and synergistic actions. J Pineal Res, 2003; 34:269–77.
Lushchak VI, Storey KB. Oxidative stress concept updated: definitions, classifications, and regulatory pathways implicated. EXCLI J, 2021; 20:956–67; https://doi.org/10.17179/excli2021-3596
Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol, 2013; 53:401–26; https://doi.org/10.1146/annurev-pharmtox-011112-140320
Maeda-Yamamoto M, Nishimura M, Kitaichi N, Nesumi A, Monobe M, Nomura S, Horie Y, Tachibana H, Nishihira J. A randomized, placebo-controlled study on the safety and efficacy of daily ingestion of green tea (Camellia sinensis L.) cv. “Yabukita” and “Sunrouge” on eyestrain and blood pressure in healthy adults. Nutrients, 2018; 10:569; https://doi.org/10.3390/nu10050569
Mahboub FA. The effect of green tea (Camellia sinensis) extract against hepato-toxicity induced by tamoxifen in rats. J Food Process Technol, 2016; 7; https://doi.org/10.4172/2157-7110.1000625
Martins A, Schimidt HL, Garcia A, Colletta Altermann CD, Santos FW, Carpes FP, Silva WC, Carpes PBM. Supplementation with different teas from Camellia sinensis prevents memory deficits and hippocampus oxidative stress in ischemia-reperfusion. Neurochem Int, 2017; 108:287–95; https://doi.org/10.1016/j.neuint.2017.04.019
Mattmiller SA, Carlson BA, Sordillo LM. Regulation of inflammation by selenium and selenoproteins: impact on eicosanoid biosynthesis. J Nutr Sci, 2013; 2:1–13; https://doi.org/10.1017/jns.2013.17
Mazur-Bialy AI, Kozlowska K, Pochec E, Bilski J, Brzozowski T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J Physiol Pharmacol, 2018; 69:117–25; https://doi.org/10.26402/JPP.2018.1.13
Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol, 2015; 89:1175–91; https://doi.org/10.1007/s00204-015-1521-x
Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension. J Am Coll Cardiol, 2018; 71:1474–82; https://doi.org/10.1016/j.jacc.2018.01.058
Misaka S, Ono Y, Uchida A, Ono T, Abe O, Ogata H, Sato H, Suzuki M, Onoue S, Shikama Y, Shimomura K. Impact of green tea catechin ingestion on the pharmacokinetics of lisinopril in healthy volunteers. Clin Transl Sci, 2021; 14:476–80; https://doi.org/10.1111/cts.12905
Mokra D, Joskova M, Mokry J. Therapeutic effects of green tea polyphenol (?)-epigallocatechin-3-gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int J Mol Sci, 2023; 24; https://doi.org/10.3390/ijms24010340
Moskaug JO, Carlsen H, Myhrstad MCW, Blomhoff R. Polyphenols and glutathione synthesis regulation. Am J Clin Nutr, 2005; 81:277–83; https://doi.org/10.1093/ajcn/81.1.277s
Mossakowski AA, Pohlan J, Bremer D, Lindquist R, Millward JM, Bock M, Pollok K, Mothes R, Viohl L, Radbruch M, Gerhard J, Bellmann-Strobl J, Behrens J, Infante-Duarte C, Mähler A, Boschmann M, Rinnenthal JL, Füchtemeier M, Herz J, Pache FC, Bardua M, Priller J, Hauser AE, Paul F, Niesner R, Radbruch H. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol, 2015; 130:799–814; https://doi.org/10.1007/s00401-015-1497-x
Nakayama T, Hashimoto T, Kajiya K, Kumazawa S. Affinity of polyphenols for lipid bilayers. BioFactors, 2000; 13:147–51.
Nunes AR, Alves MG, Tomás GD, Conde VR, Cristóvão AC, Moreira PI, Oliveira PF, Silva BM. Daily consumption of white tea (Camellia sinensis (L.)) improves the cerebral cortex metabolic and oxidative profile in prediabetic Wistar rats. Br J Nutr, 2015; 113:832–42; https://doi.org/10.1017/S0007114514004395
Opuwari C, Monsees T. Green tea consumption increases sperm concentration and viability in male rats and is safe for reproductive, liver and kidney health. Sci Rep, 2020; 10:1–14; https://doi.org/10.1038/s41598-020-72319-6
Pan B, Li H, Lang D, Xing B. Environmentally persistent free radicals: occurrence, formation mechanisms and implications. Environ Pollut, 2019; 248:320–31; https://doi.org/10.1016/j.envpol.2019.02.032
Park DH, Park JY, Kang KS, Hwang GS. Neuroprotective effect of gallocatechin gallate on glutamate-induced oxidative stress in hippocampal HT22 cells. Molecules, 2021; 26; https://doi.org/10.3390/molecules26051387
Petramfar P, Mohammadi SS, Hosseinzadeh F. Treatment of idiopathic intracranial hypotension with tea: a case report. Iran Red Crescent Med J, 2016; 18; https://doi.org/10.5812/ircmj.24620
Phimphilai S, Koonyosying P, Hutachok N, Kampoun T, Daw R, Chaiyasut C, Prasartthong-osoth V, Srichairatanakool S. Identifying chemical composition, safety and bioactivity of Thai rice grass extract drink in cells and animals. Molecules, 2021; 26(22):1–19.
Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients, 2019; 11:1–24; https://doi.org/10.3390/nu11020474
Rakshit S, Jana S, Dassarma B, Sarkar B, Samanta S. Protective role of green tea extract against cold-restraint stress induced gastric ulcerogenesis in albino rats. J Pharm Chem Biol Sci, 2018; 6:218–27.
Reddyvari H, Govatati S, Matha SK, Korla SV, Malempati S, Pasupuleti SR, Bhanoori M, Nallanchakravarthula V. Therapeutic effect of green tea extract on alcohol induced hepatic mitochondrial DNA damage in albino wistar rats. J Adv Res, 2017; 8:289–95; https://doi.org/10.1016/j.jare.2017.02.002
Reeves KG, Kanai Y. Electronic excitation dynamics in liquid water under proton irradiation. Sci Rep, 2017; 7:40379; https://doi.org/10.1038/srep40379
Regulski M, Regulska K, Stanisz B, Murias M, Gieremek P, Wzgarda A, Niznik B. Chemistry and pharmacology of angiotensin-converting enzyme inhibitors. Curr Pharm Des, 2015; 21:1764–75; https://doi.org/10.2174/1381612820666141112160013
Richi B, Kale RK, Tiku AB. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat Res Genet Toxicol Environ Mutagen, 2012; 747:62–70; https://doi.org/10.1016/j.mrgentox.2012.04.002
Sadowska-Kr?pa E, Domaszewski P, Pokora I, Zebrowska A, Gda?ska A, Podgórski T. Effects of medium-term green tea extract supplementation combined with crossfit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: a pilot study. J Int Soc Sports Nutr, 2019; 16; https://doi.org/10.1186/s12970-019-0280-0
Saravana Kumari M, Anuradha R. Effect of green tea extract on lipid peroxidation and antioxidant activity on mercuric chloride induced toxicity in rats. Int J Pharm Sci Rev Res, 2016; 36:67–72.
Shakeela Begum M, Padmavathi P, Saradamma B, Maturu P, Ananda Vardhan H, Varadacharyulu NC, Reddy DV. Effect of green tea consumption on RBC morphology, membrane properties and antioxidant status in chronic cigarette smokers. Indian J Biochem Biophys, 2018; 55:256–63.
Silveira AC, Rato L, Oliveira PF, Alves MG, Silva BM. White tea intake abrogates markers of streptozotocin-induced prediabetes oxidative stress in rat lungs’. Molecules, 2021; 26:1–12; https://doi.org/10.3390/molecules26133894
Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal, 2014; 22:505–11; https://doi.org/10.1016/j.jfda.2014.04.001
Suraphad P, Suklaew PO, Ngamukote S, Adisakwattana S, Mäkynen K. The effect of isomaltulose together with green tea on glycemic response and antioxidant capacity: a single-blind, crossover study in healthy subjects. Nutrients, 2017; 9; https://doi.org/10.3390/nu9050464
Szczeklik K, Krzysciak W, Mach P, Darczuk D, Cibor D, Rodacki T, Mach P, Darczuk D, Cibor D, Pytko-Polonczyk J, Rodacki T, Owczarek D. Alterations in glutathione peroxidase and superoxide dismutase activities in plasma and saliva in relation to disease activity in patients with Crohn’s disease. J Physiol Pharmacol, 2016; 67:709–15.
Teng Y, Wu D. Anti-fatigue effect of green tea polyphenols (-)-epigallocatechin-3-gallate (EGCG). Pharmacogn J, 2017; 13:326–31.
Thangapandiyan S, Miltonprabu S. Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: role of Nrf2/HO-1 signaling. Toxicol Rep, 2014; 1:12–30; https://doi.org/10.1016/j.toxrep.2014.01.002
Thangapandiyan S, Miltonprabu S. Epigallocatechin gallate exacerbates fluoride-induced oxidative stress mediated testicular toxicity in rats through the activation of Nrf2 signaling pathway. Asian Pac J Reprod, 2015; 4:272–87; https://doi.org/10.1016/j.apjr.2015.07.005
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol, 2007; 39:44–84; https://doi.org/10.1016/j.biocel.2006.07.001
Vrankovi? J. Age-related changes in antioxidant and glutathione S-transferase enzyme activities in the Asian clam. Biochemistry, 2016; 81:224–32; https://doi.org/10.1134/S0006297916030044
Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, de Oliveira Costa Dias G, Morel AF, Nogueira CW, Rocha JBT. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res, 2006; 1107:192–8; https://doi.org/10.1016/j.brainres.2006.05.084
Wang T, Li Q, Bi K. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci, 2018; 13:12–23; https://doi.org/10.1016/j.ajps.2017.08.004
Wang YQ, Li QS, Zheng XQ, Lu JL, Liang YR. Antiviral effects of green tea EGCG and its potential application against COVID-19. Molecules, 2021; 26:3962; https://doi.org/10.3390/molecules26133962
Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N. Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin Pharmacol Toxicol, 2015; 117:164–72; https://doi.org/10.1111/bcpt.12383
Wang Y, Wu J, Wang L, Yang P, Liu Z, Rajput SA, Hassan M, Qi D. Epigallocatechin gallate and glutathione attenuate aflatoxin B1-induced acute liver injury in ducklings via mitochondria-mediated apoptosis and the Nrf2 signalling pathway. Toxins (Basel), 2022; 14:1–14; https://doi.org/10.3390/toxins14120876
Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide, 2020; 102:39–41; https://doi.org/10.1016/j.niox.2020.06.002
Wu JH, Miao W, Hu LG, Batist G. Identification and characterization of novel Nrf2 inducers designed to target the intervening region of keap1. Chem Biol Drug Des, 2010; 75:475–80; https://doi.org/10.1111/j.1747-0285.2010.00955.x
Wu M, Wu X, Zhu J, Li F, Wei X, Wang Y. Selenium-enriched and ordinary green tea extracts prevent high blood pressure and alter gut microbiota composition of hypertensive rats caused by high-salt diet. Food Sci Hum Wellness, 2022; 11:738–51; https://doi.org/10.1016/j.fshw.2021.12.031
Xie LW, Cai S, Zhao TS, Li M, Tian Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med, 2020; 161:175–86; https://doi.org/10.1016/j.freeradbiomed.2020.10.012
Yan Z, Zhong Y, Duan Y, Chen Q, Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr, 2020; 6:115–23; https://doi.org/10.1016/j.aninu.2020.01.001
Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv JY, Zhang M. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules, 2015; 20:6626–39; https://doi.org/10.3390/molecules20046626
Yousefi T, Moazami HR. Water radiolysis by gamma –irradiation for high quality synthesis of nickel oxide nano sheet. J Nanostruct, 2019; 9:141–5; https://doi.org/10.22052/JNS.2019.01.015
Zahra KF, Lefter R, Ali A, Abdellah EC, Trus C, Ciobica A, Timofte D. The involvement of the oxidative stress status in cancer pathology: a double view on the role of the antioxidants. Oxid Med Cell Longev, 2021; 2021; https://doi.org/10.1155/2021/9965916
Zanchi MM, Manfredini V, Brum D, dos S, Vargas LM, Spiazzi CC, Soares MB, Izaguirry AP, Santos FW. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol Rep, 2015; 2:252–60; https://doi.org/10.1016/j.toxrep.2014.12.016
Zhao P, Alam MB, Lee SH. Protection of UVB-induced photoaging by fuzhuan-brick tea aqueous extract via MAPKs/Nrf2-mediated down-regulation of MMP-1. Nutrients, 2019; 11; https://doi.org/10.3390/nu11010060
Zhao T, Li C, Wang S, Song X. Green tea (Camellia sinensis): a review of its phytochemistry, pharmacology, and toxicology. Molecules, 2022; 27; https://doi.org/10.3390/molecules27123909
Zhao H, Zhu W, Jia L, Sun X, Chen G, Zhao X, Li X, Meng X, Kong L, Xing L, Yu J. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol, 2016; 89:20150665; https://doi.org/10.1259/bjr.20150665
Zhu W, Jia L, Chen G, Zhao H, Sun X, Meng X, Zhao X, Xing L, Yu J, Zheng M. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget, 2016; 7:48607–13; https://doi.org/10.18632/oncotarget.9495
Zwolak I. Epigallocatechin gallate for management of heavy metal-induced oxidative stress: mechanisms of action, efficacy, and concerns. Int J Mol Sci, 2021; 22; https://doi.org/10.3390/ijms22084027