INTRODUCTION
Ammi visnaga?L. is a medicinal plant used in traditional and modern medicines (Khalil et al., 2020). It?belongs to the Apiaceae family and is native to Asia, Europe, and the Mediterranean region of North Africa (Kamal-ad-Din, 1999). The chemical constituents of A. visnaga include furobenzopyrones (FBP), flavonoids, and essential oil, which are responsible for pharmacological action (Khalil et al., 2020). The phytoconstituents are largely responsible for treating renal colic and coronary insufficiency (Khalil et al., 2020). Ammi visnaga plant and its constituents were reported to have many biological effects such as antispasmodic effect, vasodilating effect, antidiabetic activity, treatment of vitiligo, anti-inflammatory effect, cytotoxicity effect, antioxidant activity, hair loss treatment, antimutagenic effect, immunostimulatory activity, analgesic activity, anti-hyperlipidemic, larvicidal, and insecticidal activities (Khalil et al., 2020). Few antibacterial reports exist and such activity against these microbes was majorly attributed to essential oils present in A. visnaga (Feirouz and Salima, 2014; Khalfallah et al., 2011). The experiments conducted using an organic solvent extract of A. visnaga against pathogens showed good antibacterial activity (Amin et al., 2015). In the same report, the organic extract had proteins, tannins, flavonoids, glycosides, and steroids. Such a mixture of compounds might have contributed to the antibacterial activity. Ammi visnaga contains essential oils and was reported to have antibacterial activity with Gram-negative pathogens (Khalfallah et al., 2011).
Globally, communicable disease is a leading health concern and is recognized as a significant contributor to the loss of human life (Maglangit et al., 2021). In a recent report, almost 10 million deaths were recorded, which were attributed to sepsis caused by pathogens including Escherichia coli. In general, antibiotics were considered the first line of treatment for bacterial infections. However, antibiotics misusage has led to the development of resistance (Bonomo, 2017). The pipeline for the development of antibiotics is also scarce (Martínez and Baquero, 2014). Hence, it is essential to use the existing antibiotics effectively. One such route would be using along with phytoconstituents from medicinal plants to augment the activity of the antibiotics (Dey et al., 2016; Thakur et al., 2016).
Though many chemical moieties are known to have antibacterial effects, FBPs are unique. This is because of the existence of aromatic and heterocyclic rings, fused together. FBP are compounds present in plants as secondary metabolites and are also chemically synthesized (Mao et al., 2014; Srinivasan et al., 2007). Reports on substitutions of FBP with various functional groups for different biological activities including antibacterial exists (Abu-Hashem and El-Shazly, 2015; Boddupally et al., 2019; Ragab et al., 1993).
In medicinal chemistry, heterocyclic ring systems are known to have pharmacological activity (Hiremathad et al., 2015). Among heterocyclics, benzofuran derivatives are a particular set of molecules that are seen in products of natural origin. Many researchers have reviewed the pharmacological property of benzofurans and benzopyran pharmacophores and reported to possess antibacterial activity (Dawood, 2013; Kadieva and Oganesyan, 1997; Khanam and Shamsuzzaman, 2015; Naik et al., 2015; Nevagi et al., 2015; Radadiya and Shah, 2015).
Hence, FBP could be a potential and basic chemical moiety to explore. Also, none of the reports neither discussed nor reported the effect of FBP as an antibacterial agent from A. visnaga. This study focuses on the identification, isolation, and characterization of FBP from A. visnaga and the evaluation of the antibacterial effect against E. coli.
MATERIALS AND METHODS
Chemicals and reagents
Ammi visnaga plant material was received from Herbo Nutra, India. Ammi visnaga was stored in an air-tight container at 25°C, in a dark environment, a clean, and dry place. Solvents used for extraction, and chromatography analyses were of spectroscopy grade (>98% purity, Merck, India). Escherichia coli (MTCC 443) strain was used for the antibacterial studies.
Aqueous extract preparation
Ammi visnaga plant material of various concentrations ranging between 0.50% and 25.00% w/v was prepared. Briefly, the required amount of material was weighed in an analytical balance and transferred to a 250 ml conical flask containing 100 ml of deionized water. The contents were stirred in a magnetic stirrer for 60 minutes at 1,200 rpm (Remi, 1-MLH, India). Then, the contents were filtered (Whatman filter paper No.4) and the filtrates were transferred to an air-tight, ultraviolet (UV) protected DURAN® amber glass bottle and stored in the refrigerator at 5°C ± 2°C. Prior to analysis, the contents were allowed to reach room temperature.
Sequential organic solvent extract preparation
Briefly, 10 g of the plant material was weighed into a 250 ml conical flask. The solvents used for the extractions are methanol, tetrahydrofuran (THF), and chloroform. In brief, 100 ml of methanol was added to 10 g of plant material, and the mixture was stirred in a magnetic stirrer for 60 minutes at 1,200 rpm (Remi, 1-MLH, India) at 25°C ± 2°C. Then, the Whatman filter paper (No.4) was used to filter the contents and the filtrate (methanolic extract) was collected. To the residue, 100 ml of THF was added, stirred, and filtered, as explained before. The filtrate was collected (THF extract). Then, to the residue, chloroform was added, and the steps were repeated to collect the filtrate (chloroform extract). The collected extracts were concentrated using Rota-vapor under vacuum with heating at 50°C to 1 ml of the respective solvents. The concentrated extracts were transferred to an air-tight, UV-protected DURAN® amber glass bottle and stored in the refrigerator at 5°C ± 2°C. Prior to analysis, the contents were allowed to reach room temperature.
Zone of inhibition studies
The zone of inhibition studies was carried out using the well diffusion method to evaluate the antimicrobial efficacy of the various extracts (aqueous and organic solvents) against E. coli (Wiegand et al., 2008). The overnight grown E. coli were normalized to 0.5 McFarland standard (1 × 106 cfu/ml) using Mueller Hinton broth (HiMedia Laboratories Private Limited, India) and swabbed aseptically using sterile cotton swabs on sterile Mueller Hinton agar plates. The colony count was confirmed by serially diluting the normalized cultures using 0.9% saline and by pour plating on HiCrome coliform agar media (HiMedia Laboratories Private Limited, India). For positive control (PC), sterile ciprofloxacin susceptibility discs (5 μg, HiMedia Laboratories Private Limited, India) were then placed on the center of each of the plates and 100 μl of the extract was loaded aseptically into the wells. The incubation was carried out overnight at 37°C and the zone that got inhibited was measured using a scale (mm).
Growth studies
The ability of the aqueous extract to affect the growth of E. coli was studied using Mueller Hinton broth (Wiegand et al., 2008). The bacterial suspension was grown for 18 hours, and normalized to 5 × 105 cfu/ml using sterile Mueller Hinton broth. The aqueous extracts were added to Mueller Hinton broth containing normalized E. coli culture at the 1% concentration. The flasks were incubated at 37°C overnight under stirring. After 18 hours, the optical density (OD) was taken at 620 nm using the UV-VIS spectrophotometer (Shimadzu).
Gas chromatography (GC)-MS analysis
The THF extract of A. visnaga was analyzed using Agilent Technologies 7890B GC system connected to a 5977B, Agilent Technologies mass spectrophotometer (MS) (Olasehinde et al., 2022). The GC was fitted with a capillary column (Agilent, part No.: 122-5532UI, 30 m × 250 μm × 0.25 μm, 0.25 mm ID). As a carrier gas, helium was employed at a flow rate of 1 ml/minute and the total run time was 60 minutes. The temperatures of the injector and detector were 280°C. The oven temperature was set to rise by 3°C/minutes from 60°C to 250°C. For the analysis, a 2 μl injection volume was used. A split ratio of 110:1 was used for the split injection. The mass spectra were captured at 70 eV (electron ionization, EI) and scanned between 40 and 500 m/z. Based on mass spectral patterns and those chemical spectral databases available in the National Institute of Standards and Technology (NIST) electronic library, the compounds were identified (NIST 14 Mass Spectral Library version 2.2). The spectra of the unidentified chemicals were compared to the spectrum of recognized compounds stored in the NIST collection. This allowed for the determination of the compounds’ names, molecular weights, and molecular formulas.
C18 solid phase extraction (SPE) purification
The THF extract of A. visnaga was subjected to C18 SPE as per the method described by Bruni et al. (2019), with modifications. The C18 SPE column (Agilent, Bond Elut C18 cartridge, part No.: 12102118) was used for the purification. Briefly, the THF extracts were diluted with water at a ratio of 1:1. Then, a pre-equilibrated C18 SPE column was taken and washed thrice with 10% aqueous methanol. Then, the sample was loaded, and allowed the solvent flowed out by gravity. The column was then three times rinsed with 1 ml of 100% water. Later, 1 ml of 100% THF (THF fraction) was used to elute the compounds and the steps are repeated thrice with 100% THF. The THF fractions were pooled, concentrated to 1 ml using Rota-vapor under vacuum, and used for high-performance liquid chromatography (HPLC), Fourier transform infrared (FTIR), and minimum inhibitory concentration (MIC) studies.
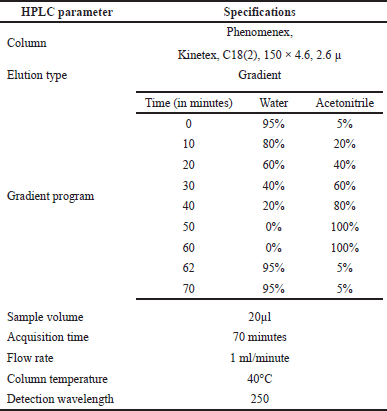 | Table 1. HPLC conditions. [Click here to view] |
HPLC analysis
The HPLC analysis was performed as per the method reported by Zgorka et al. (1998) with modifications. The chromatographic system consisted of Agilent LC, 1200 Infinity II model, equipped with a UV detector and interfaced with Openlab CDS software. Then, the separation of compounds was performed using the chromatographic conditions using the step gradient program listed in Table 1. About 1.5 ml of A. visnaga THF fraction obtained after the C18-SPE purification step was taken, diluted 10 times with acetonitrile, and transferred to amber HPLC vials with a cap. Then, the analyses were carried out with the described method and parameters (Table 1).
Fourier transform infrared
The FTIR spectra were recorded with a Spectrum Two (Perkin-Elmer, USA) spectrometer (Abousamra et al., 2016). The A. visnaga THF fraction was taken, and a drop was placed in the sample loading area. The scanning speed was at 0.2 cm/seconds within the optimum scan range from 500 to 4,000 cm−1 and the software spectrum IR (Ver 10.6.0) was used for interpreting the spectra.
MIC determination of THF fraction
The antimicrobial activity of the THF fraction was evaluated by determining the MIC against E. coli using the reported procedure (Wiegand et al., 2008). The E. coli was grown for 18 hours, normalized to 5 × 105 cfu/ml using sterile Mueller Hinton broth, and 100 μl was loaded into the 96 well tissue culture plates. The THF fraction was added from a concentration of 0.16 to 20 μg/μl using Mueller Hinton broth and 100 μl of each of the concentrations was loaded into the respective wells. The initial OD of the plates was read at 620 nm using the SpectraMax® Plus 384 Absorbance Plate Reader (Molecular Devices, USA) and the plates were incubated at 37°C overnight under static conditions. The MIC determination was based on the final OD at 620 nm, by visual observation for growth in the 96 well plates, and by colony count. The colony count was performed by serially diluting the normalized cultures using 0.9% saline and pour plating using HiCrome coliform agar.
Statistical analysis
The statistical analyses were carried out using the Statistical Analysis System from STATGRAPHICS® Centurion XVI program, Version 16.2.04 (Statgraphics Technologies, Inc., The Plains, VA). Each variable’s mean and standard deviation were computed. A one-way analysis of variance was used to assess the data with a 95% confidence level. A p-value of 0.05 or less was regarded as statistically significant. There were no data points left out of the study.
RESULTS
Effect of the aqueous extract against E. coli
The impact of different concentrations of aqueous extracts of A. visnaga on the growth of E. coli is shown in Table 2. After 18 hours, the growth was not seen in groups treated with >15% extract, which showed an OD of <0.140. However, the control group which was not treated showed an OD of 1.973. This indicates that the compounds present in the aqueous extract have inhibitory effects against E. coli. Though the growth was seen in groups treated between 0.50% and 12.50%, less growth was observed in the 12.50% treated group. However, no major difference in the growth of E. coli was observed in groups treated between 0.50% and 5.00% when compared with the control. Since the results were positive, the zone of inhibition studies was carried out and the results of various concentrations of aqueous extracts are shown in Figure 1. On analyzing the zone of inhibition, we could see the zone of inhibition beyond 15%, which agreed with the growth results (Fig. 1 and Table 2).
Effect of organic solvent extracts against E. coli
The effectiveness of A. visnaga organic solvent extracts against E. coli is shown in Figure 2. Amongst the solvents used, it was clear that the THF extract showed a high zone of inhibition (52 mm). Such results suggest that the active compounds in THF were found to have good activity against E. coli. This could be due to the efficient extraction of active compounds in THF solvent. The methanol extract showed an 18 mm zone, which must be attributed to the poor extraction of active components in the methanol. The chloroform extracts also did not show significant activity (13 mm zone).
Identification of active compounds
GC-MS analysis
The THF extract was further subjected to GC-MS analysis and the processed chromatogram is shown in Figure 3. Table 3 shows the mass ions of the peaks identified in the extracts. Based on the results, 26 peaks were observed, and the mass of those peaks ranged between 50 and 300 m/z. The obtained information of mass and molecular information was subjected to the mass spectral database and the information about the compounds was extracted and listed in Table 4. The substances numbered 3, 4, and 6 were found to be solvent THF and its by-product that was used for the extraction (Table 4). From the list, 10 compounds have contributed to an area of >1%, and the reports of such compounds were looked at. Amongst, FBP such as 7H-Furo[3,2-g][1]benzopyran-7-one, 4-methoxy (compound#23), and 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy (compound#25) were reported to be present in A. visnaga (Khalil et al., 2020). These compounds are derivatives of 7H-Furo[3,2-g][1]benzopyrans.
HPLC analysis
Since FBPs (compounds#23 and 25) eluted at the later stage of the chromatographic analysis and were eluted closer to each other, a simple purification step using C18 SPE cartridges was performed. The compounds purified using the C18-SPE step (THF fraction) were subjected to HPLC analysis, and the outcomes are shown in Figure 4 and Table 5. From the chromatogram, the compound eluted at RT 21.686 minutes contributed to 91% area. These results indicate that the THF fraction had the compound to the extent of >90% purity.
Mass analysis
To identify the compound eluted at RT 21.686 minutes, the sample was subjected to mass analysis and the mass ion spectrum is shown in Figure 5. The major fragment ions were seen at m/z 246 and 231, followed by minor fragment ions at m/z 203, 188, and 175. The presence of these major and minor fragment ions confirms the presence and identity of 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy (NIST, 2021).
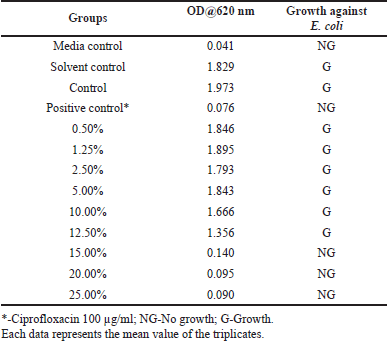 | Table 2. The effect of various concentrations of aqueous extracts of A. visnaga against E. coli. [Click here to view] |
FTIR analysis
Further, the THF fraction was subjected to FTIR analysis to understand the functional groups present in the sample, and the result is shown in Figure 6. The prominent peaks were observed at 3276cm−1, 2958cm−1, 2878cm−1, 1189cm−1, 1054cm−1, and 923 cm−1. Such peaks indicate the presence of functional groups such as aromatic ketones, aliphatic hydroxyl groups, alkyl groups, and cyclic aromatic rings. The peak at 1,054 cm−1 was majorly contributed by THF, a solvent used for the extraction.
MIC determination of THF fraction
The THF fraction which contained 91% of 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy was subjected to antibacterial studies against E. coli to know the MIC, and the results are shown in Figure 7. From the results, it was very evident that at 10 and 20 μg/μl concentrations, full inhibition was seen. Also, the growth was partially inhibited at 5 μg/ μl, suggesting the MIC was at 10 μg/ μl. The results also suggest that a dose-dependent inhibition against E. coli was observed. The PC that was used in this study was ciprofloxacin, which did not show any growth confirming its antibiotic property. The media control did not show any growth, indicating no external contamination occurred during the study. The solvent (THF) that was used in this study, did not have an effect on the growth of the E. coli.
DISCUSSION
Plants are the richest sources of secondary metabolites such as alkaloids, terpenoids, flavonoids, polyphenols, etc., and are known to have medicinal properties. These compounds differ in their physico-chemical properties and are extracted based on their solubility parameter (Musa et al., 2011). The solvents which differ in polarity between polar and non-polar were considered for extracting the actives from A. visnaga. Initially, a polar solvent (deionized water) was used to extract the active compounds. After 18 hours of incubation with the aqueous extract, the 15% treated group showed an OD of 0.14, which was lesser than the control OD of 1.973 (Table 2). Such lower OD suggests that the extract has the potential to inhibit the E. coli growth. To reconfirm the findings, zone of inhibition results was carried out and a visible zone of 10.5 mm was seen for the 15% treated group (Fig. 1). On increasing the concentrations of the aqueous extract to 20% and 25%, the zone of inhibition was found to be 12.50 and 14.00 mm, respectively. Though statistical significance was observed between the groups, the increase in the zone of inhibition was found to be marginal. However, all the tested concentrations exhibited a lesser zone of inhibition when compared with PC. Such activity at higher concentrations must be attributed to the poor solubility of the active ingredients present in the plants. In plants, the compounds having higher log p values were reported to have poor aqueous solubility (Del Río et al., 2014). Because of this, the activity was not seen at lower concentrations. Also, these results indicate that the compound contributed to the antibacterial property has less solubility in water, suggesting the need for organic solvents to extract active ingredients.
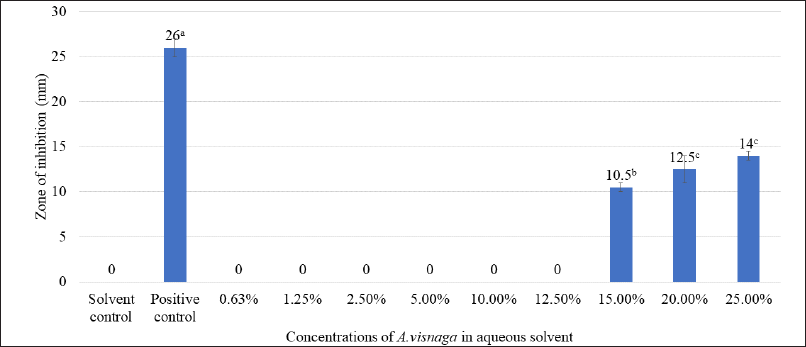 | Figure 1. Antibacterial effect of various concentrations of aqueous extracts of A. visnaga. The mean of the value ± SD (n = 3) is used to express each experimental data set. Different alphabets in superscript served as indicators of a significant difference between the groups (p < 0.05). PC-positive control, Ciprofloxacin. Solvent control-100% water. [Click here to view] |
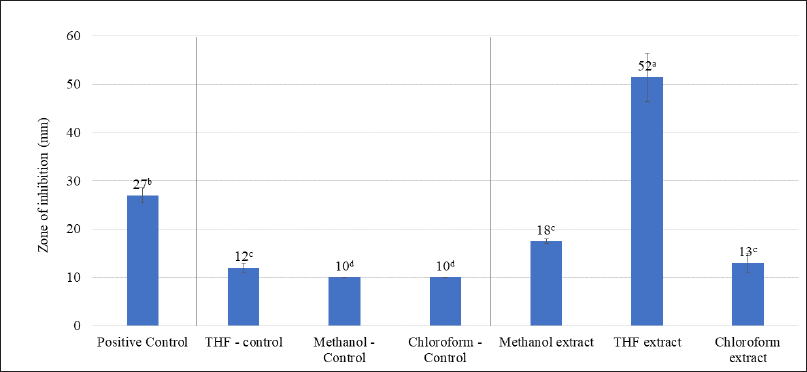 | Figure 2. Antibacterial effect of organic solvent extracts of A. visnaga. The mean of the value ± SD (n = 3) is used to express each experimental data set. Different alphabets in superscript served as indicators of a significant difference between the groups (p < 0.05). Positive control-Ciprofloxacin. [Click here to view] |
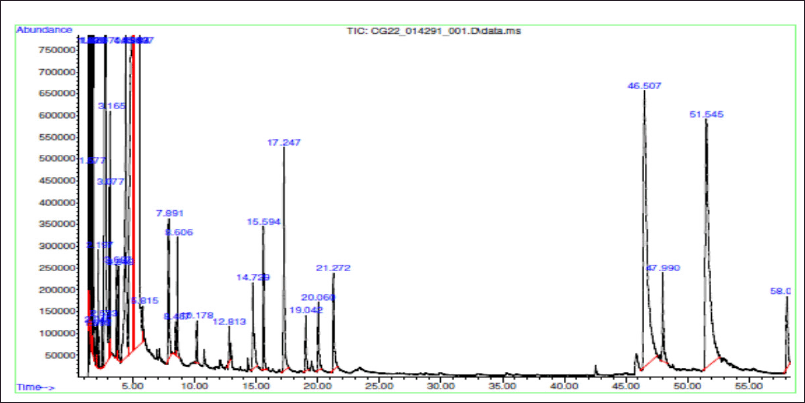 | Figure 3. GC-MS analysis of THF extract of A. visnaga. [Click here to view] |
The active ingredients were often extracted using organic solvents with varying degrees of polarity, such as methanol, ethanol, isopropyl alcohol, THF, dichloromethane, and chloroform. In this study, the sequential extraction procedure was carried out, to avoid the carry-over of the actives to the various solvents when extracted individually. The sequential extraction of actives was performed using methanol, THF, and chloroform which ranged from highly polar to non-polar organic solvents, and the results are shown in Figure 2. Amongst, the THF extract showed a 52 mm zone of inhibition against E. coli, and it was found to be much more efficacious and significantly higher than any of the tested groups (p < 0.05, Fig. 2). The antibacterial activity of compounds isolated from plant sources were evaluated by several authors using zone of inhibition test and efficacious leads were newly identified (Archana and Bose, 2022; Arif et al., 2022; Eloff, 2019; Gonelimali et al., 2018; Saquib et al., 2019).
As the initial experiments were positive, an attempt was made to identify the compounds present in the THF extract that are responsible for the activity. The chromatographic techniques coupled with MS along with database search help to identify and characterize the compounds (Kanthal et al., 2014). The THF extract was subjected to GC-MS analysis (Fig. 3) and mass spectral database searches were carried out to identify the compounds present in the THF extract (Table 4). In the extract, 26 different compounds were present and 2 FBP derivatives were seen (Fig. 3 and Table 4). The FBPs that were seen are 7H-Furo[3,2-g][1]benzopyran-7-one, 4-methoxy-, and 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy. In a study reported by Gunaydin and Beyazit (2004) furanochromones (or furobenzopyrans, FBP) and one furanochromone glycoside along with essential oils were extracted from A. visnaga’s fruits, which were confirmed through spectroscopy and chromatographic studies. On comparing the compounds reported by Gunaydin and Beyazit (2004) and compounds listed in Table 4, two FBPs were present in the current study, which confirms and agrees with the presence of FBPs in A. visnaga. Alaatabi et al. (2020) has conducted a GC-MS study to profile the phytoconstituents in the methanolic extract of A. visnaga. The compounds that were found in the majority were khellin along with visnagin. Khellin and visnagin have FBP as a common core chemical group. Based on the reports from Gunaydin and Beyazit (2004) and Alaatabi et al. (2020), the plant extract that was used in the current study confirms the identity of A. visnaga.
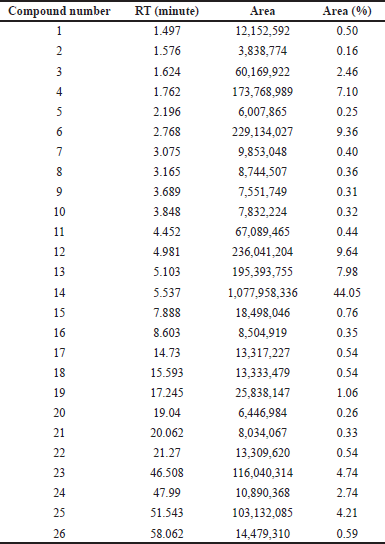 | Table 3. Compounds identified in THF extract of A. visnaga through GC-MS. [Click here to view] |
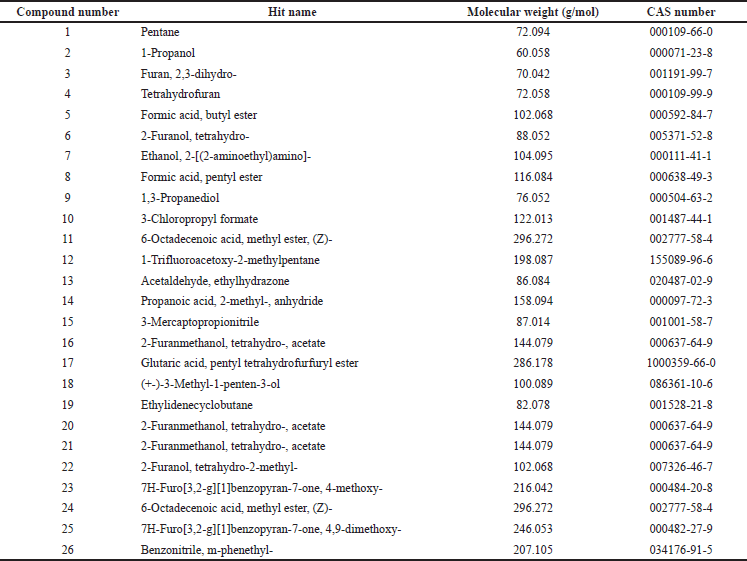 | Table 4. Compounds identified in THF extract of A. visnaga after performing NIST mass spectral database search. [Click here to view] |
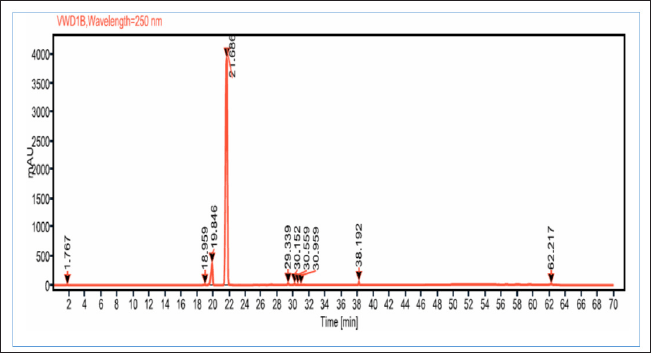 | Figure 4. HPLC chromatogram of THF fraction obtained after C18 SPE extraction with THF extract of A. visnaga. [Click here to view] |
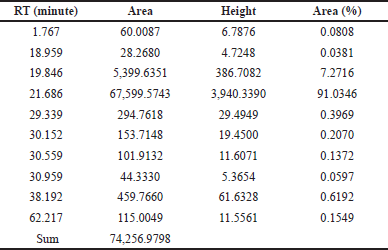 | Table 5. Peak information of THF fraction of A. visnaga subjected to HPLC analysis after C18 SPE extraction. [Click here to view] |
The phytoconstituents of A. visnaga were reported by many authors and regulatory bodies (WHO, 2007). Ammi visnaga contained phytoconstituents such as FBP (up to 4%), which includes khellin, visnagin, and their derivatives. Additionally, coumarins (0.2%–0.5%), with the predominant one being pyranocoumarin (visnadin, 0.3%), and oils up to 18% were also reported (Al-Snafi, 2013). Based on the results from GC-MS, many compounds were present along with FBP in the THF extract (Table 4).
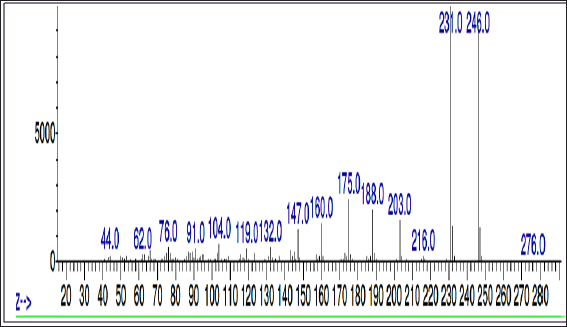 | Figure 5. Mass spectrum of THF fraction of A. visnaga obtained after C18 SPE extraction [Click here to view] |
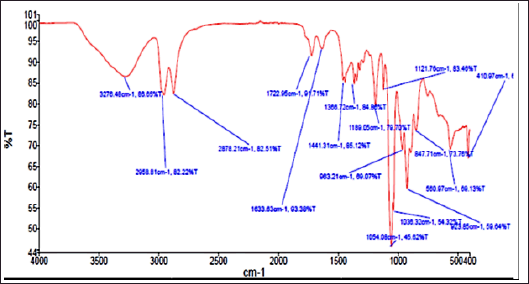 | Figure 6. FTIR graph of THF fraction of A. visnaga obtaind after C18 SPE extraction. [Click here to view] |
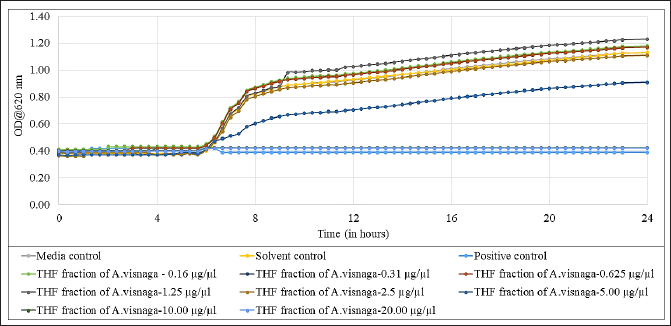 | Figure 7. Antibacterial effect of THF fraction of A. visnaga obtained after C18 SPE extraction against E. coli. Each experimental result is presented as the mean of the three values. Media control-Mueller Hinton Broth; Solvent control-THF; Positive control, Ciprofloxacin 100 μg/ml. [Click here to view] |
As the crude extract contains a mixture of compounds, it would be possible that the observed effect was due to a combination of compounds. To evaluate the FBPs for antibacterial activity, it is essential to isolate and purify them. SPE is one such technique used to separate and isolate the compounds from the mixture (Poole, 2003). In natural product isolation, SPE was used to isolate the phenols, flavones, and lignans from the plant sources (Mateos et al., 2001). In the review article published by Bruni et al. (2019) C18 SPE cartridges were used to isolate the furanocoumarins from the plant sources. Also, attempts have been made to purify the naturally occurring coumarins by SPE, which contains a benzopyran-2-one nucleus, and the results were positive in getting the purified compound (Del Río et al., 2014). On basis of these reports, a purification step using C18 SPE cartridges was done, and the compounds of FBP were extracted with THF (Fig. 4 and Table 5). The purified THF fraction was subjected to HPLC, MS, and FTIR analysis. In the HPLC analysis, almost 91% of the area was contributed by a single compound eluted at 21.68 minutes (Fig. 4 and Table 5). Such results indicate that the purification happened in the C18-SPE step yielding the purity to the extent of >90%. The fraction was also subjected to MS analysis, and the compound had an m/z of 246.053 (Fig. 5). The analysis revealed the presence of major fragment ions at m/z 246 and 231 followed by minor fragment ions at m/z 203, 188, and 175 (Fig. 5). The compound 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy was reported to have m/z 246 and similar fragment ions (NIST, 2021). Also, reports on A. visnaga confirm the presence of this compound in the different parts of the plant including fruits (Khalil et al., 2020). Based on our results, published reports, and NIST database search, we confirm that the FBP present in THF fraction was 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy (Al-Snafi, 2013; Khalil et al., 2020; NIST, 2021).
The THF fraction was subjected to FTIR analysis (Fig. 6) and transmittance at 1,054 cm−1 indicating the C–O stretching of primary alcohols; 2,958 and 2,878 cm−1 indicating the C–H stretching of alkyl groups; 3,276 cm−1 indicating O–H stretching of substituted alcohols; 1,441 cm−1 indicating C–H bending due to presence of methyl/methylene groups were noticed (Fig. 6; Uddin, 2012). On analyzing the chemical structure of 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy, and the functional groups observed in FTIR analyses, were-confirmed the identified compound. Then, the THF fraction which contained 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy was subjected to antibacterial studies against E. coli and the MIC was found to be at 10 μg/μl (Fig. 7). At 5 μg/μl, a slow growth rate was observed, suggesting this compound has altered the growth without exerting cidal action (Fig. 7). In a study reported by Elsherif et al. (2020), pyrazolines incorporated with benzofuran showed antimicrobial activity against some of the pathogenic microorganisms including E. coli. Researchers have explored the compounds at sub-MIC concentrations to control the virulence of pathogens (Das et al., 2021; Khan et al., 2022; Liu et al., 2021; Mohabi et al., 2017; Pandit et al., 2013; Sagar et al., 2022). In such a scenario, the virulence of the pathogens was suppressed without affecting the growth of the pathogens (Zhou et al., 2017). Upon analyzing the reports on controlling the virulence at sub-MIC concentrations and the results of this study (Fig. 7), 7H-Furo[3,2-g][1]benzopyran-7-one, 4,9-dimethoxy has the high potential to control the virulence.
CONCLUSION
The dried plant material of A. visnaga was subjected to various extraction techniques and the FBP was identified, purified, and characterized using chromatographic and spectroscopic techniques. The FBP compound was found to be 5H-Furo[3,2-g][1]benzopyran-5-one, 4,9-dimethoxy. The experiments revealed that 5H-Furo[3,2-g][1]benzopyran-5-one, 4,9-dimethoxy exerted an antibacterial effect against E. coli from 10 μg/μl onwards.
FUTURE STUDIES
Since 5H-Furo[3,2-g][1]benzopyran-5-one, 4,9-dimethoxy possessed antibacterial activity, its effect towards controlling the virulence factors such as the formation of biofilm and endotoxin, quenching the quorum sensing pathways in Gram-negative pathogens will be explored along with derivatives and similar compounds. Such studies would help in utilizing the existing antibiotics to treat bacterial infections, effectively and circumvent antibiotic resistance.
LIST OF ABBREVIATIONS
FBP, Furobenzopyrans; FTIR, Fourier transform infrared spectroscopy; GC, Gas chromatography; HPLC, High performance liquid chromatography; MIC, Minimum inhibitory concentration; MS, Mass spectrometry; NIST, National institute of standards and technology; OD, optical density; EI, Electronic ionization; SPE, solid phase extraction THF, Tetrahydrofuran; THF extract, A. visnaga extracted with tetrahydrofuran; THF fraction, THF extract subjected to C18 SPE and eluted with THF; PC, Positive control.
ACKNOWLEDGMENT
I would like to express my sincere acknowledgment to Kemin Industries South Asia Pvt. Ltd. for supporting me in carrying out these studies. Also, I would acknowledge Mr. Debarchan Chakraborty, a student of the department of Bio-engineering, VISTAS for his help.
AUTHOR CONTRIBUTIONS
Rajendra Moorthy, R. contributed to experimental design and execution, data collection, analysis, manuscript design, preparation, and revision of the manuscript. Brindha Devi, P. contributed to data interpretation and analysis, revision, and approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interest.
ETHICAL APPROVALS
We hereby confirm that lab animals, and humans are not involved in this study.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abousamra MM, Basha MO, El Awdan S, Ismail NS. Effect of methyl-Β-cyclodextrin complexation on the hypoglycemic and hypolipidemic effects of khellin: experimental study. Int J Pharm Pharm Sci, 2016; 8(11):165–72. CrossRef
Abu-Hashem AA, El-Shazly M. Synthesis, reactions and biological activities of furochromones: a review. Eur J Med Chem Rep, 2015; 90:633–65. CrossRef
Alaatabi RM, Almousawi UM, Mosa MN, Hamarashid SH. Phytochemical screening by using TLC and GC-MS methods for qualitative determination of compounds in Ammi visnaga. extract. Plant Arch, 2020; (2):4326–30.
Al-Snafi AE. Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga. A review. Int J Pharm Ind Res, 2013; 3(3):257–65.
Amin JN, Murad A, Motasem AM, Ibrahem SR, Ass’ad JM, Ayed AM. Phytochemical screening and in-vitro evaluation of antioxidant and antimicrobial activities of the entire Khella plant (Ammi visnaga. L.) a member of palestinian flora. Int J Pharmacogn Phytochem Res, 2015; 7:137–43.
Archana H, Bose VG. Evaluation of phytoconstituents from selected medicinal plants and its synergistic antimicrobial activity. Chemosphere, 2022; 287:132276. CrossRef
Arif M, Wang X, Kazi MS, Ullah Khan S, Saeed S, Khan AM, Khan RA, Afzal M, Nawaz AF, Zia MA, O. Elansary H. Antimicrobial activities of different solvent extracts from stem and seeds of Peganum Harmala L. PLoS One, 2022; 17(4):e0265206. CrossRef
Boddupally S, Jyothi P, Rao MV, Rao KP. Design and synthesis of antimicrobial active (E)-(3-(Substituted-styryl)-7H-furo [2, 3-f] chromen-2-yl)(phenyl) methanone derivatives and their in silico molecular docking studies. J Heterocyclic Chem, 2019; 56(1):73–80. CrossRef
Bonomo RA. β-Lactamases: a focus on current challenges. Cold Spring Harb Perspect Med, 2017; 7(1):a025239. CrossRef
Bruni R, Barreca D, Protti M, Brighenti V, Righetti L, Anceschi L, Mercolini L, Benvenuti S, Gattuso G, Pellati F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules, 2019; 24(11):2163. CrossRef
Das S, Chourashi R, Mukherjee P, Kundu S, Koley H, Dutta M, Mukhopadhyay AK, Okamoto K, Chatterjee NS. Inhibition of growth and virulence of Vibrio cholerae by carvacrol, an essential oil component of Origanum spp. J Appl Microbiol, 2021; 131(3):1147–61. CrossRef
Dawood KM. Benzofuran derivatives: a patent review. Expert Opin Ther Pat, 2013;23(9):1133–56. CrossRef
Del Río JA, Díaz L, García-Bernal D, Blanquer M, Ortuno A, Correal E, Moraleda JM. Furanocoumarins: biomolecules of therapeutic interest. Stud Nat Prod Chem, 2014; 43:145–95. CrossRef
Dey D, Ghosh S, Ray R, Hazra B. Polyphenolic secondary metabolites synergize the activity of commercial antibiotics against clinical isolates of β-Lactamase-producing Klebsiella pneumoniae. Phytother Res, 2016; 30(2):272–82. CrossRef
Eloff JN. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement Altern Med, 2019; 19(1):1–8. CrossRef
Elsherif MA, Hassan AS, Moustafa GO, Awad HM, Morsy NM. Antimicrobial evaluation and molecular properties prediction of pyrazolines incorporating benzofuran and pyrazole moieties. J Appl Pharm Sci, 2020; 10(2):037–43. CrossRef
Feirouz B, Salima KG. Antibacterial activity and chemical composition of Ammi visnaga L. essential oil collected from Boumerdes (Algeria) during three periods of the plant growth. J Essent Oil Bear Plants, 2014; 17(6):1317–28. CrossRef
Gunaydin K, Beyazit N. The chemical investigations on the ripe fruits of Ammi visnaga (Lam.) Lamarck growing in Turkey. Nat Prod Rep, 2004; 18(2):169–75. CrossRef
Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol, 2018; 9:1639. CrossRef
Hiremathad A, Patil MR, Chethana KR, Chand K, Santos MA, Keri RS. Benzofuran: an emerging scaffold for antimicrobial agents. RSC Adv, 2015; 5(117):96809–28. CrossRef
Kadieva MG, Oganesyan ET. Methods for the synthesis of benzofuran derivatives. Chem Heterocycl Compd, 1997; 33(11):1245–58. CrossRef
Kamal-ad-Din HB. Wild medicinal plants in Egypt: an inventory to support conservation and sustainable use. Academy of Scientific Research et Technology, Cairo, Egypt, 1999.
Kanthal LK, Dey A, Satyavathi K, Bhojaraju P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacogn Res, 2014; 6(1):58. CrossRef
Khalfallah A, Labed A, Semra Z, Kaki B, Kabouche A, Touzani R, Kabouche Z. Antibacterial activity and chemical composition of the essential oil of Ammi visnaga L.(Apiaceae) from Constantine, Algeria. Int J Med Aromat Plants, 2011; 1(3):302–5.
Khalil N, Bishr M, Desouky S, Salama O. Ammi visnaga L., a potential medicinal plant: a review. Molecules, 2020; 25(2):301. CrossRef
Khan MA, Khan HM, Ganie IB, Kumar S, Shahzad A, Celik I, Shahid M. Anti-quorum sensing, antibiofilm, and antibacterial activities of extracts of Centella asiatica L. leaves, and in vitro derived leaves-calli through tissue culture: a potential for biofouling-prevention. Biofouling, 2022; 38(7):715–28. CrossRef
Khanam H, Shamsuzzaman. Bioactive benzofuran derivatives: a review. Eur J Med Chem, 2015; 97:483–504. CrossRef
Liu B, Zhang X, Ding X, Wang Y, Zhu G. Regulatory mechanisms of sub-inhibitory levels antibiotics agent in bacterial virulence. Appl Microbiol Biotechnol, 2021; 105(9):3495–505. CrossRef
Maglangit F, Yu Y, Deng H. Bacterial pathogens: threat or treat (a review on bioactive natural products from bacterial pathogens). Nat Prod Rep, 2021; 38(4):782–821. CrossRef
Mao Z, Sun W, Fu L, Luo H, Lai D, Zhou L. Natural dibenzo-α-pyrones and their bioactivities. Molecules, 2014; 19(4):5088–108. CrossRef
Martínez JL, Baquero F. Emergence and spread of antibiotic resistance: setting a parameter space. Ups J Med Sci, 2014; 119(2):68–77. CrossRef
Mateos R, Espartero JL, Trujillo M, Ríos JJ, León-Camacho M, Alcudia F, Cert A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J Agric Food Chem, 2001; 49(5):2185–92. CrossRef
Mohabi S, Kalantar-Neyestanaki D, Mansouri S. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by Quercus infectoria gall extracts. Iran J Microbiol, 2017; 9(1):26.
Musa KH, Abdullah A, Jusoh K, Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Anal Methods, 2011; 4(1):100–7. CrossRef
Naik R, Harmalkar DS, Xu X, Jang K, Lee K. Bioactive benzofuran derivatives: Moracins A–Z in medicinal chemistry. Eur J Med Chem Rep, 2015; 90:379–93. CrossRef
Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal significance of benzofuran. Eur J Med Chem Rep, 2015; 97:561–81. CrossRef
NIST. NIST Chemistry WebBook, SRD 69. 2021. Available via https://webbook.nist.gov/cgi/cbook.cgi?ID=C482279&Mask=200#Mass-Spec (Accessed 26 December 2021).
Olasehinde OR, Afolabi OB, Owolabi OV, Akawa AB, Omiyale OB. GC–MS analysis of phytochemical constituents of methanolic fraction of Annona muricata leaf and its inhibition against two key enzymes linked to type II diabetes. Sci Afr, 2022; 16:e01178. CrossRef
Pandit S, Chang KW, Jeon JG. Effects of Withania somnifera on the growth and virulence properties of Streptococcus mutants and Streptococcus sobrinus at sub-MIC levels. Anaerobe, 2013; 19:1–8. CrossRef
Poole CF. New trends in solid-phase extraction. Trends Analyt Chem, 2003; 22(6):362–73. CrossRef
Radadiya A, Shah A. Bioactive benzofuran derivatives: an insight on lead developments, radioligands and advances of the last decade. Eur J Med Chem Rep, 2015; 97:356–76. CrossRef
Ragab FA, Hussein MM, Hanna MM, Hassan GS, Kenawy SA. Synthesis, anticonvulsant and antimicrobial activities of certain new furochromones. Pharmazie, 1993; 48(11):808–11.
Sagar PK, Sharma P, Singh R. Inhibition of quorum sensing regulated virulence factors and biofilm formation by Eucalyptus globulus against multidrug-resistant Pseudomonas aeruginosa. J Pharmacopuncture, 2022; 25(1):37. CrossRef
Saquib SA, AlQahtani NA, Ahmad I, Kader MA, Al Shahrani SS, Asiri EA. Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontal pathobionts: an in vitro microbiological study. Antibiotics (Basel), 2019; 8(3):89. CrossRef
Srinivasan KK, Neelima Y, Alex J, Sreejith G, Ciraj AM, Rao JV. Synthesis of novel furobenzopyrone derivatives and evaluation of their antimicrobial and antiinflammatory activity. Indian J Pharm Sci, 2007; 69(2):326. CrossRef
Thakur P, Chawla R, Goel R, Narula A, Arora R, Sharma RK. Augmenting the potency of third-line antibiotics with Berberis aristata: in vitro synergistic activity against carbapenem-resistant Escherichia coli. J Glob Antimicrob Resist, 2016; 6:10–6. CrossRef
Uddin J. (Ed.). Macro to nano spectroscopy. BoD–Books on demand. InTech, Rijeka, Croatia, 2012. CrossRef
WHO. WHO monographs on selected medicinal plants. World Health Organization, Geneva, Switzerland, vol. 3, 2007.
Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc, 2008; 3:163–75. CrossRef
Zgorka G, Dragan T, G?owniak K, Basiura E. Determination of furanochromones and pyranocoumarins in drugs and Ammi visnaga fruits by combined solid-phase extraction–high-performance liquid chromatography and thin-layer chromatography–high-performance liquid chromatography. J Chromatogr Open, 1998; 797(1–2):305–9. CrossRef
Zhou J, Bi S, Chen H, Chen T, Yang R, Li M, Fu Y, Jia AQ. Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Front Microbiol, 2017; 8:769. CrossRef