INTRODUCTION
Diabetes mellitus is a worldwide public health condition with a growing prevalence and an urgent global concern (Glovaci et al., 2019). From slightly over 100 million in 1980 to over 420 million in 2017, projected to reach over 629 million in less than three more decades, the prevalence has more than tripled in the last several decades (Global Burden of Disease Collaborative Network, 2015). Possible causes of the rather drastic rise in prevalence might be increased urbanization, a sedentary lifestyle, increased consumption of processed diets, and many other socioeconomic factors (Basu et al., 2013). Diabetes mellitus usually goes undetected for many years in patients, and complications arise due to this delay in making a diagnosis. These complications, either microvascular or macrovascular though varied, are a significant reason why the global deaths attributable to diabetes mellitus is over 5 million annually (Glovaci et al., 2019). An example of a microvascular complication of diabetes is diabetic neuropathy, a significant cause of disability, diabetic foot ulcer, and amputation in hospitals. Diabetic peripheral neuropathy (DPN) accounts for about 75% of diabetic neuropathy, with pain being its most common symptom (Selvarajah and Tesfaye, 2012; Zheng et al., 2017).
There is still much uncertainty surrounding the development of DPN at the molecular level. However, there is evidence that hyperglycemia is crucial in initiating and accelerating the course of diabetes mellitus and diabetic neuropathy. In the nervous system, uncontrolled hyperglycemia targets both the axon (due to its access to the blood supply of the nerve) and the Schwann cells that cover it, thereby destroying axonal integrity and damaging the peripheral fibers of sensory nerve cells leading to pain (Edwards and Vincent, 2008; Zabihi et al., 2021). Hyperglycemia causes a rise in the metabolic activities of the mitochondria and initiates oxidative stress by overwhelming the normal endogenous antioxidants like glutathione, catalase, and superoxide dismutase (SOD) (Prabodha et al., 2018).
Furthermore, hyperglycemia also adversely drives other metabolic mechanisms, such as the polyol route, which result in excessive/inappropriate stimulation of the protein kinase C pathway and the buildup of advanced glycation end products (Prabodha et al., 2018). Historically, neuropathic pain has been managed by using anticonvulsants like gabapentin and pregabalin, as well as tricyclic antidepressants like amitriptyline and nortriptyline, and serotonin-norepinephrine reuptake inhibitors such as duloxetine and venlafaxine (Singh et al., 2014). There is, however, a paucity of literature on natural plant extracts that can control underlying hyperglycemia, mitigate free radicals, and modulate neuropathic pain. Ficus exasperata, commonly known as “sandpaper tree,” is a deciduous shrub or tree that can grow up to 20 m in height; it has smooth gray bark and very rough leaves. Flavonoids, tannins, saponins, alkaloids, and cyanogenic glycosides have all been identified by phytochemical and toxicological investigations of F. exasperata leaf and stem extracts (Ijeh and Ukweni, 2007). Diabetes treatment with F. exasperata has been previously described (Deepa et al., 2018). The antioxidant property and antinociceptive effect of its bioactive compounds have also been investigated (Anigboro et al., 2019; Popwo et al., 2020). However, the role of F. exasperata in streptozocin (STZ)-induced diabetic neuropathic pain has not been reported. Hence, this study sought to investigate the possible role of F. exasperata in modulating neuropathic pain and alleviating oxidative stress in male Wistar rats.
MATERIALS AND METHODS
Experimental design
This study compared healthy normal rats with treated and untreated diabetic rats with neuropathy. Pregabalin, a commonly used medication, and an aqueous extract of F. exasperata were given to the treated rats. In the experiment, there were a total of 36 male Wistar rats that ranged in weight from 120 to 170 g. The animals were housed in the animal house of the College of Health Sciences at the University of Ilorin. There, they were given access to natural light and dark cycles, a suitable room temperature, sufficient ventilation, and adequate spacing between them. During the acclimatization period of 2 weeks, rats were given free access to regular rat pellets and purified water. Following acclimatization, the baseline blood glucose level and thermal allodynia (measured by the ice-cold test and the hot plate test) were examined. After this, a single injection of STZ into the intraperitoneal cavity at a dose of 50 mg/kg b.w. was used to induce type 2 diabetes. Following successful induction of diabetes as confirmed by fasting blood glucose level of at least 13.9 mmol/l, a period of 30 days was observed to allow signs of peripheral neuropathy to set in. This was confirmed by a repeated pain threshold test via assessment of thermal allodynia using ice-cold and hot plate tests. The animals in groups 3 and 4 were then treated with pregabalin 0.71 mg/kg b.w. and aqueous extract of F. exasperata 200 mg/kg b.w., respectively. All drugs were administered orally using an oral cannula once daily for 21 days.
Animal grouping
Using random selection, the animals were divided into four groups of nine animals each. Group 1 (healthy rats) was treated with normal saline 1 ml/kg b.w., group 2 (diabetic rats) was treated with normal saline 1 ml/kg b.w., group 3 (diabetic rats) was treated with pregabalin 1.5 mg/kg b.w., and group 4 (diabetic rats) was treated with F. exasperata 200 mg/kg b.w.
Estimation of plasma glucose
The rats fasted 10–12 hours before induction of diabetes with STZ, and their blood glucose was estimated before and after (72 hours) induction of diabetes. The estimation was carried out using a blood glucose test strip (ACON laboratories). The on-call plus visual test system was used to measure the glucose concentration in the capillary whole blood. A diabetic state was confirmed at a fasting plasma glucose level of ≥13.9 mmol/l.
Induction of diabetes mellitus
After baseline fasting plasma glucose had been checked, diabetes mellitus was induced by a single intraperitoneal injection of 50 mg/kg of STZ. Rats were observed for 30 days for features of DPN to set in.
Confirmation of DPN
The onset of DPN was confirmed by assessing the pain threshold via thermal allodynia using ice-cold and hot plate tests.
Ice cold test
Cold allodynia was assessed using the ice-cold test. A block of ice was placed directly on the center of the left hind paw of each rat for 60 seconds. The latency (time taken to observe a nocifensive behavior and generate responses like paw withdrawal/licking and jumping) was recorded accordingly using a stopwatch. The test was aborted if the rat did not show any response after the 60 seconds elapsed.
Hot plate test
Rats were placed individually in a chamber on a hot plate whose temperature was regulated at 55°C ± 2°C. The time it took for responses like jumping, paw licking, scratching at the walls of the chamber, and so on to occur was recorded using a stopwatch. The test also lasted for 60 seconds per rat and was aborted if the rat did not show any response.
Plant extraction
Local farmers in Ilorin, Kwara State, Nigeria, provided the fresh leaves of the F. exasperata plant used in this study. The Herbarium of the Department of Plant Biology at the University of Ilorin was responsible for the identification and authentication of the leaves and the assignment of the voucher number: UIL/001/1296. The leaves were air-dried for 12 days, pounded into the powdery form using a ceramic mortar and pestle, and then soaked in three and a half liters of distilled water for 12 hours. The solution was then extracted by filtration using filter paper as the filter medium. A water bath was used in order to evaporate the filtrate until it was completely dry at 60°C. The aqueous extract was then dissolved in normal saline for daily administration.
Drug and extract administration
Following successful induction of diabetes mellitus and the onset of diabetic peripheral neuropathic pain, a standard drug, pregabalin, and the aqueous extract of F. exasperata were administered orally once daily for 3 weeks at doses of 0.71 mg/kg b.w. and 200 mg/kg b.w., respectively, using an oral cannula.
Animal euthanasia
At the completion of the experiment, a single intraperitoneal injection of ketamine/xylazine 100/12.5 mg/kg b.w. was used to humanely euthanize the animals. Immediately after euthanasia, under sterile conditions, a plain bottle was used to collect the blood that was taken after a heart puncture and was allowed to coagulate. Clotted blood sample was centrifuged at 1,500 rpm for 5 minutes, and serum was collected in a plain bottle. The serum was stored at 4°C until it was assayed for glutathione (GSH) and SOD.
Brain sample collection
Under sterile conditions, the brain was excised, and the hippocampus was collected on ice. It was then homogenized in a 0.25 M sucrose buffer solution. The homogenates were then centrifuged at 2,000 rpm for 5 minutes and assayed for malondialdehyde (MDA) and nitric oxide (NO).
Sciatic nerve collection
The left sciatic nerve of each rat was excised under sterile conditions and homogenized in 0.25 M sucrose buffer solution. The homogenates were then centrifuged at 2,000 rpm for 5 minutes and assayed for brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF).
Skin sample collection
Under sterile conditions, skin snip was excised from the back, midway between the head and the tail, after shaving the fur of each rat. It was then homogenized in a 0.25 M sucrose buffer solution. The homogenates were then centrifuged at 2,000 rpm for 5 minutes and assayed for calcitonin gene-related peptide (CGRP) and substance P (SP).
Statistical analysis
Data were expressed as mean ± SE of the mean on bar charts. Comparison between different groups was analyzed using one-way analysis of variance with GraphPad Prism version 5.0. The level of significance was set at p < 0.05.
RESULTS
Plasma glucose
Figure 1 shows the effect of the aqueous extract of F. exasperata leaf on the plasma glucose level of rats in the experimental groups before and after induction of DPN, as well as after treatment. There was a significant (p < 0.05) increase in the plasma glucose level after induction of diabetes when compared to the baseline. This was reversed following treatment, with a more pronounced decrease in plasma glucose in the group treated with an aqueous extract of F. exasperata leaf than in the pregabalin-treated group.
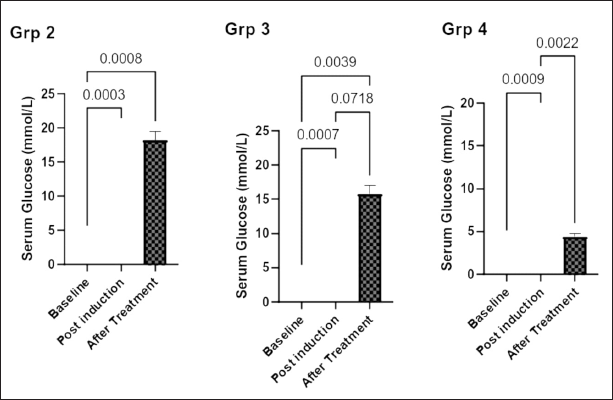 | Figure 1. Effect of aqueous extract of Ficus exasperata leaf on plasma glucose level (mmol/l) of diabetic peripheral neuropathic male Wistar rats. Data expressed are mean ± SEM, n = 7. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. a, p < 0.05 versus baseline glucose level. [Click here to view] |
Pain threshold (thermal allodynia)
Ice-cold test
Figure 2a shows the pain threshold across the four groups using the ice-cold test on day 21 and day 28 after induction of diabetes. On day 21, group 1 showed a significant increase in pain threshold (7.61 ± 0.43 seconds) compared with group 2 (3.51 ± 0.46 seconds) (p = 0.0014). Group 2 showed a significantly lower pain threshold than groups 3 (9.9 ± 0.54 seconds) and 4 (9.04 ± 0.72 seconds) (p = 0.0011 and p < 0.0030, respectively). On day 28 after induction of diabetes, group 1 showed a significantly lower pain threshold (6.44 ± 0.36 seconds) than group 4 (9.26 ± 0.63) (p = 0.0314). Group 2 also showed a significantly lower threshold (2.16 ± 0.36) than groups 3 and 4 (p = 0.0006 and p = 0.0004, respectively).
Hot plate test
The results of the pain threshold on the hot plate test across the four groups are shown in Figure 2b. The baseline measurement on day 1 showed no significant difference across the four groups. On day 28, the result showed that there was a significantly raised pain threshold in groups 1, 3, and 4 compared with group 2 (p = 0.0008, p < 0.00001, and p < 0.0001, respectively), and there was a significant higher pain threshold in group 3 than that in group 4 (p = 0.0150). The result seen on day 35 was similar to that observed on day 28.
Serum biomarkers
Super-oxide dismutase
Figure 3a shows the serum concentration of SOD among the four groups. There was a significantly higher level of SOD in groups 1 and 4 (4.26 ± 0.53 and 4.48 ± 0.64 U/mg, respectively) than that in group 2 (0.65 ± 0.59 U/mg) (p = 0.0145 and p = 0.0434, respectively).
Glutathione
Figure 3b shows a significantly higher serum level of GSH in group 1 (4.12 ± 0.53 μmol/l) than that in groups 2 and 3 (1.18 ± 0.19 and 1.69 ± 0.14 μmol/l, respectively) (p = 0.0107 and p = 0.0421, respectively). The serum level of GSH in group 4 (3.23 ± 0.42 μmol/l) was significantly higher (p < 0.0106) than that in group 2.
Brain biomarkers
Malondialdehyde
Figure 4a shows that the brain homogenate concentration of MDA in group 2 was significantly higher than that in groups 1 and 4 (p = 0.0036 and p = 0.0220, respectively).
Nitric oxide
Figure 4b shows that the brain NO concentration was significantly lower in groups 1, 3, and 4 than that in group 2 (p = 0.0007, p = 0.0018, and p = 0.0003, respectively).
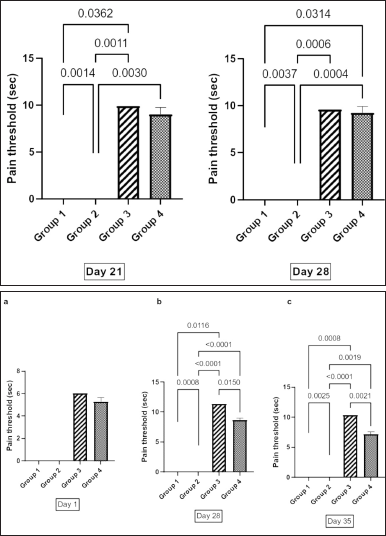 | Figure 2. (a) Analgesic effect of aqueous extract of Ficus exasperata leaf on pain threshold of diabetic rat using ice cold test. Values are expressed as mean ± SEM, n = 7. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. p < 0.05. (b) Analgesic effect of aqueous extract of Ficus exasperata leaf on pain threshold of diabetic rat using hot plate test (Seconds). Data expressed are mean ± SEM, n= 7. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. p < 0.05. [Click here to view] |
Sciatic nerve biomarkers
Brain-derived neurotrophic factor
Figure 5a shows that the concentration of brain BDNF was significantly higher in groups 1 and 4 than in groups 2 and 3 (p = 0.0005, p < 0.0001, p = 0.0011, and p < 0.0248, respectively).
Nerve growth factor
Figure 5b shows that the concentration of NGF was significantly higher in group 2 than that in groups 1 and 4 (p = 0.0049 and p = 0.0018, respectively). Similarly, there was a significant elevation of NGF in group 3 compared with groups 1 and 4 (p = 0.0009 and p = 0.0145, respectively).
Skin biomarkers
Calcitonin gene-related peptide
Figure 6a shows that the concentration of skin CGRP in groups 1, 3, and 4 was significantly lower than that in group 2 (p = 0.0028, p = 0.0155, and p = 0.0109. respectively). Similarly, the concentration of CGRP in groups 1 and 4 was significantly lower than that in group 3 (p < 0.0137 and p = 0.0413, respectively).
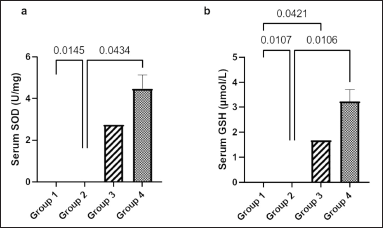 | Figure 3. Effect of Ficus exasperata leaf aqueous extract on serum SOD (a) and GSH (b) concentration of streptozocin-induced diabetic male Wistar rats. Values are expressed as mean ± SEM with n = 5. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. [Click here to view] |
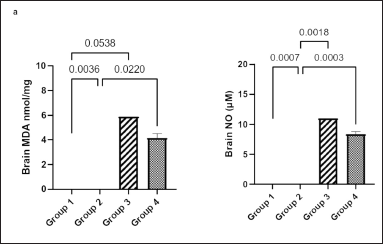 | Figure 4. Effect of Ficus exasperata leaf aqueous extract on brain MDA (a) and NO (b) concentrations of streptozocin-induced diabetic male Wistar rats. Values are expressed as mean ± SEM with n = 5. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. [Click here to view] |
Substance P
Figure 6b shows that the concentration of skin substance P in group 2 was significantly higher than that in the other three groups (p = 0.0013, p = 0.0064, and p = 0.0019, respectively).
DISCUSSION
Different combating pathways have been documented for the etiology of diabetic neuropathy. They range from the role of increased reactive oxygen species (Vats et al., 2004) to the role of the polyol pathway (Gabbay, 2004), development of advanced glycation end products, the elevation of proinflammatory cytokines like interleukin (IL-6), and tumor necrosis factor-α (Akintoye et al., 2018; Bishnoi et al., 2011), downregulation of anti-inflammatory cytokines like IL-10 (Akintoye et al., 2018), and increase of insulin resistance (Akintoye et al., 2020; Alzamil, 2020), among others. This research was aimed at investigating the possible role of F. exasperata in modulating neuropathic pain of STZ -induced neuropathic pain in male Wistar rats.
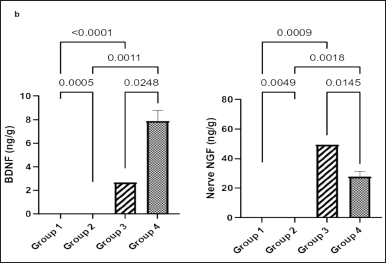 | Figure 5. Effect of Ficus exasperata leaf aqueous extract on sciatic nerve BDNF (a) and NGF (b) concentrations of streptozocin-induced diabetic male Wistar rats. Values are expressed as mean ± SEM with n = 5. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. [Click here to view] |
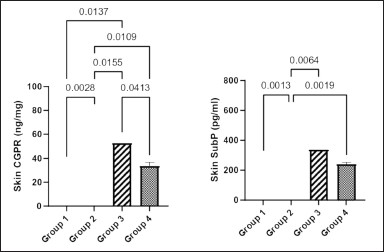 | Figure 6. Effect of Ficus exasperata leaf aqueous extract on skin CGRP (a) and SP (b) concentrations of streptozocin-induced diabetic male Wiatar rats. Values are expressed as mean ± SEM with n = 5. Data were analyzed by one-way ANOVA followed by Bonferroni’s multiple post-hoc test. [Click here to view] |
The underlying mechanism of F. exasperata’s hypoglycemic impact is unknown at this time, but plausible theories include insulin effect enhancement, either by increasing pancreatic insulin secretion from cells of the islet of Langerhans or its release from bound insulin (Peri and Satheesh, 2004). According to a study conducted by Kazeem et al. (2013), aqueous F. exasperata extract (FEE) is a moderate inhibitor of amylase and a potent inhibitor of glucosidase. Inhibition of these enzymes delays carbohydrate digestion, which in turn lowers the rate of glucose uptake and, subsequently, the postprandial blood glucose spike (Kwon et al., 2007). Furthermore, the polyol pathway, which is at the center of many diabetic neuropathy complications pathways (Lu et al., 2018; Niimi et al., 2018), will be greatly reduced since the serum glucose level is under control by reducing the entry of glucose into the vascular compartment via its gastric entry route. This study showed that aqueous FEE has a potent hypoglycemic effect as seen in Figure 1 when compared with other treatment groups. This is similar to the reports by Adewole et al. (2011) and Deepa et al. (2018). Deepa postulated that the hypoglycemic property of aqueous FEE is via its insulin secretion enhancement, which may result from one of its phytochemical constituents: alkaloids, flavonoids, saponins, tannins, and glycosides (Adewole et al., 2011; Obatomi et al., 1996). Though previous research reports had suggested a correlation between hyperglycemia and increased pain perception (DPN), which is the commonest diabetic complication (Akintoye et al., 2020), this was also noticed in this study as presented in Figure 1. Compared with the standard drug pregabalin, an antidepressant drug that is legally used in the management of DPN, aqueous FEE showed a better antinociceptive property that is likely to be time-dependent, as noticed in Figure 2a, as well as its effect in Figure 2b, which showed the effect of aqueous FEE in the hot plate pain threshold model. The results in Figures 3a and b demonstrate the strong antioxidant property of FEE as reported in previous scientific journals (Abotsi et al., 2010; Enogieru et al., 2015; Kazeem et al., 2013; Sirisha et al., 2010). As the disease progresses, antioxidant potential decreases depending on the level of glycemic control, which reverted toward the normal glycemic range in the result. Both serum SOD and GSH levels were significantly higher than those of the diabetic and pregabalin-treated rats. SOD is an enzyme that helps maintain antioxidant molecules such as GSH, and it detoxifies free radical entities in cells, tissues, and extracellular fluids. Decreased SOD expression leads to decreased mitochondrial GSH and increased oxidative stress. This is consistent with the observation of Sirisha et al. (2010) that F. exasperata and other Ficus sp. increase the serum GSH level (Sirisha et al., 2010).
A hydroxyl group in flavonoids gives them scavenging power and aids in limiting lipid peroxidation, making flavonoids one of the most diverse and ubiquitous classes of natural substances (Mayur et al., 2010). In contrast, tannin is made up of a core glucose molecule derivative at its hydroxyl groups with one galloyl residue, and it serves as an antioxidant by inhibiting the generation of hydroxyl radicals in the presence of copper ions (Andrade et al., 2005). The result in Figures 4a and b shows the antioxidative property of aqueous FEE in the brain homogenate. There was a significantly lower MDA level in the aqueous FEE-treated group than that in the diabetic rats. However, the finding of NO in the brain homogenate showed a significantly lower concentration in the aqueous FEE-treated group than that in the diabetic and pregabalin-treated rats. Likewise, this study demonstrated that aqueous FEE preserved the BDNF secretion in the nerve when compared to that of the other groups. The concentrations of CGRP in the skin and NGF in the nerve of aqueous FEE-treated rats were significantly lower than those of the diabetic and pregabalin-treated rats, while the skin concentration of SP was only significantly lower than that of the diabetic, untreated rats. Previous researchers have reviewed the possible roles of these cytokines in the pathogenesis of DPN (Iyengar et al., 2019). As such, they are used as neuropathy makers (Choi and Nardo, 2018; Shillo et al., 2021). The ameliorating effect of aqueous FEE is probably due to its high content of flavonoids, which is a strong, natural, antioxidant compound.
CONCLUSION
The findings of this study suggest that the aqueous extract of F. exasperata leaves has potent hypoglycemic, antiallodynic, and antioxidant properties. Thus, it is a potential remedy for the complication of diabetes mellitus DPN, particularly in males.
ACKNOWLEDGMENT
The authors would like to acknowledge the laboratory staff of the Department of Physiology, University of Ilorin, Ilorin, Nigeria, for their technical support in the successful completion of this work.
AUTHORS’ CONTRIBUTION
All authors contributed to various extents to the conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting of the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTERESTS
The authors of this work declare that they do not have any conflict of interest regarding the study.
ETHICAL APPROVALS
The institutional guidelines and regulations of the University of Ilorin, Ilorin, Nigeria, and was assigned the approval number: UERC/ ASN/2019/1554 on February 14, 2019, by the University Ethical Review Committee.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abotsi WM, Woode E, Ainooson GK, Amo-Barimah AK, Boakye-Gyasi E. Antiarthritic and antioxidant effects of the leaf extract of Ficus exasperata P. Beauv. (Moraceae). Pharmacogn Res, 2010; 2(2):89. CrossRef
Adewole SO, Adenowo TK, Naicker T, Ojewole JA. Hypoglycaemic and hypotensive effects of Ficus exasperata Vahl. (Moraceae) leaf aqueous extracts in rats. Afri J Trad Comp Alt Med, 2011; 8(3):275–83. CrossRef
Akintoye OO, Oniyide AA, Owoyele BV. A study of pain threshold, interleukins and NLR in diabetic poly neuropathy in a selected Nigerian population. Niger J Physiol Sci, 2018; 33(2):151–7.
Akintoye OO, Owoyele BV, Fabunmi OA, Raimi TH, Oniyide AA, Akintoye AO, Ajibare AJ, Ajayi DD, Adeleye GS. Diabetic neuropathy is associated with increased pain perception, low serum beta-endorphin and increase insulin resistance among Nigerian cohorts in Ekiti State. Heliyon, 2020; 6(7):e04377; doi:10.1016/j.heliyon.2020.e04377. CrossRef
Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes, 2020:5076858; doi:10.1155/2020/5076858. CrossRef
Andrade Jr RG, Dalvi LT, Silva Jr JM, Lopes GK, Alonso A, Hermes-Lima M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch Biochem Biophy, 2005; 437(1):1–9. CrossRef
Anigboro AA, Avwioroko OJ, Ohwokevwo OA, Pessu B. Bioactive components of Ficus exasperata, Moringa oleifera and Jatropha tanjorensis leaf extracts and evaluation of their antioxidant properties. EurAs J Biosci, 2019;13(2): 1766–7.
Basu S, Stuckler D, McKee M, Galea G. Nutritional determinants of worldwide diabetes: an econometric study of food markets and diabetes prevalence in 173 countries. Public Health Nutr, 2013; 16(1):179–86. CrossRef
Bishnoi M, Bosgraaf CA, Abooj U, Zhong L, Premkumar LS. Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: role of transient receptor potential vanilloid 1 (TRPV1) and inflammatory mediators. Mol Pain, 2011; 7:1744–8069. CrossRef
Choi JE, Nardo AD. Skin neurogenic inflammation. Semin Immunopathol, 2018; doi: 10.1007/s00281-018-0675-z. CrossRef
Deepa P, Sowndhararajan K, Kim S, Park SJ. A role of Ficus species in the management of diabetes mellitus: a review. J Ethnopharmacol, 2018; 215:210–32. CrossRef
Edwards J, Vincent A. Diabetic neuropathy: mechanisms to management. Elsevier, Amsterdam, The Netherlands, 2008. CrossRef
Enogieru A, Momodu O, Omoruyi S, Om’iniabohs F. Changes in biochemical markers of kidney function and antioxidant status of diabetic rats treated with aqueous leaf extracts of Ficus exasperata (Vahl). Afr J Biomed Res, 2015; 18:61–7.
Gabbay KH. Aldose reductase inhibition in the treatment of diabetic neuropathy: where are we in 2004? Curr Diab Rep, 2004; 4(6):405–8. CrossRef
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2015 (GBD 2015) population estimates 1970–2015. Institute for Health Metrics and Evaluation (IHME), Seattle, WA, 2015.
Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep, 2019; 21(4):1–8; doi: 10.1007/S11886-019-1107-Y. CrossRef
Herzog R, Cummins T, Waxman S. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol, 2001; 86:1351–64. CrossRef
Ijeh I, Ukweni A. Acute effect of administration of ethanol extacts of F. exasperata vahl on kidney function in albino rats. J Med Plant Res, 2007; 1(2):27–9.
Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache, 2019; 59(5):659–81; doi: 10.1111/head.13529. CrossRef
Kazeem MI, Oyedapo BF, Raimi OG, Adu OB. Evaluation of Ficus exasperata Vahl. leaf extracts in the management of diabetes mellitus in vitro. J Med Sci, 2013; 13(4):269. CrossRef
Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans, and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food, 2007; 10(2):266–75. CrossRef
Lu Q, Hao M, Wu W, Zhang N, Isaac AT, Yin J, Zhu X, Du L, Yin X. Antidiabetic cataract effects of GbE, rutin and quercetin are mediated by the inhibition of oxidative stress and polyol pathway. Acta Bioch Pol, 2018; 65(1):35–41; doi: 10.18388/abp.2016_1387. CrossRef
Mayur B, Sandesh S, Shruti S, Sung-Yum S. Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L. J Med Plants Res, 2010; 4(15):1547–53.
Niimi N, Yako H, Takaku S, Kato H, Matsumoto T, Nishito Y, Watabe K, Ogasawara S, Mizukami H, Yagihashi S, Chung SK, Sango K. A spontaneously immortalized Schwann cell line from aldose reductase-deficient mice as a useful tool for studying polyol pathway and aldehyde metabolism. J Neurochem, 2018; 144(6):710–22. CrossRef
Obatomi DK, Aina VO, Temple VJ. Effects of African mistletoe on blood pressure in spontaneously-hypertensive rats. Int J Pharmacol, 1996; 34:124–7. CrossRef
Peri L, Satheesh MA. Antidiabetic activity of Boerhaavia diffusa L.: effect on hepatic enzymes in experimental diabetes. J Ethnopharmacol, 2004; 91:109–13. CrossRef
Popwo Tameye SC, Ndom JC, Nguemfo EL, Wansi JD, Vardamides JC, Azebaze AGB. Ficusanol, a new cinnamic acid derivative and other constituents from the roots of Ficus exasperata Vahl. (Moraceae) with antioxidant and cytotoxic activities. Trends Phytochem Res, 2020; 4(1):3–8.
Prabodha LBL, Sirisena ND, Dissanayake VHW. Susceptible and prognostic genetic factors associated with diabetic peripheral neuropathy: a comprehensive literature review. Int J Endocrinol, 2018; doi: 10.1155/2018/8641942. CrossRef
Selvarajah S, Tesfaye S. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Wiley Online Library, Hoboken, NJ, pp 8–14, 2012. doi: 10.1002/dmrr.2239. CrossRef
Shillo P, Yiangou Y, Donatien P, Greig M, Selvarajah D, Wilkinson ID, Anand P, Tesfaye S. Nerve and vascular biomarkers in skin biopsies differentiate painful from painless peripheral neuropathy in type 2 diabetes. Front Pain Res, 2021; 2:731658. CrossRef
Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Resol, 2014; 80:21–35. CrossRef
Sirisha N, Sreenivasulu M, Sangeeta K, Madhusudhana C. Antioxidant properties of Ficus species—a review. Int J Pharmcol Tech Res, 2010; 2(4):2174–82.
Vats V, Yadav P, Grover J. Effect of T. foenumgraecum on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J Ethnopharmacol, 2004; 90:155–60. CrossRef
Zabihi M, Ranjbar AM, Mosaddegh MH, Zare N. Analgesic effects of the Cressa Cretica extract on induced neuropathic pain in rats, and the potential role of opioid receptors. Iran J Toxicol, 2021; 15(4):241–8. CrossRef
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol, 2017; 14(2):88–98; doi: 10.1038/nrendo.2017.151. CrossRef