INTRODUCTION
In recent times, the usage of fixed dose combination (FDC) to target multiple receptors for the treatment of chronic illnesses like cancer, cardiovascular disease and diabetes, are frequently adopted (Maillard and Burnier, 2007). FDC helps in reducing multiple dosing, thereby resulting in better tolerability, synergistic effects, patient adherence improvement, and patient compliance, besides cost-effectiveness and extended life cycle management. (Arya et al., 2019). However, the presence of multiple drugs in a single composition raise challenges associated with physiochemical characteristics, such as solubility. Improvement of physical properties is of significant interest to pharmaceuticals since several medicines are formulated as solid forms. Moreover, the physical properties of solids combined with drug products will have a direct impact on the processing, delivery, and performance of the formulation (Kengar et al., 2019). To enhance physiochemical properties, various techniques such as salt formation, complexation, and cocrystal formation are widely accepted and implemented in various drug developments (Karimi-Jafari et al., 2018). Drug-drug cocrystal preparation is one of the novel approaches that reduce the load of improvement otherwise required for the physiochemical properties of these drugs taken independently for FDC (Drozd et al., 2017). Cocrystals are defined as neutral crystalline single-phase solid materials comprised of two or more diverse molecular and/or ionic compounds, typically in a fixed stoichiometric ratio. Drug substances with complementary functional groups that can contribute to the formation of supramolecular synthones are apt candidates to form cocrystals (Aitipamula et al., 2012). The formation of supramolecular synthones can take place by common functional groups like carboxylic acids, amides, and alcohols (Shan and Zaworotko, 2003). Carboxylic acid is one of the most widely focused and studied functional groups in pharmaceutical cocrystals, which tends to form supramolecular heterosynthones with aromatic nitrogen and chloride anions (Shan and Zaworotko, 2003). Thus, the synthesis of cocrystal is feasible due to the favorable molecular structures of Telmisartan (TEL) and Hydrochlorothiazide (HCT) as given in Figure 1 (drugbank.com).
The presence of the carboxylic acid group in TEL and aromatic nitrogen and chloride anion in HCT makes both moieties suitable to make supramolecular heterosynthone.
TEL and HCT FDC is an established and effective combination and is indicated for the treatment of hypertension (Neutel et al., 2003). Both the moieties possess low solubility, and for enhancement of solubility, the development of individual cocrystals of both TEL and HCT has been reported in the literature (Kundu et al., 2018) (Gopi et al., 2017). Few studies have been published suggesting the formation of TEL and HCT cocrystal, but limited only to solubility enhancement part and none of them have discuss about the preparation of dosage form (tablets) using these cocrystals and its added advantages to address the concern of moisture sensitivity (Kulkarni et al., 2020) (Mei et al., 2017). TEL is insoluble in the physiological pH range between 3 and 7, and HCT possesses high solubility at alkaline pH. To enhance the solubility of TEL and HCT, the commercially available tablet dosage form (Mecardis® HCT and Cresar® H) use inorganic alkalizers (Kundu et al., 2018). Due to their hygroscopic nature, alkalizers like sodium hydroxide are known for their moisture uptake tendency. Moreover, the moisture sensitivity of these tablets is also mentioned on the product information leaflet of the product.
The purpose of this research is to synthesize drug-drug cocrystals which can have improved physicochemical properties, particularly solubility of TEL and HCT. The formulation of cocrystal produced is further intended for tablet dosage form without the inclusion of any alkalizer and which can help in solving the issue of the formulation’s sensitivity to moisture.
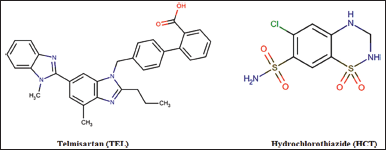 | Figure 1. Molecular structure of TEL and HCT [Click here to view] |
MATERIALS AND METHODS
Drug substance and solvent
TEL (CAS 144701-48-4), HCT (CAS 58-93-5), Ethanol (CAS 64-17-5) and Methanol (CAS 67-56-1) used in experimentation were provided by Piramal Pharma Solutions, Ahmedabad, India as gift samples. All other chemicals and solvents used in the investigation were of analytical grade.
Cocrystal synthesis
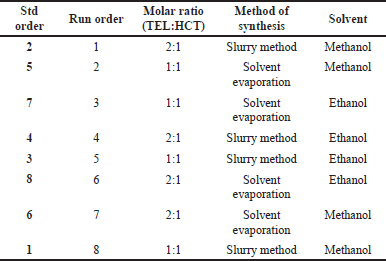
The Design of Experiment (DoE) has been applied as a systematic approach toward the synthesis of (TEL:HCT) cocrystal. A full factorial study using three factors at two levels was considered (Table 1). Molar ratios, synthesis methodologies, and solvents used in the preparation were studied at two different levels to identify the most suitable combination for cocrystal formation.
The experimental design was prepared using Minitab17 statistical software (Table 2). Eight randomized experiments were generated through the usage of software and executed as per run order.
Slurry method
The TEL:HCT cocrystals were prepared using the slurring method. To synthesize cocrystals for DoE experiment run 5 and 8, the composition consists of equimolar quantities of TEL (12 mg, 1 mM) and HCT (6 mg, 1 mM) was considered. For DoE experiment run 1 and 4, a molar ratio of 2:1, TEL (24 mg, 2 mM) and HCT (6 mg, 1 mM) was taken. For run 1 and 8, both the drug substances were added to 20 ml of methanol and for run 4 and 5, 20 ml of ethanol was used. The slurry was stirred using a magnetic stirrer at 200 RPM for 72 hours, maintained at a temperature of 25°C ± 0.5°C. The slurry obtained was filtered using 0.5μm parchment paper and vacuum dried in an oven (DZF-6010, Labsnova, China ) at 50°C for 30 minutes to obtain a dry powder.
 | Table 1. Factors and levels set for DoE trials. [Click here to view] |
 | Table 2. DoE trials as per standard order and run order. [Click here to view] |
Solvent evaporation
In the case of the solvent evaporation method, for run 2 and 3, equimolar quantities of TEL (12 mg, 1 mM) and HCT (6 mg, 1 mM), and for run 6 and 7, the molar ratio of 2:1, TEL (24 mg, 2 mM) and HCT (6 mg, 1 mM) were incorporated. For run 2 and 7, both the drug substances were added to 20 ml of methanol, and for run 3 and 6, 20 ml of ethanol was used. The mixture was heated at 50°C in rotavapor (Rotavapor® R-100, Buchi, Switzerland) till the whole solvent evaporated, followed by vacuum drying in an oven at 50°C for 30 minutes to obtain a dry powder.
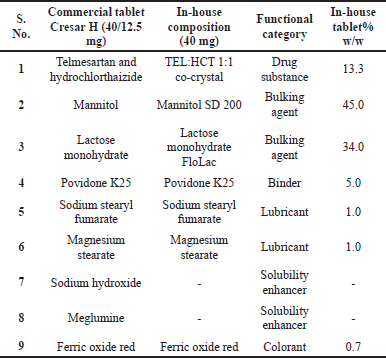 | Table 3. The qualitative composition of commercial tablets and in-house tablets. [Click here to view] |
Tablet manufacturing
Tablets were prepared using similar excipients as per commercially available Cresar H tablets (Table 3), except for the alkalizing agents (sodium hydroxide and meglumine), used to enhance drug substance solubility. In-house tablets were prepared using the direct compression method using a small R&D tablet compression machine (XL 100, Korsch AG, Germany).
TEL:HCT 1:1 cocrystal along with bulking agents (Mannitol SD 200, lactose monohydrate (FLoLac), binder (Povidone K25), and colorant (Ferric oxide red) were passed through #30 mesh (600 microns) and blended in Turbula 3D shaker mixer (TURBULA®, Willy A. Bachofen AG, Switzerland) at 22 RPM for 10 minutes. The mixture is then lubricated using sodium stearyl fumarate and magnesium stearate in a Turbula blender at 22 RPM for 5 minutes. The final lubricated blend is then compressed using a tablet compression machine (XL 100, Korsch AG, Germany).
Cocrystal characterization
Powders obtained after drying from experiments were subjected to characterization using different techniques.
Melting point
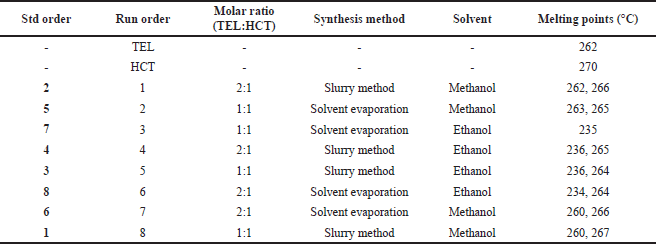
The melting temperature of the powder prepared from DoE experimentation was measured using melting point apparatus (Supertek®CH11513B, India). The characteristic melting points of TEL, HCT, and cocrystal prepared with different molar concentrations of TEL and HCT were recorded.
Differential scanning calorimeter (DSC)
Instrument model AQ20 from TA Instruments, USA, has been used to perform DSC analysis. A sample of around 2 mg was placed in an aluminum pan and a heating rate of 10°C/minutes was applied under a nitrogen atmosphere. DSC was performed in the range of 0°C to 400°C temperature for the cocrystal samples prepared from DoE runs along with individual TEL and HCT. The data was processed by Platinum Advantage software.
X-ray powder diffraction (XRPD)
X’Pert Pro X-ray diffractometer from Malvern PANalytical, UK, was equipped with a CU anode, a wavelength of 0.154 nm, maximum 2.2 kW, 60 kV, long fine focus ceramic tube, type PW3373/00, which was used to analyze cocrystal samples prepared from DoE runs along with TEL, HCT, and physical mixture of drug samples. Illumination was done on the 15 mm sample size and analyzed from 5° to 40° in 2θ. X’Pert High Score software was used to refine the captured XRPD patterns.
 | Table 4. The melting point of TEL, HCT, and DoE trials. [Click here to view] |
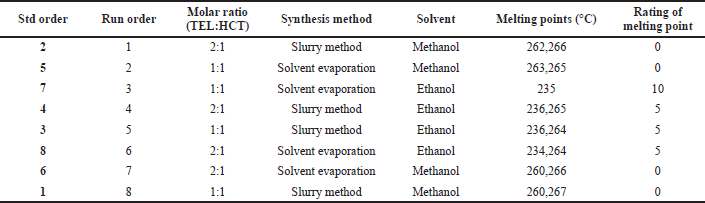 | Table 5. DoE trials with melting point and rating to melting point as a response. [Click here to view] |
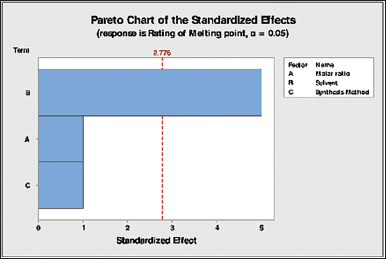 | Figure 2. Pareto chart of standardized effects for the response in melting point rating. [Click here to view] |
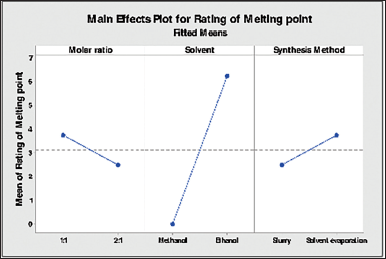 | Figure 3. Main effects for the response in melting point rating [Click here to view] |
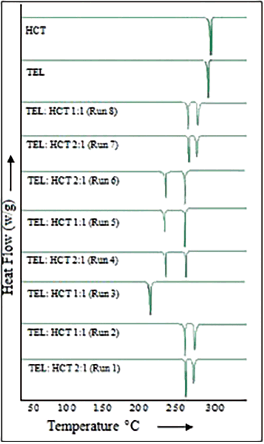 | Figure 4. TEL, HCT, and DoE run samples of DSC endotherms. [Click here to view] |
Saturation solubility
Saturation solubility of TEL, HCT, TEL:HCT 1:1 cocrystals, In-house tablets, and marketed tablets were performed based on the saturation solubility method by dissolving an excess amount of sample in 20 ml each of different buffers (pH 1.2, pH 4.5, pH 6.8) and water (Jhaveri et al., 2020). Samples in different buffers and water were placed in a conical flask and shaking was carried out using a portable shaker (New Brunswick™ Innova-2000, Canada) at 250 RPM for 24 hours at room temperature (25°C ± 0.5°C). Resultant samples were filtered through a 0.45 μm membrane filter (Millipore, Bedford, MA, USA) and quantitative analysis was done using a Ultra violet (UV) spectrophotometer (1800, Japan).
Dynamic vapor sorption (DVS)
A gravimetric sorption technique was performed on commercially available Cresar H tablets and In-house tablets using DVS Intrinsic Plus, Surface Measurement Systems, UK. The In-house tablets and Cresar H tablets were tested individually by keeping them on balance, which was tared and allowed to stabilize initially to 0% RH. The 15 mg of powdered tablet sample was accurately weighed, and the sample was re-equilibrated to the humidity condition (0% RH) and then the final weights were noted. The software used to analyze the samples was Version 5.1.0.5, standard DVS. The samples were run at 25°C, with the RH range starting at 0% and ending at 90%, with the humidity interval set at 10%.
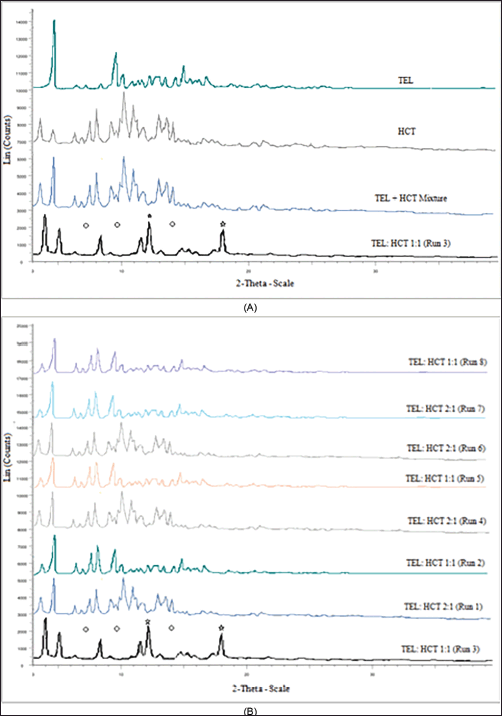 | Figure 5. (a) XRPD patterns for TEL, HCT, and TEL + HCT mixture and TEL:HCT 1:1 cocrystal. (b) XRPD patterns for TEL:HCT 1:1 cocrystal (Run3) and other DoE runs. [Click here to view] |
Assay and dissolution testing
The assay of TEL and HCT was determined in a 1:1 ratio cocrystal sample. In-house tablets and marketed tablets were used in saturation solubility and dissolution testing using a UV spectrophotometer (UV-1800 Shimadzu 1800). The maximum absorbance value (λmax) for TEL and HCT was observed at 296.2 and 271.3 nm, respectively.
In-house manufactured tablets, commercial Cresar H tablets, TEL:HCT 1:1 cocrystal, TEL, and HCT were subjected to dissolution using USP type II apparatus (paddle) in 0.1 N HCl 900 ml at 37°C ± 0.5°C at a paddle speed of 50 RPM. Samples were collected at 0–60 minutes time points and analyzed suitably using a UV spectrophotometer (Shimadzu UV 1800).
Cocrystal stability
Stability of TEL:HCT 1:1 cocrystals were performed by keeping samples in a glass vial in long-term (25°C/60% RH) condition for 12 months and in accelerated (40°C/75% RH) condition for 6 months (ICH Q1A). Samples were tested for Assay and XRPD for any changes in the crystal lattice.
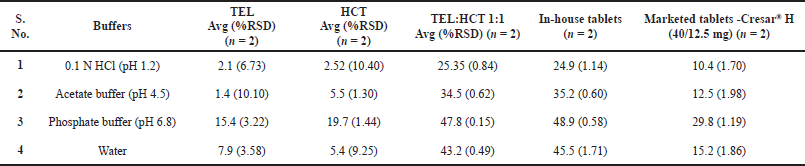 | Table 8. Saturation solubility comparison of TEL:HCT 1:1 cocrystal, TEL, HCT, In-house tablets, and Cresar® H tablets. [Click here to view] |
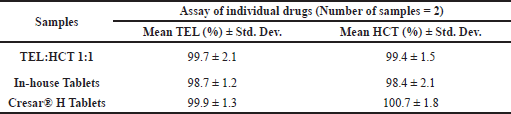 | Table 9. Average assay of TEL and HCT in TEL:HCT 1:1 cocrystal. [Click here to view] |
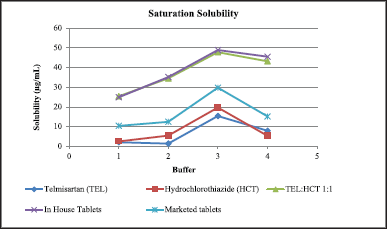 | Figure 6. Graph showing saturation solubility of TEL, HCT, and TEL:HCT 1:1, In-house tablets, commercial tablets cocrystal at different pH [Click here to view] |
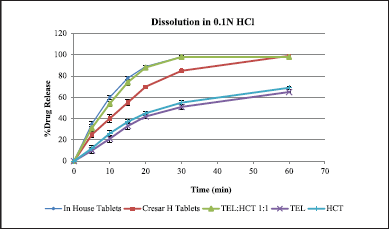 | Figure 7. Dissolution graph of in-house tablets and Cresar H tablets in 0.1N HCl. [Click here to view] |
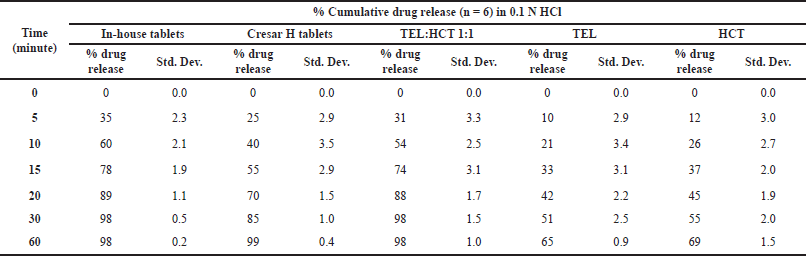 | Table 10. Dissolution of in-house tablets, Cresar® H tablets, Tel:HCT 1:1, TEL, and HCT in 0.1N HCl. [Click here to view] |
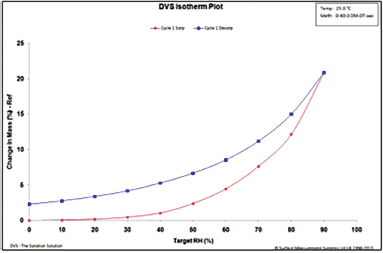 | Figure 8. DVS isotherm of Cresar H tablets. [Click here to view] |
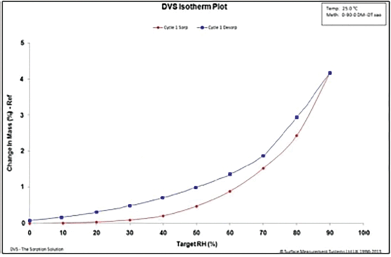 | Figure 9. DVS isotherm of in-house tablets. [Click here to view] |
In-vivo pharmacokinetic study
The In-vivo assessment was conducted on albino rats weighing 190 ± 20 g. For the study, ten rats were taken and divided into five groups with two in each group. The first group of animals was administered with pure TEL, the second group was administered with pure HCT, the third group is administered with cocrystal of TEL:HCT 1:1, the fourth group is provided with tablet composition having DS in the form of TEL:HCT 1:1 cocrystal and the fifth group is treated as control. Samples were administered considering dose of 1 mg/kg via a feeding tube. The blood samples were collected at 0, 10, 15, 30, 60, 120, 240, 360, and 480 minutes through the tail vein in a centrifuge tube (Ganesh et al., 2019). To separate plasma from blood, collected blood samples were centrifuged at 4,000 RPM for 10 minutes. Plasma samples were then analyzed for drug content using a UV spectrophotometer (UV-1800 Shimadzu 1800, Japan). UV spectroscopy involves estimation of both the drugs simultaneously without any interference is adapted from work established using multivariate calibration method and proven to be a valid alternate to HPLC method (Lakshmi and Lakshmi, 2010).
RESULT AND DISCUSSION
Melting point analysis
As shown in Table 4, the melting point was determined for powdered cocrystal samples obtained after performing various DoE trials along with pure drug samples of TEL and HCT.
From the melting point data, TEL and HCT show sharp melting points at 262°C and 270°C respectively. From DoE trials, samples exhibit multiple melting points, except one sample prepared with the solvent evaporation method using ethanol as solvent and drug substances in the molar ratio of 1:1 gives a sharp melting point at 235°C. This distinct melting point indicates the presence of a new crystalline lattice. On the other hand, for other samples, multiple melting points indicate the presence of a physical mixture of two components representing TEL and HCT, respectively. Although, for samples prepared using ethanol, irrespective of ratio and method, they did display one melting point near 235°C.
Melting point data of DoE trials were taken as a response to the DoE trials conducted. For statistical purposes in Minitab 17 software, ratings of melting points were generated to obtain single quantitative numbers. For samples exhibiting observed with two melting points near the melting point of TEL or HCT is rated as 0, while the one with two melting points that are distinct from TEL and HCT is rated as 5, and the one with a single melting point different from TEL and HCT melting point is rated as 10 (Table 5).
From the analysis of variance (ANOVA), the factor that is significantly (p-value <0.05) influencing the cocrystal formation is the solvent used in synthesis, as it shows a p-value of 0.007 (Table 6). The same is depicted in the Pareto chart as provided in Figure 2.
The main effects plot for melting point as response for three different factors i.e., Molar ratio, solvent used and synthesis method used are captured in Figure 3. For solvent used in cocrystal synthesis, ethanol out of both the solvents found to be significantly impacting on cocrystal formation as compared to methanol. For molar ratios, 1:1 molar ratio shows significant impact as compared to 1:2 molar ratio. For method of synthesis, solvent evaporation as a method of synthesis show more impact than the slurry method. Overall, cocrystal prepared in 1:1 ratio using ethanol as solvent in solvent evaporation synthesis impacts melting point response.
As a multiple response prediction, ethanol as a solvent is recommended for TEL:HCT cocrystal formation in a molar ratio of 1:1 using the solvent evaporation method.
Differential scanning calorimeter
The cocrystals synthesized for TEL, HCT, and TEL:HCT cocrystals prepared from DoE trials were tested for thermal behavior and endotherm as reported in Table 7.
TEL, HCT, and TEL:HCT 1:1 (Run 3) molar ratio were shown to have separate endotherms at 278.8°C, 285.3°C, and 240.5°C, respectively (Fig. 4). A single endotherm exhibited for TEL:HCT at a 1:1 molar ratio indicates the formation of a new crystal lattice. This endotherm is distinct from the TEL and HCT endotherms, indicating the absence of both the starting materials in the cocrystal. Furthermore, samples from other DoE runs were also measured for DSC and found to have two endotherms, indicating the presence of two different crystal lattices in the same sample.
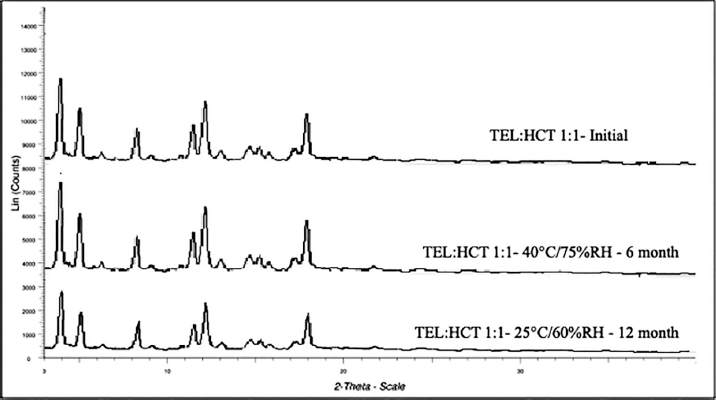 | Figure 10. XRPD scans of samples kept at long-term and accelerated stability study as compared to the initial sample. [Click here to view] |
 | Table 11. Assay of samples charged on stability for TEL:HCT 1:1 cocrystals. [Click here to view] |
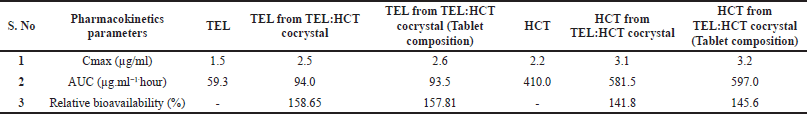 | Table 12. Pharmacokinetic parameters measured for TEL, HCT and TEL:HCT cocrystal from in-vivo study. [Click here to view] |
X-ray powder diffraction
XRPD pattern of TEL, HCT, TEL-HCT physical mixture, and TEL:HCT 1:1 (Run 3) screened for cocrystal formation are provided in Figure 5a. From the different patterns, the TEL:HCT 1:1 molar ratio (Run 3) displayed a unique pattern, as new characteristic peaks were observed for TEL:HCT 1:1 at 13.3 and 18.0, while many of the peaks like those at 7.2, 9.8, 14.4, and 15.1 which were originally present in TEL or HCT disappeared. Similar differences in diffraction peaks were observed when run 3 was compared to other DoE run samples (Fig. 5b). A unique diffraction pattern observed for TEL:HCT 1:1 (Run 3) confirms the formation of the cocrystal.
Saturation solubility study
Saturation solubility of TEL:HCT 1:1 cocrystal, TEL, HCT, In-house tablets, and marketed tablets (Cresar® H) in 0.1 N HCl (pH 1.2), acetate buffer (pH 4.5), phosphate buffer (pH 6.8), and water were determined and reported in Table 8.
A comparison of the saturation solubility of different samples is provided in Figure 6. The saturation solubility of TEL:HCT 1:1 showed similar solubility across different pH, which was found to be multifold higher as compared to TEL and HCT alone. Marketed tablets show better solubility as compared to TEL and HCT but are not as good as cocrystal and In-house tablets.
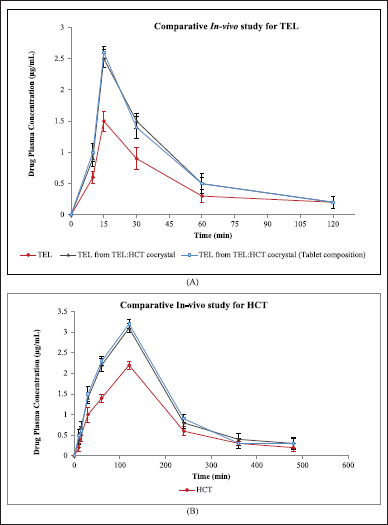 | Figure 11. (a) Comparative In-vivo study for TEL, TEL from TEL:HCT cocrystal and TEL from TEL:HCT cocrystal (Tablet composition). (b) Comparative In-vivo study for HCT, HCT from TEL:HCT cocrystal and HCT from TEL:HCT cocrystal (Tablet composition). [Click here to |
Assay and dissolution
Individual drug assays in TEL:HCT 1:1 cocrystal were performed, and TEL content was found to be 99.7% and HCT content was found to be 99.4%, as shown in Table 9.
From the dissolution data generated for In-house tablets, Cresar H tablets, TEL:HCT 1:1, TEL and HCT tablets in 0.1 N HCl, a faster dissolution rate for In-house tablets and TEL:HCT 1:1 cocrystal as compared to TEL, HCT and Cresar tablets was observed. This higher dissolution rate indicates an improvement in the solubility of drug substances in 0.1 N HCl (Table 10 and Fig. 7). The dissolution profile demonstrated by both, In-house tablets and Cresar® H tablets, even comply with the dissolution acceptance criteria recommended by Food and Drug Administration guidance document for immediate release solid dosage form drug products containing high solubility drug substances (FDA, 1997) (Jacob and Nair, 2018).
Dynamic vapor sorption
During the sorption cycle of the DVS study, it can be observed that there is a 20% increase in the mass of the Cresar H tablets (Fig. 8), as compared to 5% for the in-house tablets (Fig. 9), as RH reaches 90%. At the end of the desorp cycle, Cresar H tablets had a permanent mass gain of up to 2.5% (Fig. 8), whereas In-house tablets had a change in mass (%) of close to 0% (Fig. 9).
Cocrystal stability
From the drug:drug cocrystal stability study of TEL:HCT 1:1 cocrystal, based on XRPD scan results, no changes in crystal lattice were observed in accelerated and long-term stability conditions in 6 and 12 months, respectively (Fig. 10). Additionally, no significant change in assay results values of the stability sample as compared to that assay of the initial sample (Table 11). These results indicate stable and robust cocrystal lattice formation.
In-vivo pharmacokinetic study
Pharmacokinetic parameters like peak plasma concentration (Cmax), area under plasma concentration –time curve (AUC), and relative bioavailability (%) of TEL, HCT, TEL and HCT as cocrystal, and cocrystal in the tablet are reported in Table 12, Figure 11a and b. PKSolver (add-Ins program in Microsoft Excel 2017) is used for pharmacokinetic data analysis. Results indicate an increase in drug plasma concentration (Cmax) of TEL alone (1.5 μg/ml) to TEL in cocrystal (2.5 μg/ml) and HCT alone (2.2 μg/ml) to HCT in cocrystal (3.1 μg/ml). Similarly, an increase in AUC (μg.ml−1·hour) and relative bioavailability (%) was observed for cocrystals in comparison to pure drugs. Comparable results were observed for cocrystal and cocrystal in tablet composition for all the pharmacokinetic parameters.
CONCLUSION
An attempt was been made to develop a cocrystal of TEL and HCT to enhance the solubility in FDC. Improvement in solubility through the cocrystallization technique sequentially helped to remove alkalizers typically used in commercially available tablets that make the formulation susceptible to moisture uptake. A DoE study was applied to determine the most suitable molar ratio, solvent for synthesis, and method of preparation in a systematic manner. The formation of TEL:HCT 1:1 cocrystals was confirmed using DSC and XRPD studies performed on crystalline phase obtained after synthesis. A saturated solubility study of TEL, HCT, and TEL:HCT 1:1 cocrystals were conducted, and a multiple-fold increase in solubility was observed in buffers of different pH and water. The tablets were manufactured using TEL:HCT 1:1 cocrystals, keeping all the excipients similar to marketed tablets except the alkalizers (sodium hydroxide and meglumine). Based on the outcome of saturation solubility, dissolution, and DVS studies, it is indicated that there is improvement in both solubility, dissolution, pharmacokinetic parameters and no moisture uptake of in-house tablets prepared using TEL:HCT 1:1 cocrystal. This experimental work involving the application of DoE study in the preparation of cocrystals was found to be systematic and effective to screen cocrystals and opens a new approach to leverage DoE studies in upcoming work in cocrystal engineering.
LIST OF ABBREVIATIONS
AUC, Area under curve; DoE, Design of experiment; DSC, Differential scanning calorimeter; DVS, Dynamic vapor sorption; FDC, Fixed-dose combination; HCT, Hydrochlorothiazide; ICH, International council for harmonization; TEL, Telmisartan; UV, Ultra violet; XRPD, X-ray powder diffraction.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no financial support/funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study was approved by Institutional ethical committee with No.: IP/PCEU/FAC/27/2020/030.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR, Desiraju GR, Dikundwar AG, Dubey R, Duggirala N, Ghogale PP. Polymorphs, salts, and cocrystals: what’s in a name? Cryst Growth Des, 2012; 12(5):2147–52. CrossRef
Arya DS, Chowdhury S, Chawla R, Das AK, Ganie MA, Kumar KP, Nadkar MY, Rajput R. Clinical benefits of fixed dose combinations translated to improved patient compliance. J Assoc Physicians India, 2019; 67(12):58–64.
Drozd KV, Manin AN, Churakovb AV, Perlovicha GL. Novel drug-drug cocrystals of carbamazepine with para-aminosalicylic acid: screening, crystal structures and comparative study of carbamazepine cocrystal formation thermodynamics. CrystEngComm, 2017; 19:4273. CrossRef
Drug Substance Structures. Available via https://go.drugbank.com/drugs (Accessed 23 January 2023).
FDA. Dissolution testing of immediate-release solid oral dosage forms. U.S. Department of Health, Food and Drug Administration, Center for Drug Evaluation and Research, 1997.
Ganesh M, Ubaidulla U, Rathnamb G, Jang HT. Chitosan-Telmisartan polymeric cocrystals for improving oral absorption: in vitro and in vivo evaluation. Int J Biol Macromol, 2019; 131:879–85. CrossRef
Gopi SP, Banik M, Desiraju GR. Cocrystal and salt forms of furosemide: solubility and diffusion variations. Cryst Growth Des, 2017; 17(1):308–16. CrossRef
ICH Q1A(R2). Stability testing of new drug substances and products Q1A(R2); Current Step 4 version dated 6 February 2003. Paris, France, ICH.
Jacob S, Nair AB. An updated overview with a simple and practical approach for developing in vitro–in vivo correlation. Drug Dev Res, 2018; 79(3):97–110. CrossRef
Jhaveri M, Nair AB, Shah J, Jacob S, Patel V, Mehta T. Improvement of oral bioavailability of carvedilol by liquisolid compact: optimization and pharmacokinetic study. Drug Deliv Transl Res, 2020; 10(4):975–85. CrossRef
Karimi-Jafari M, Padrela L, Walker GM, Croker DM. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications. Cryst Growth Des, 2018; 18(10):6370–87. CrossRef
Kengar Manohar D, Howal Rohit S, Aundhakar Dattatray B, Nikam Amit V, Hasabe Priyajit S. Physico-chemical properties of solid drugs: a review. Asian J Pharm Tech, 2019; 9(1):53–9. CrossRef
Kulkarni A, Swapnil S, Vishal H, Ritesh B. Novel pharmaceutical cocrystal of Telmisartan and hydrochlorothiazide. Asian J Pharm Clin Res, 2020; 13(3) :104–12. CrossRef
Kundu S, Kumari N, Soni SR, Ranjan S, Kumar R, Sharon A, Ghosh A. Enhanced solubility of Telmisartan phthalic acid cocrystals within the pH range of a systemic absorption site. ACS Omega, 2018; 3:15380−8. CrossRef
Lakshmi KS, Lakshmi S. Design and optimization of a chemometric-assisted spectrophotometric determination of Telmisartan and hydrochlorothiazide in pharmaceutical dosage form. J Young Pharm, 2010; 2(1):85–9. CrossRef
Maillard MP, Burnier M. Is the fixed-dose combination of Telmisartan and hydrochlorothiazide a good approach to treat hypertension? Vasc Health Risk Manag, 2007; 3(3):265.
Mei X, Wang J, Yu Q. Shanghai Institute of Materia Medica of CAS. Cocrystal of Telmisartan and hydrochlorothiazide. US20200181121 and CN107501192. 2017-08-15.
Neutel JM, Kolloch RE, Plouin PF, Meinicke TW, Schumacher H. Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension—a randomized ABPM study. J Hum Hypertens, 2003; 17(8):569–75. CrossRef
Shan N, Zaworotko MJ. Polymorphic crystal forms and cocrystals in drug delivery (crystal engineering). In: Abraham DJ (ed.). Burger’s medicinal chemistry and drug discovery, Wiley, Hoboken, NJ, 2003.