INTRODUCTION
Millions worldwide use paracetamol (acetaminophen, 4-hydroxyacetanilide) as an analgesic and antipyretic. Although the usage of paracetamol at the therapeutic doses is safe, long-term usage of paracetamol could lead to hepatotoxicity and nephrotoxicity, which is a rising concern about paracetamol toxicity worldwide (Latif et al., 2021). Its metabolism is primarily associated with glucuronidation and sulfate conjugation. But 5%–10% of paracetamol is metabolized by cytochrome P-450 2E1 (CYP2E1). CYP2E1 metabolism is responsible for the formation of a reactive electrophilic intermediate metabolite called N-acetyl-p-benzoquinoneimine (NAPQI). NAPQI exhibits toxicity and inactivates the cellular proteins due to its oxidative nature. Therefore, leading to the death of the cells, causing nephrotoxicity and hepatotoxicity (Pathan et al., 2013). In addition, paracetamol is a P-glycoprotein (P-gp) substrate and inhibitor (Novak et al., 2013).
Diosmin is a naturally occurring citrus bioflavonoid and glycoside found in various plant materials. Diosmin has been reported to exhibit a broad spectrum of pharmacological properties such as anticancer, lipid-lowering (Mohamed and Tawakkol, 2013), neuroprotective, hepatoprotective (Abdel-Salam et al., 2012), and antioxidants. It was reported that diosmin acts as an anti-inflammatory agent and protects by alleviating inflammation by regulating TNF-α and NF-kB activation in ethanol-induced hepatic injury (Tahir et al., 2013). Previous studies reported that diosmin was an inhibitor of P-gp (Neerati and Bedada, 2015; Yoo et al., 2007) and CYP2E1 (Rajnarayana et al., 2008; Tahir et al., 2013). However, there is no scientific evidence of diosmin, reducing NAPQI levels via the inhibition of CYP2E1. Therefore, we evaluated the long-term effect of diosmin on the paracetamol metabolism mediated via CYP2E1 in this study using rats.
MATERIALS AND METHODS
Chemicals and reagents
Paracetamol was gifted by Lancer Pharmaceuticals Pvt. Ltd, Baddi, India, and approached Sigma Chemical Co. to purchase diosmin and NAPQI. Finar Chemicals Ltd, Ahmadabad, India, was approached to purchase Methanol, Triethylamine, Orthophosphoric acid, and water for high-pressure liquid chromatography (HPLC) analysis. Transasia Bio-Medicals Limited, Solan, Himachal Pradesh, India, was approached to purchase standard kits to perform the biochemical analysis. An analytical grade of chemicals and reagents was used in this study.
Animal experiments
Animal tests were performed as indicated by Committee for the purpose of control and supervision of experiments on animals guidelines. Animal studies were conducted with the approval and support of the KVSR Siddhartha College of Pharmaceutical Sciences (SCOPS), Vijayawada, Andhra Pradesh, India. The protocol approval number was KVSRSCOPS/29-03-2019-003. Mahaveer was approached to purchase male Wistar rats (180–220g). Animal food was purchased from (Hindustan Switch, Mumbai, India). Male Wistar rats (six for each group) were provided with food and water at the animal house of the institution.
Experimental design
Paracetamol and diosmin were suspended in 1% sodium carboxymethyl cellulose (SCMC) for oral administration. Four groups of male Wistar rats with six rats in each group were divided and treated consecutively once daily for 28 days with the following.
Group I: (Control): Treated with 0.5 ml of vehicle (1% SCMC, oral)
Group II: Treated with paracetamol (300 mg/kg, oral)
Group III: Treated with diosmin (25 mg/kg, oral), for 15 minutes followed by paracetamol (300 mg/kg)
Group IV: Treated with diosmin (50 mg/kg, oral), for 15 minutes followed by paracetamol (300 mg/kg).
Blood samples were collected from the tail vein in heparinized Eppendorf tubes at 0.25, 0.5, 1.0, 1.5, 3.0, 6.0, and 8.0 hours for the determination of paracetamol and NAPQI in plasma. The plasma separation was performed by centrifugation (Remi, R4C Compact model, Mumbai, India) at 5,000 rpm for 6 minutes and was stored at −20°C until analysis. At the end of the study, 1 ml of the blood sample was collected from each rat via retro-orbital venous plexus on the 28th day and the serum was separated for biochemical analysis.
Biochemical analysis
The biochemical parameters were estimated from the centrifuged serum. The serum glutamic-pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase, total bilirubin (TB), indirect bilirubin (IB), albumin, creatinine, lactate dehydrogenase (LDH), uric acid, and total proteins (TP) levels were estimated.
Histopathological examination
Tissue samples for the histopathological examinations were prepared as described by (Sobeh et al., 2018). Before processing the tissue samples in paraffin-embedded blocks, the liver and kidney samples of the animals used in the study were fixed in 10% neutral buffered formalin. Followed by the blinded histological examination, hematoxylin-eosin was used to cut and stain the sections (5 µm thick). Electronic light microscopy (Olympus BX-50 Olympus Corporation, Tokyo, Japan) was used to examine the glass slides. Vascular congestion, tissue degeneration, steatosis, and sinusoidal dilation were considered to evaluate the histopathology of the liver. Similarly, a photomicroscope was used to observe the sections of the kidneys. To consider the kidneys’ normal cytoarchitecture, the presence of glomeruli, convoluted tubules, interstitium, and capillaries was observed. By contrast, kidney damage was considered to observe hemorrhage cellular necrosis, glomerular hypercellularity, tubular degeneration, and capillary congestion.
Paracetamol and NAPQI extraction from the plasma
Paracetamol and NAPQI were extracted from the rat plasma using a single-step liquid-liquid extraction method (Pingili et al., 2019). Briefly, 100 µl plasma and 100 µl of 0.3 M phosphate buffer of pH 5.0 were added and gently mixed and followed by a mixture of 5 ml of isopropyl alcohol and chloroform (5:95 v/v). The entire mixture of solutions was vortexed and centrifuged at 3,000 rpm for 5 minutes using a Remi vortex mixer. The resulting supernatant of 1.4 µl was removed, dried, and regenerated in the mobile of 30 µl. The HPLC analysis was performed using 20 µl of the obtained sample.
Analytical methods
The quantification of paracetamol and NAPQI plasma concentration was performed as described in the previous studies (Flores-Perez et al., 2011; Pingili et al., 2019). A Shimadzu HPLC system with Thermo Electron Corp, Beverly, MA) and a dual-wavelength photodiode array detector (PDA) detector (SPD-10A VP), liquid chromatography (LC)-20AT VP pump, C18 column (ODS Thermo Hypersil, 250 × 4.6 mm, 5.0 μm. Data collection and processing were performed using LC solution software. A 20:40:40 ratio of methanol, water, and buffer was used as the mobile phase. A 0.45 μm nylon Millipore membrane was used to vacuum-filter the mobile phase. Ultrasonication (Remi, Mumbai, India) for 20 minutes was performed to degas the mobile phase before being used. About 20 µl of samples was injected and the effluent was monitored at 205 nm with a PDA detector at a 1 ml/minute flow rate. The NAPQI and paracetamol retention times were obtained at 3.919 and 6.938 minutes (Fig. 1).
Method validation
NAPQI calibration samples of 1, 2, 4, 6, and 8 µg/ml were prepared from the appropriate working solutions. Graphs were plotted between concertation and peak area to obtain the calibration curves. To perform the method validation, % of relative standard deviation between the five standards on the same day and five different days was determined to analyze intra-day and interday precision, respectively.
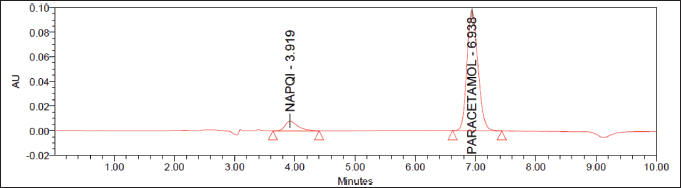 | Figure 1. Representative chromatograms of NAPQ1 (3.919) and paracetamol (6.938). [Click here to view] |
Calculation of pharmacokinetic parameters
Thermo Kinetica software was used to perform non-compartmental pharmacokinetics (PK) analysis with the plasma concentrations of paracetamol and NAPQI. Pharmacokinetic parameters such as concentration of maximum plasma (Cmax), time to reach the maximum plasma concentrations (Tmax), AUC, AUC from zero to the last sampling time (AUC0–t), AUC from time zero to infinite (AUC0–α), apparent terminal half-life (t1/2), clearance (CL/F), apparent volume of distribution (Vz/F), apparent volume of distribution at steady state (Vss), and mean residence time (MRT) were obtained for each rat.
Statistical analysis
Statistical analysis was performed with Graph Pad Prism 5.0 software. One-way ANOVA followed by Dunnett’s test was used to compare plasma concentrations of paracetamol and NAPQI. A two-way ANOVA followed by the Bonferroni test was used to perfume multiple comparisons. The test was considered statistical significance for a p-value of <0.05.
RESULTS
Effect of diosmin on the pharmacokinetics of paracetamol
The pharmacokinetic parameters of paracetamol alone and in combination with diosmin (25 and 50 mg/kg) are shown in Figure 2A. The time profiles comparing paracetamol and in combination with diosmin showed that co-administration of diosmin substantially improved the plasma concertation of paracetamol, compared to paracetamol alone (Fig. 2A). The mean pharmacokinetic parameters are described in Table 1. The results indicated that diosmin significantly increased the Cmax and AUC0–α of paracetamol from 3.546 ± 0.452 to 4.944 ± 0.581 μg/ml at the dose of 50 mg/kg and 1,422.18 ± 173.926 to 1,730.87 ± 193.921 μg. minute/ml with diosmin 25 mg/kg and 1,978.95 ± 231.472 μg. minute/ml with diosmin 50 mg/kg, respectively.
The AUC0–12 of paracetamol increased significantly from 1,382.89 ± 157.356 to 1,634.78 ± 183.764 μg, minute/ml with diosmin 25 mg/kg, and 1,847.79 ± 216.935 μg, minute/ml with diosmin 50 mg/kg. The t1/2 of paracetamol after diosmin treatment was longer than the paracetamol control group. The t1/2 was increased from 106.385 ± 12.463 to 143.846 ± 16.342 with 25 mg/kg diosmin and 152.799 ± 17.327 minutes with 50 mg/kg. The MRT of paracetamol was higher when paracetamol was co-administered with diosmin. It significantly increased from 288.737 ± 30.241 to 315.537 ± 32.427 hours when treated with diosmin 25 mg/kg and 329.043 ± 33.945 minutes when treated with diosmin 50 mg/kg.
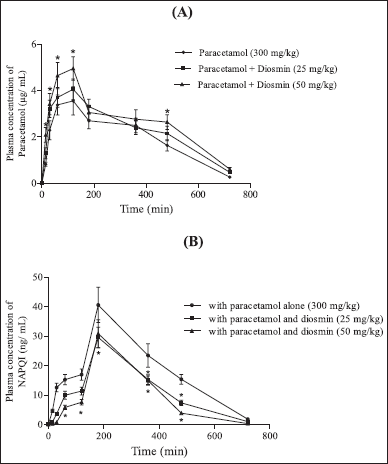 | Figure 2. Profiles of mean plasma concentration time A. Paracetamol; B. NAPQI following an oral administration of paracetamol to rats with or without diosmin (n = 6). (•) Control (paracetamol); (?) with 25 mg/kg diosmin and paracetamol (300 mg/kg); (?) with 50 mg/kg diosmin and paracetamol (300 mg/kg). All values are Mean ± SD. Bars represent the SD. *p < 0.001, NSp > 0.05 compared to the paracetamol control group (Two-way ANOVA followed by Bonferroni post-tests to compare each column to column). [Click here to view] |
The CL/F of paracetamol was decreased from 0.011 ± 0.003 to 0.009 ± 0.008 ml/hour/kg with diosmin 25 mg/kg and to 0.008 ± 0.001 ml/hour/kg with diosmin 50 mg/kg. The Vss/F of paracetamol was decreased from 3.248 ± 0.421 to 2.916 ± 0.173 ml/hour/kg with diosmin 25 mg/kg and to 2.66 ± 0.312 ml/hour/kg with diosmin 50 mg/kg. The Vz/F of paracetamol was increased from 1.726 ± 0.192 to 1.918 ± 0.201 (diosmin 25 mg/kg) and 1.782 ± 0.194 (diosmin 50 mg/kg) when administered with diosmin.
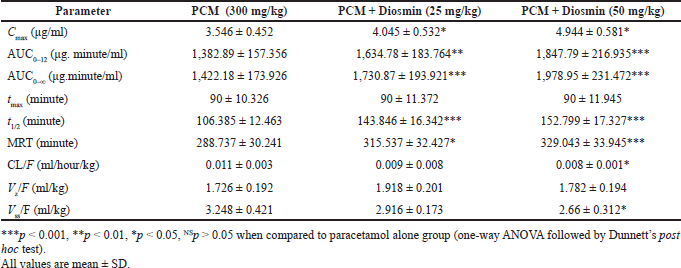 | Table 1. Pharmacokinetic parameters of paracetamol (300 mg/kg) following an oral administration of paracetamol to rats with and without diosmin (25 and 50 mg/kg). [Click here to view] |
Effect of diosmin on the pharmacokinetics of NAPQI
The NAPQI pharmacokinetic parameters following paracetamol oral administration to rats with or without diosmin (25 and 50 mg/kg) were depicted in Table 2. Pharmacokinetic parameters of NAPQI such as Cmax, AUC0–12, AUC0–∞, t1/2, and MRT were significantly decreased in the rats treated with diosmin compared to the paracetamol control group. The Cmax was significantly decreased from 40.567 ± 5.764 to 29.567 ± 3.957 ng/ml with diosmin 25 mg/kg and 30.854 ± 3.875 ng/ml with diosmin 50 mg/kg. The AUC0–12 of NAPQI decreased significantly from 13,088.8 ± 1,397.863 to 8,350.73 ± 983.745 ng minute/ml with diosmin 25 mg/kg and to 7,306.51 ± 795.733 ng minute/ml with diosmin 50 mg/kg. Diosmin treatment significantly decreased the AUC0–α of NAPQI from 13,335.3 ± 1,401.742 to 8,475.1 ± 932.324 ng minute/ml with diosmin 25 mg/kg and to 7,337.11 ± 812.746 ng minute/ml with diosmin 50 mg/kg. The MRT of NAPQI was 295.913 ± 34.673 minutes, which was decreased to 283.248 ± 32.424 minutes (at diosmin 25 mg/kg) and 264.235 ± 29.523 minute (at diosmin 50 mg/kg). The t1/2 of NAPQI decreased from 94.140 ± 12.537 to 89.146 ± 10.322 minutes when co-administered with diosmin at 25 mg/kg and 65.4431 ± 7.326 minute with diosmin at 50 mg/kg.
Effect of diosmin on liver function tests and its structure
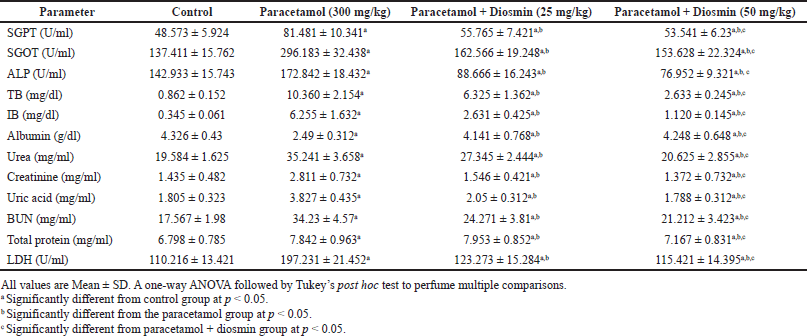
To further investigate the protective pharmacological activities of diosmin, the functioning markers and structure of the liver were compared and analyzed between the different groups. The liver function markers between the control and paracetamol treated (300 mg/kg) are shown in Table 3. The values in the paracetamol-treated group indicated a significant elevation in serum SGPT, SGOT, alkaline phosphatase (ALP), TB, and IB by 67.74%, 115.54%, 20.92%, 110.01%, and 171.13%, along with severe damage associated with vascular congestion, sinusoidal dilatation, ballooning degeneration, and steatosis (Fig. 3). In combination of diosmin (50 mg/kg) with paracetamol significant decreased the albumin levels and restored the liver functioning markers by significantly decreasing the serum levels of SGPT, SGOT, ALP, TB, and IB by 52.18%, 92.79%, 124.61%, 293.46%, and 458.48%, respectively. The restoration of liver functioning markers and albumin levels leads to scattered cytoplasmic vacuolization, which shows the protective effect of diosmin on hepatic cells (Fig. 3).
Effect of diosmin on kidney function markers and its structure
Another study evaluated the kidney functioning markers in the rats treated with 300 mg/kg of paracetamol and paracetamol co-administered with diosmin (25 and 50 mg/kg). Compared to the control group, the rats treated with paracetamol showed a significant increase in the renal function markers serum urea, creatinine, uric acid, blood urea nitrogen (BUN), and TP by 79.79%, 95.88%, 112.02%, 94.85%, and 15.35%, respectively, as shown in Table 3. Similarly compared to the paracetamol-treated group, rats co-administered with diosmin (25 and 50 mg/kg) showed a significant decline (p < 0.01) in the levels of these renal biochemical parameters. Further, the animals treated with paracetamol alone showed numerous tubular casts, degenerated tubular structure, glomerular atrophy, leukocyte infiltration, and visible pathological changes (Fig. 4). On treatment with diosmin (25 and 50 mg/kg), the renal lesions were remarkably ameliorated.
DISCUSSION
Intestinal P-gp and drug-metabolizing enzymes (DMEs) modulate the PK and the first-pass metabolism of several drugs. These DMEs are reported to be inhibited by flavonoids, a natural phytochemical ubiquitously found in fruits, vegetables, and several daily consumables. Several studies have reported the pharmacological properties and uses of flavonoids. It was also reported that flavonoids act as protective agents against several lifestyle diseases (Kumar and Pandey, 2013; Panche et al., 2016). Apart from the protective properties of flavonoids, consuming flavonoid-rich foods during medication resulted in drug interactions and altered the metabolism of drugs by the CYP enzymes and P-gp as reported in our previous publications (Pingili et al., 2016, 2021; Sridhar et al., 2014; Surya Sandeep et al., 2014; Vemulapalli, 2016). Baicalin (Jang et al., 2003), genistein (Fan et al., 2013), quercetin, and chrysin (Pingili et al., 2015) were among the few flavonoids investigated to understand the inhibitory effects on the P-gp CYP2E1 and other CYP enzymes.
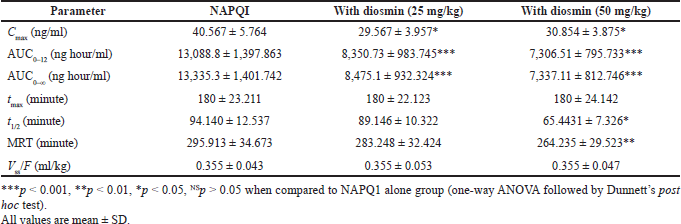 | Table 2. NAPQI pharmacokinetic parameters after the administration of paracetamol orally to rats with and without diosmin (25 and 50 mg/kg). [Click here to view] |
 | Table 3. Effect of diosmin (25 and 50 mg/kg) on the serum biochemical parameters in paracetamol (300 mg/kg) induced hepatotoxicity and nephrotoxicity in wistar rats. [Click here to view] |
In this study, we examined the effect of diosmin, a known CYP2E1 (Rajnarayana et al., 2008; Tahir et al., 2013) and P-gp inhibitor (Neerati and Bedada, 2015; Yoo et al., 2007) on the pharmacokinetics of paracetamol, a CYP2E1 and P-gp substrate in rats (Manyike et al., 2000). We found that the AUC and Cmax of paracetamol, when administered orally, were significantly enhanced by co-administration of diosmin at 25 and 50 mg/kg. The results are consistent with the studies conducted in several groups (Bedada et al., 2017; Neerati and Bedada, 2015; Rajnarayana et al., 2008). Thus, the preclinical and clinical studies conducted in both rats and humans support that diosmin possesses the P-gp inhibitory activity.
A set of in-vitro studies conducted on fexofenadine (P-gp substrate) combined with diosmin showed a significant change in the absorption and pharmacokinetic paraments of fexofenadine. Interestingly, there has been an increase in the apparent permeability and intestinal transport of fexofenadine across the duodenum, jejunum, and ileum. Similarly, there is a significant increase in the Cmax and the AUC of the fexofenadine pre-treated with diosmin with no changes in the t1/2, tmax, and elimination rate constant (Kel) of fexofenadine. These metabolic changes of fexofenadine in rats were accounted for due to P-gp inhibition (Neerati and Bedada, 2015).
A similar trend in the fexofenadine kinetic parameters was observed in human studies conducted with the diosmin. Although upon treatment with diosmin, a significant change was not observed in the tmax, t1/2, and renal clearance. The fexofenadine control group showed Cmax and AUC of fexofenadine of 523.28 ng/ml and AUC of 3,459.48 ng hour/ml and in combination with diosmin showed a Cmax and AUC of fexofenadine of 780.63 ng/ml and 64.4% 5,687.74 ng hour/ml, which is a 49.2% and 64.4% increase in the presence of diosmin. Simultaneously presence of diosmin led to a decrease in the CL/F of fexofenadine significantly by 41.3% (37.03 vs. 21.75 l/hour). These significant improvements in the pharmacokinetic parameters of fexofenadine in the presence of diosmin are attributed to diosmin-mediated inhibition of P-gp efflux in humans (Bedada et al., 2017).
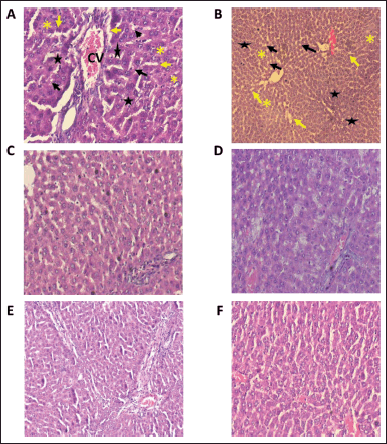 | Figure 3. Histopathological examination of hepatoprotective activity of diosmin when administered along with paracetamol. A, B: Represents the liver histology section of rats treated with paracetamol; ballooning degeneration (black arrows), vascular congestion (star), sinusoidal dilatation (yellow arrows), central vein (CV), and steatosis; (clear vacuoles contained lipid in life) (yellow asterisk) were seen in paracetamol treated group C, D: Represents the liver histology section of rats treated with paracetamol and diosmin 25 mg/kg; E, F: Represents the liver histology section of rats treated with paracetamol and diosmin 50 mg/kg. The images from A–F shows a significant improvement (C–F) in the hepatic markers with scattered cytoplasmic vacuolization indicating the hepatic protective property of diosmin. [Click here to view] |
It was reported that diosmin pre-treatment in humans before the administration of chlorzoxazone (CYP2E1 substrate) led to an increase in AUC, Cmax, and t1/2 and a concomitant reduction in CL/F significantly. Diosmin pre-treatment leads to decreased overall excretion (0–8 hours) of 6-hydroxy-chlorzoxazone and chlorzoxazone, indicating the role of diosmin inhibiting the microsomal CYP2E1-mediated hydroxylation of chlorzoxazone (Rajnarayana et al., 2008). These results are further supported by studies conducted on evaluating the systemic exposure of paracetamol in combination with dose-dependent quercetin (P-gp and CYP inhibitor). As expected, quercetin enhanced the paracetamol systemic exposure. The underlying mechanism was believed to be P-gp inhibition, CYP2E1, and other CYP enzymes by quercetin (Pingili et al., 2015). In our study, upon treating with diosmin 25 and 50 mg/kg, there is a 1.18-fold, 1.33-fold, 1.14-fold, and 1.39-fold increase in AUC0–12, Cmax of paracetamol. The above results indicate the role of diosmin in inhibiting paracetamol metabolism
Long-term usage of paracetamol at the therapeutic dose is one of the major causes of rinsing hepato and nephrotoxicity cases apart from overdosage. Glucuronidation (40%–67%) and sulfation (20%–46%) are major contributors to the metabolism of Paracetamol (Bateman et al., 2014; Vliegenthart et al., 2015). However, the reaming 5%–15% of the paracetamol oxidation results in the formation of NAPQI, a toxic metabolite, and it was reported that the oxidation was carried via CYP2E1 CYP1A2 and CYP3A4. It was reported that the formation of NAPQI is an electrophile oxidant and highly reactive. The formation of this toxic metabolite NAPQI is the core reason behind the hepatic and nephrotoxicity of paracetamol (Bender et al., 2004). Herbal drugs have been a long tradition to treat liver diseases followed in Eastern medicine. Silymarin, quercetin, and chrysin flavonoids are used exclusively for treating liver disease (Rumack, 2004).
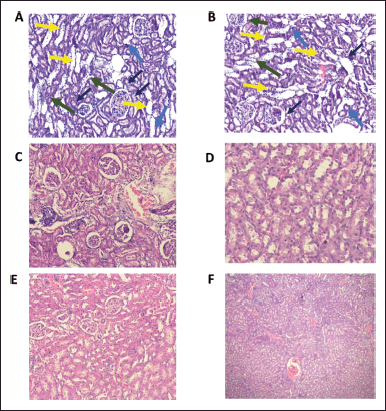 | Figure 4. Histopathological examination of the nephroprotective activity of diosmin when administered along with paracetamol. A, B: Represents the kidney histology section of rats treated with paracetamol; pathological changes, including glomerular atrophy (black arrows), degenerated tubular structure (yellow arrows), leukocyte infiltration (green arrows), and numerous tubular casts (blue arrows) can be seen. C, D: Represents the kidney histology section of rats treated with paracetamol and diosmin 25 mg/kg; E, F: Represents the liver histology section of rats treated with paracetamol and diosmin 50 mg/kg. The images from A to F shows a significant improvement in the kidney functioning markers (C–F), with renal lesions, which were being remarkably ameliorated by treating with diosmin (25 and 50 mg/kg)—indicating the nephroprotective activity of diosmin. [Click here to view] |
The formation of NAPQI in the presence of diosmin in combination with paracetamol was investigated in this current study. Interestingly the results highlight a 0.797-folds and 0.713 folds decrease in the Cmax and AUC0–∞. of NAPQI when pre-treated with diosmin. In addition, the formation of NAPQI was further decreased when diosmin was co-administered in rats. These results must be supported by previous findings across various groups. Prior studies by different groups have reported the hepatic and nephrotoxicity protective agents like moutan cortex extract, baicalin, lipopolysaccharide-binding protein, Phyllanthus urinaria extracts aminotriazole and ozagrel hydrochloride; Ornithogalum saundersiae, dioscin, lupeol, α- and β-amyrin, S-allyl mercapto cysteine, berberine, propylene glycol, ebselen, allylsulfide, allyl mercaptan, allylmethylsuhide, diallyl sulfone, fucoidan, and genistein (Fan et al., 2013; Hau et al., 2009; Hong et al., 2012; Janbaz and Gilani, 2000; Jang et al., 2003; Jing et al., 2015; Kumari and Kakkar, 2012; Oliveira et al., 2005; Qiu-Ju et al., 1994; Su et al., 2005; Sumioka et al., 2001; Tomishima et al., 2013; Ying et al., 2012; Zhao et al., 2012). The inhibition of the formation of NAPQI via CYP2E1 by the above agents resulted in the reduction of paracetamol-induced hepatotoxicity.
Liver enzymes were measured to assess the function and integrity when treated with paracetamol and with or without diosmin. The increased serum levels of SGPT confirmed the liver dysfunction in our study; SGOT, ALP, TP, and LDH were increased due to the leakage of damaged hepatocytes into the bloodstream upon treatment with paracetamol. The histology findings of this current study showed that upon treatment with diosmin, integrity, and the functioning of the liver were improved, and these findings align with the previous studies (Ali et al., 2018; Perumal et al., 2015; Tahir et al., 2013).
Co-administration of diosmin improved liver function biomarkers and defects compared to paracetamol significantly. It can be concluded that diosmin exhibited hepatoprotective effects via modulating Keap-1/Nrf-2 and P38-MAPK/NF-κB/inducible nitric oxide synthase (iNOS) pathway against cholestatic liver cirrhosis (Ali et al., 2018). Another study revealed that diosmin management suppressed biochemical and morphological characteristics due to the pathogenesis of hepatocytes induced by N-Nitrosodiethylamine (NDEA) and suppressed by the management with diosmin (200 mg/kg/b.w/p.o) for 28 days. Diosmin proved its therapeutic efficiency by restoring the structural modifications and biochemical enzymes damaged by NDEA
A study evaluated the protective role of diosmin against ethanol-induced nephrotoxicity in animals (Perumal et al., 2015; Tahir et al., 2013). The study concluded that diosmin attenuates the liver damage induced by ethanol through different mechanisms. That resulted in suppressing inflammatory markers and inhibiting oxidative stress markers. Liver damage induced by ferrous sulfate was investigated in the presence of diosmin to determine the protective effect. A reduction in the hepatocyte membrane markers ranging from 24% to48%, oxidative (hepatic MDA) and inflammatory (nitrogen oxides) markers, respectively, dyslipidemic markers showing 34% and 32% were observed and 35% and 39% reductions regarding serum TC and TG, respectively, at p < 0.05. Histopathological and immunohistochemical investigations of iNOS and endothelial nitric oxide synthase in liver sections were supported biochemical findings. In conclusion, diosmin may have an excellent hepatoprotective effect, mainly through antioxidant and anti-inflammatory potentials via the modulation of nitric oxide synthase expression (Abdel-Reheim et al., 2017). The hepatoprotective effects of diosmin were simultaneously confirmed by alleviating histopathological changes induced by paracetamol in the present study.
In our study, the kidney functioning parameters were assessed by evaluating the following markers; LDH, creatinine, uric acid, urea, blood urea nitrogen, and TP. These results were compared between the groups treated with paracetamol alone and paracetamol co-administered with diosmin. Interestingly, elevated kidney functioning markers were observed in the paracetamol treatment group, and diosmin treatment restored the renal functioning markers. These findings align with previous reports (Rehman et al., 2013). A previous study shows that cell death caused due to trichloroethylene can be reverted by diosmin. Therefore, these studies investigated the biomarkers related to trichloroethylene toxicity, carcinogenicity, renal toxicity, nephrotoxicity, hepatotoxicity, and mutagenicity (Rehman et al., 2013).
The beneficial role of diosmin is a never-ending list, and the study conducted by (Abdel-Daim et al., 2017) is another example. The role of diomsin was investigated against methotrexate (MTX)-induced renal injury in mice. Similar to the previous studies, the combination treatment of oral disomin and intraperitoneal MTX shows that in comparison to MTX alone treatment, diomsin combination treatment decreased MTX-induced biomarkers like interleukin-1-beta, interleukin-6) and TNFα, which are plasmas oro-inflammatory cytokines. Furthermore, the cellular metabolic biomarkers including urea creatinine, LDH, aminotransferases serum alkaline phosphatase, and creatine kinases were significantly decreased.
Combination treatment of MTX with diosmin increased the glutathione-related free radical scavengers, including superoxide dismutase and catalase. In addition, the malondialdehyde and nitric oxide levels in the tissues were significantly reduced compared to the MTX treatment alone. A combination treatment of MTX-diosmin further reduced histopathological alterations and restored the standard renal, hepatic, and cardiac tissue architecture. The histopathological examination confirmed this. The following findings support the protective nature of diosmin in improving the cytotoxicity caused due to the administration of MTX (Abdel-Daim et al., 2017).
Diosmin treatment in the animals affected with aflatoxin reduced the tissues and blood levels of MDA, nitric oxide, and 4-NHE. In addition, diosmin treatment also resulted in improved biochemical parameters; aflatoxin-induced oxidative stress, and liver and kidney damage (Eraslan et al., 2017). A study has shown that the treatment of diosmin reversed the renal toxicity induced by oral exposure to Cadmium (Cd). Administration of CdCl2 resulted in elevated levels of urinary constituents and altered biochemical enzymes suggesting kidney damage and renal toxicity due to the administration of cadmium chloride. Surprisingly when co-administered with diosmin for 28 days (40 mg/kg) as a treatment process for the cadmium poisoning of animals, the swiftness of recuperation was rapid. Diosmin co-administration also results in reverting the altered biochemical enzymes. The finding of this study is an encouraging finding supporting that diosmin may reduce renal toxicity induced by cadmium and an alternate treatment for the environmental disorder caused by cadmium (Sindhu et al., 2015).
The significant findings of this present study are that diosmin co-administered with paracetamol leads to an increase in the absorption of paracetamol and a simultaneous reduction in NAPQI plasma concentration in a dose-dependent manner. Interestingly, diosmin ameliorated paracetamol-induced hepatotoxicity and nephrotoxicity. Furthermore, long-term usage of diosmin helped to revert the hepatic and nephrotoxicity caused by the paracetamol. The present study results align with previous reports described as follows: flavonoids chrysin and quercetin remarkably have improved the absorption of paracetamol and diminished the concentration of NAPQI in the rat plasma due to inhibition of CYP2E1. Similarly, chrysin and quercetin exhibited hepatic and nephroprotective activities against paracetamol-induced toxicity (Pingili et al., 2019).
CONCLUSION
The results of the present study suggested that diosmin attenuated paracetamol-induced hepato- and nephrotoxicity via inhibition of CY2E1-mediated metabolism of paracetamol in rats by reducing the formation of NAPQI. Because of the protective nature of diosmin, it can be used in paracetamol poisoning in addition to N-acetylcysteine. These results support the idea of flavonoids as a supplement before taking clinical drugs to reduce the side effects caused by them. Future studies are required to investigate the other mechanisms of diosmin and its role in reducing paracetamol-induced toxicity.
ACKNOWLEDGMENTS
The authors are very grateful to the management of SVKM’s NMIMS Deemed to be University and KVSR SCOPS, Vijayawada, for providing necessary facilities and their encouragement in completing this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare no conflict of interest with anyone or any Institute.
ETHICAL APPROVALS
The approval and support of the KVSR Siddhartha College of Pharmaceutical Sciences (SCOPS), Vijayawada, Andhra Pradesh, India. The protocol approval number was KVSRSCOPS/29-03-2019-003.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Daim MM, Khalifa HA, Abushouk AI, Dkhil MA, Al-Quraishy SA. Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxid Med Cell Longev, 2017; 2017:3281670.
Abdel-Reheim MA, Messiha BAS, Abo-Saif AA. Hepatoprotective effect of diosmin on iron-induced liver damage. Int J Pharmacol, 2017; 13:529–40.
Abdel-Salam OM, Youness ER, Mohammed NA, Abd-Elmoniem M, Omara E, Sleem AA. Neuroprotective and hepatoprotective effects of micronized purified flavonoid fraction (Daflon®) in lipopolysaccharide-treated rats. Drug Discov Ther, 2012; 6:306–14.
Ali FE, Azouz AA, Bakr AG, Abo-Youssef AM, Hemeida RA. Hepatoprotective effects of diosmin and/or sildenafil against cholestatic liver cirrhosis: the role of keap-1/Nrf-2 and P38-MAPK/NF-κB/iNOS signaling pathway. Food Chem Toxicol, 2018; 120:294–304.
Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, Dow M, Coyle J, Cranfield KR, Hook C, Sandilands EA, Veiraiah A, Webb D, Gray A, Dargan PI, Wood DM, Thomas SH, Dear JW, Eddleston M. Effect of the UK’s revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol, 2014; 78:610–8.
Bedada SK, Boga PK, Kotakonda HK. The effect of diosmin on the pharmacokinetics of fexofenadine in healthy human volunteers. Xenobiotica, 2017; 47:230–5.
Bender RP, Lindsey RH, Burden DA, Osheroff N. N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry, 2004; 43:3731–9.
Eraslan G, Sarica ZS, Bayram LC, Tekeli MY, Kanbur M, Karabacak M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int, 2017; 24:27931–41.
Fan YJ, Rong Y, Li PF, Dong WL, Zhang DY, Zhang L, Cui MJ. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem Toxicol, 2013; 55:172–81.
Flores-Perez C, Chavez-Pacheco JL, Ramirez-Mendiola B, Alemon-Medina R, Garcia-Alvarez R, Juarez-Olguin H, Flores-Perez J. A reliable method of liquid chromatography for the quantification of acetaminophen and identification of its toxic metabolite N-acetyl-p-benzoquinoneimine for application in pediatric studies. Biomed Chromatogr, 2011; 25:760–6.
Hau DK, Gambari R, Wong RS, Yuen MC, Cheng GY, Tong CS, Zhu GY, Leung AK, Lai PB, Lau FY, Chan AK, Wong WY, Kok SH, Cheng CH, Kan CW, Chan AS, Chui CH, Tang JC, Fong DW. Phyllanthus urinaria extract attenuates acetaminophen induced hepatotoxicity: involvement of cytochrome P450 CYP2E1. Phytomedicine, 2009; 16:751–60.
Hong SW, Lee HS, Jung KH, Lee H, Hong SS. Protective effect of fucoidan against acetaminophen-induced liver injury. Arch Pharm Res, 2012; 35:1099–105.
Janbaz KH, Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia, 2000; 71:25–33.
Jang SI, Kim HJ, Hwang KM, Jekal SJ, Pae HO, Choi BM, Yun YG, Kwon TO, Chung HT, Kim YC. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol, 2003; 25:585–94.
Jing Y, Wu K, Liu J, Ai Q, Ge P, Dai J, Jiang R, Zhou D, Che Q, Wan J, Zhang L. Aminotriazole alleviates acetaminophen poisoning via downregulating P450 2E1 and suppressing inflammation. PLoS One, 2015; 10:e0122781.
Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J, 2013; 2013:162750.
Kumari A, Kakkar P. Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci, 2012; 90:561–70.
Latif AAE, Assar DH, Elkaw EM. Protective role of Chlorella vulgaris with thiamine against paracetamol induced toxic effects on haematological, biochemical, oxidative stress parameters and histopathological changes in Wistar rats. Sci Rep, 2021; 11:391.
Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther, 2000; 67:275–82.
Mohamed D, Tawakkol SM. Fluorimetric determination of diosmin and hesperidin in combined dosage forms and in plasma through complex formation with terbium. Bull Fac Pharm Cairo Univ, 2013; 51:81–8.
Neerati P, Bedada SK. Effect of diosmin on the intestinal absorption and pharmacokinetics of fexofenadine in rats. Pharmacol Rep, 2015; 67:339–44.
Novak A, Carpini GD, Ruiz ML, Luquita MG, Rubio MC, Mottino AD, Ghanem CI. Acetaminophen inhibits intestinal p-glycoprotein transport activity. J Pharm Sci, 2013; 102:3830–7.
Oliveira FA, Chaves MH, Almeida FR, Lima Jr RC, Silva RM, Maia JL, Brito GA, Santos FA, Rao VS. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol, 2005; 98:103–8.
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci, 2016; 5:e47.
Pathan M, Khan M, Moregaonkar S, Somkuwar A, Gaikwad N. Amelioration of paracetamol induced nephrotoxicity by Maytenus emarginata in male Wistar rats. Int J Pharm Pharm Sci, 2013; 5:471–4.
Perumal S, Babu LH, Langeswaran K, Kumar S, Balasubramanian M. Pharmacological potential of natural flavonoid diosmin against n-nitrosodiethylamine induced hepatocellular carcinogenesis in wistar albino rats. Int J Res Biosci, 2015; 4:25–36.
Pingili RB, Pawar AK, Challa SR. Systemic exposure of paracetamol (acetaminophen) was enhanced by quercetin and chrysin co-administration in Wistar rats and in vitro model: risk of liver toxicity. Drug Dev Ind Pharm, 2015; 41:1793–800.
Pingili RB, Pawar AK, Challa SR. Effect of chrysin on the formation of N-acetyl-p-benzoquinoneimine, a toxic metabolite of paracetamol in rats and isolated rat hepatocytes. Chem Biol Interact, 2019; 302:123–34.
Pingili RB, Vemulapalli S, Dirisala VR, Mullapudi SS, Gullapalli Y, Kilaru NB. Effect of naringenin on the pharmacokinetics of metoprolol succinate in rats. Xenobiotica, 2021; 51:926–32.
Pingili R, Vemulapalli S, Mullapudi SS, Nuthakki S, Pendyala S, Kilaru N. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev Ind Pharm, 2016; 42:1110–7.
Qiu-Ju L, Bessems JG, Commandeur JN, Adams B, Vermeulen NP. Mechanism of protection of ebselen against paracetamol-induced toxicity in rat hepatocytes. Biochem Pharmacol, 1994; 48:1631–40.
Rajnarayana K, Venkatesham A, Nagulu M, Srinivas M, Krishna DR. Influence of diosmin pretreatment on the pharmacokinetics of chlorzoxazone in healthy male volunteers. Drug Metabol Drug Interact, 2008; 23:311–21.
Rehman MU, Tahir M, Quaiyoom Khan A, Khan R, Lateef A, Hamiza OO, Ali F, Sultana S. Diosmin protects against trichloroethylene-induced renal injury in Wistar rats: plausible role of p53, bax and caspases. Br J Nutr, 2013; 110:699–710.
Rumack BH. Acetaminophen misconceptions. Hepatology, 2004; 40:10–5.
Sindhu K, Revathy R, Selvam M, Sathish S, Balasubramanian MP. Anti-nephrotoxic perception of diosmin, a citrus flavonoid inhibiting cadmium chloride induced oxidative stress in experimental rats. J Chem Pharm Res, 2015; 7:1512–8.
Sobeh M, Esmat A, Petruk G, Abdelfattah MA, Dmirieh M, Monti DM, Abdel-Naim AB, Wink M. Phenolic compounds from Syzygium jambos (Myrtaceae) exhibit distinct antioxidant and hepatoprotective activities in vivo. J Funct Foods, 2018; 41:223–31.
Sridhar V, Surya Sandeep M, Ravindra Babu P, Naveen Babu K. Evaluation of first-pass cytochrome P4503A (CYP3A) and P-glycoprotein activities using felodipine and hesperetin in combination in Wistar rats and everted rat gut sacs in vitro. Phytother Res, 2014; 28:699–705.
Su GL, Gong KQ, Fan MH, Kelley WM, Hsieh J, Sun JM, Hemmila MR, Arbabi S, Remick DG, Wang SC. Lipopolysaccharide-binding protein modulates acetaminophen-induced liver injury in mice. Hepatology, 2005; 41:187–95.
Sumioka I, Matsura T, Yamada K. Therapeutic effect of S-allylmercaptocysteine on acetaminophen-induced liver injury in mice. Eur J Pharmacol, 2001; 433:177–85.
Surya Sandeep M, Sridhar V, Puneeth Y, Ravindra Babu P, Naveen Babu K. Enhanced oral bioavailability of felodipine by naringenin in Wistar rats and inhibition of P-glycoprotein in everted rat gut sacs in vitro. Drug Dev Ind Pharm, 2014; 40:1371–7.
Tahir M, Rehman MU, Lateef A, Khan R, Khan AQ, Qamar W, Ali F, O’Hamiza O, Sultana S. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-alpha and NF-kappaB activation. Alcohol, 2013; 47:131–9.
Tomishima Y, Ishitsuka Y, Matsunaga N, Nagatome M, Furusho H, Irikura M, Ohdo S, Irie T. Ozagrel hydrochloride, a selective thromboxane A(2) synthase inhibitor, alleviates liver injury induced by acetaminophen overdose in mice. BMC Gastroenterol, 2013; 13:21.
Vemulapalli S. Influence of pomegranate juice on the CYP3A4-mediated metabolism and p-glycoprotein mediated transport of saquinavir in vivo and ex vivo models. Indones J Pharm, 2016; 27:152.
Vliegenthart AD, Antoine DJ, Dear JW. Target biomarker profile for the clinical management of paracetamol overdose. Br J Clin Pharmacol, 2015; 80:351–62.
Ying W, Yan-Ling W, Li-Hua L, Ji-Xing N. Protective effect of Ornithogalum saundersiae Ait (Liliaceae) against acetaminophen-induced acute liver injury via CYP2E1 and HIF-1α. Chin J Nat Med, 2012; 10:177–84.
Yoo HH, Lee M, Chung HJ, Lee SK, Kim DH. Effects of diosmin, a flavonoid glycoside in citrus fruits, on P-glycoprotein-mediated drug efflux in human intestinal Caco-2 cells. J Agric Food Chem, 2007; 55:7620–5.
Zhao X, Cong X, Zheng L, Xu L, Yin L, Peng J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol Lett, 2012; 214:69–80.