INTRODUCTION
Mainly two types of organisms found are on this planet: eukaryotes, which have a well-defined nucleus enclosed with nuclear membrane, and prokaryotes, which have no well-defined nucleus (Mayr, 1998; Stanier and van Niel, 1962). Prokaryotic cells are typically unicellular and micrometer in size. Eukaryotic cells are much larger in shape, size, and genome too. Additionally, there are extranuclear cell organelles viz. mitochondria and chloroplasts (de Duve, 1996). Eukaryotes are considered to have evolved from prokaryotes, and in this context Yamaguchi et al. (2012) reported a unique organism “Parakaryon myojinensis” with cellular structures appearing to have intermediate features between prokaryotes and eukaryotes. Furthermore, microbes are also classified as prokaryotic and eukaryotic microbes. Bacteria, archaebacteria, cyanobacteria, actinomycetes, and yeast come under the category of prokaryotic microbes whereas fungi and protozoans come under the category of eukaryotic microbes.
Nanotechnology is an application and manufacturing-based technology of the 21st century involving multidisciplinary research along with understanding and control of several substances into nano size. Nanotechnology describes the change in the properties like magnetic, electric, and mechanical with the change of material shape and size into nano scale. Nanoparticles (NPs) range from submicron size to several hundred nanometers. They are also reported in a range less than 10–20 nm where a significant change in the properties was observed (Cao, 2004; Charinpanitkul et al., 2008; Naito et al., 2007).
Basically, NP biosynthesis approach includes two methods i.e., top-down and bottom-up illustrated in Figure 1. Top-down methods include lithography, milling, and repeated quenching. In this method, bulky metals break down at nanoscale. Controlled particle size and their structure are tedious to obtain through top-down approach. Researchers in this field mostly follow the bottom-up approach in which materials are synthesized from bottom: molecule-by-molecule, atom-by-atom, and cluster-by-cluster (Cao and Wang, 2011; Cele, 2020).
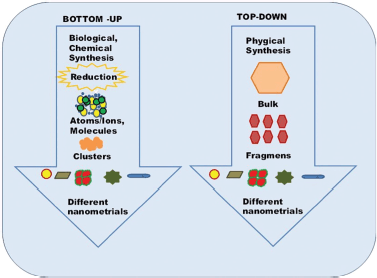 | Figure 1. Bottom to up and top to down method for the synthesis of NPs. [Click here to view] |
The NPs are synthesized by the process of oxidation/reduction mediated through intracellular or extracellular metabolites produced by microbes, called green synthesis. The synthesis process is specific with specific microorganism along with environmental conditions, leading to variation in shapes and sizes (Salem and Fouda, 2020). In addition, the chemical method of NPs’ synthesis also includes the bottom-up approach. Numerous physical, biological, and chemical techniques are being utilized for the fabrication of nanomaterials with specific size and shapes (Grzelczak et al., 2008).
Living cell-based green synthesis of metal NPs is a favorable and unique tool in the field of nanobiotechnology. This is an environment resilient, safe, economic, and easier method to obtain NPs with enhanced productivity and purity. Several reports in previous investigations explained that green synthesis of metal NPs, such as zinc nanoparticles, iron nanoparticles (FeNPs), and copper oxide nanoparticles (CuO-NPs) have obtained by various prokaryotic and eukaryotic microorganisms (Abdelhakim et al., 2019; John et al., 2020; Korbekandi et al., 2014; Mahdavi et al., 2013; Rahman et al., 2009; Srivastava et al., 2013). For NP synthesis, high temperature or pressure is not needed to green synthesis method and harmful substances for reduction and stabilization as well (Usman et al., 2019). Green synthesis approach includes the NP synthesis intracellularly or extracellularly depending on the location of NPs’ synthesis. Using extracellular methodology Majeed et al. (2021) fabricated iron oxide nanoparticles (IONPs) from Proteus vulgaris. When IONPs were used against pathogenic Staphylococcus aureus (methicillin resistant S. aureus) the significant and satisfactory antibacterial activity was observed. Nanobiotechnology is boosting in several fields of science like biomedical (Bin-Meferij and Hamida, 2019; Iqtedar et al., 2019), environment monitoring (Majumder et al., 2019), catalysis (Mishra et al., 2014), vegetables and food preservation (Fayaz et al., 2009), chemical industries (Khan et al., 2017) and agriculture (Ramírez-Rodríguez et al., 2020; Shebl et al., 2019). Nanomedicines are becoming an area of interest for researchers that hold tremendous potential for the disease diagnosis.
Numerous applications of NPs in various fields, such as development of regenerative medicine, drug delivery, wound healing, catalysis, waste-water treatment, development of nano-fertilizers, as antioxidants diminishing reactive oxygen species (ROS), drug delivery, anti-cancer agents, and in several industries like pharmaceutical, textiles, and cosmetics (Bin-Meferij and Hamida, 2019; Chandrakala et al., 2022; Mustapha et al., 2022; Seqqat et al., 2019).
Therefore, the current review illuminates the mechanism, necessity, and application of metal NPs’ synthesis with the help of prokaryotic and eukaryotic microbes. Previous investigations revealed the mechanism and method of metal NPs biosynthesis, but there are several issues to be illustrated related to microbial biosynthesis of metal NPs, and we try to emphasize those points too here.
USE OF PROKARYOTIC MICROBES FOR NP BIOSYNTHESIS
Prokaryotes have advantages including simple morphology, well-known biological processes, and simple production of bioactive molecules. So in recent years these microorganisms have attracted many scientists for the fabrication of NPs (Ag, Au, Fe, Zn, Co, Mg, Cu, Mn, etc). During the cellular synthesis of NPs, the target ions are grabbed by microbial cells from their surroundings, and afterward, this is converted into elemental metal in the presence of enzymes released as a result of cellular activities. This process of NP biosynthesis is categorized into intracellular and extracellular formation, which is related to the site of NP synthesis (Fig. 2). NPs can be synthesized on either the inner side of the cell or the outer side of the cell (in the medium). Ions transport inside the microbial cells, and in the presence of intracellular enzymes NPs are synthesized. In the extracellular synthesis of NPs, the metal ions are trapped on the microbial cell surface where reduction took place in the presence of extracellular enzymes, leading to synthesis (Zhang et al., 2011). Numerous biomolecules that are present inside the microbial culture or on the surface of the microorganisms work as oxidants or reductants as well as capping agents on the target ions from their surroundings.
The biological synthesis of metal NPs depends upon the organized utilization of microorganisms, like bacteria, cyanobacteria, extremophiles, algae, and fungi (Abdollahnia et al., 2020; Husain et al., 2019; Majeed et al., 2021; Patel et al., 2021; Srivastava et al., 2013). In bio-based synthesis, no chemical stabilizers are used, the biomolecules of microorganisms can carry out this role themselves and control the effect of synthesized NPs such as shape, size, etc. (Ovais et al., 2018).
NPs’ synthesis from bacteria
Bacteria are the first group of microorganisms utilized in the very early research on the fabrication of metal NPs because bacteria can synthesize NPs of different sizes and shape with the relative simplicity of their cultivation and manipulation (Selvarajan et al., 2013). Curiously, silver ions that are toxic to most microbial cells can change into silver nanoparticles (AgNPs) after their reduction in the presence of bacterial cells. Pseudomonas strain separated from an association of Antarctic marine ciliate Euplotes focardii, which were used for the fabrication of AgNPs with well-defined sizes and unique structures (John et al., 2020). Researchers now follow new methods for the development of NPs specifically targeting them for biomedical applications. Obtained AgNPs were spherical polydisperse. Synthesized AgNPs revealed a higher antimicrobial activity to the pathogens, including Escherichia coli, S. aureus, and Candida albicans (John et al., 2020). In addition, endophytic actinomycetes were tested for the formation of copper nanoparticles (CuNPs) by Hassan et al. (2018). Actinomycetes were isolated from medicinal plant (Convolvulus arvensis) leaves. Biosynthesized CuNPs were potent in killing many pathogenic agents. In another example, CuO-NPs synthesized using Proteus mirabilis, showed antagonistic activity against prokaryotic and eukaryotic microorganisms. Synchronous biosynthesis of zero-dimensional (inside the cell) and one-dimensional (outside the cell) CuO-NPs is reported first time by Eltarahony et al. (2018). Synthesized CuO-NPs were revealing surface plasmon resonance at 275 nm and spherical shape when synthesized inside the cell while surface plasmon resonance was at 430 nm and needle or wire shape structure when synthesized outside the cell. In previous research, Bacillus thuringiensis was utilized for the fabrication of si AgNPs by Subramaniam Ramachandran (2014). The size range was from 43.52 to 142.97 nm. We have listed the biosynthesis of metal NPs utilizing bacteria in Table 1.
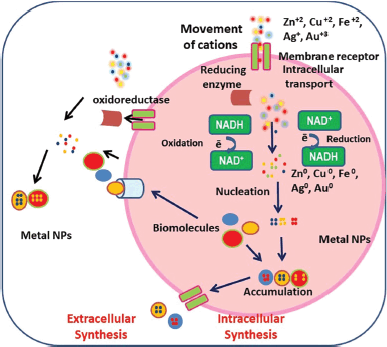 | Figure 2. Generalized depiction of the NPs biosynthesis at out-side and in-side the cell. [Click here to view] |
NP synthesis from cyanobacteria (Blue green algae)
Cyanobacteria are a great alternative among microorganisms for the biosynthesis of NPs with different dimensions because being perseverance suppliers for different metabolic products with outstanding biomedical applicability (Roychoudhury et al., 2016). Moreover, cyanobacterial (Microchaete NCCU-342) species are explored for the synthesis of different NPs like AgNPs. This bio-fabrication has paved the way to save the environment through simplicity, rapid rate of synthesis, and eco-friendliness. Synthesized NPs were 60–80 nm in size, appropriate for the degradation of methyl red (MR) within 2 hours (Husain et al., 2019). In one of the types of research, the synthesis of gold nanoparticles (AuNPs) includes the protein extract of Spirulina platensis with 10 mM tetrachloroauric acid (Suganya et al., 2015). After the addition of 1 N NaOH green color changed to greenish yellow instantly, reaction was carried out for 3 hours with constant stirring. After the completion of AuNP synthesis, the color of the reaction mixture turned ruby red. Synthesized NPs are further characterized by UV-visible spectroscopy and other characterization methods. The stability of synthesized NPs was estimated at different temperatures (4°C, 15°C, 25°C, 60°C, and 80°C) to monitor λ max. UV-Vis spectrum was predicting that AuNPs are stable at 4°C–60°C and stability was affected by higher temp. Singh et al. (2014) reported on the use of the cell-free extract of a strain of cyanobacterium Anabaena L31 for the fabrication of pure zinc oxide nanoparticles (ZnONPs). Additionally, reported the formation of UV-absorbing water-soluble compound shinorine conjugate of ZnONPs–shinorine. The conjugate of ZnONPs with shinorine revealed the significant antioxidant property by reduction of ROS produced by cyanobacteria. At the same time, Cepoi et al. (2014) examined S. platensis and Nostoc linckia species of cyanobacteria as an element for the biosynthesis of NPs because of the presence of many bioactive elements. In an earlier study, the effect of H+ ion concentration on the morphology of NPs was observed along with a screening of potential algal strains (Sharma et al., 2019).
Based on screening three algal strains Phormidium valderianum, Phormidium tenue and Microcoleus chthonoplastes were found to be suitable for the biosynthesis of AuNPs intracellularly. Only P. valderianum, was found as able to synthesize AuNPs with different sizes at different pH. A diversified morphology of NPs was observed after characterization by UV-visible spectroscopy, X ray diffraction (XRD) as well as transmission electron microscopy (TEM) studies (Parial et al., 2012). Lengke et al. (2006a), used two different salts of gold as precursors [AuCl4−] and [Au (S2O3)23−] and proved the intracellular synthesis of Au-NPs ranges from 10 to 25 nm in size. In this study Plectonema boryanum UTEX485 was used as a model organism that is found to be predominant in water bodies. Cubical and octahedral nanoplates were observed after characterization by TEM. In addition, Lengke synthesized platinum and AgNPs with a diameter of 30 nm–0.3 µm and 1–40 nm, respectively (Lengke et al., 2006b, 2007).
Numerous reports have described the formulation and characterization of NPs from BGA like Phormidium (Rahman et al., 2009), N. linckia (El-Naggar et al., 2017), Microchaete NCCU-342 (Husain et al., 2019), Synechococcus (Keskin et al., 2016), Anabaena and Calothrix (Brayner et al., 2009), Oscillatoria limnetica (Hamouda et al., 2019), and Desertifilum IPPAS B-1220 (Hamida et al., 2020). The formation of NPs either from biomass or from the supernatant of cyanobacteria is not illustrated in detail with a clear methodology and description in several research articles. The synthesis from supernatant is still not reported and is less explored. In another study of micronutrient NP synthesis, we have observed that from both biomass and supernatant of Oscillatoria and Phormidium there is a proper and adequate synthesis of micronutrient NPs also (unpublished data).
NP synthesis from actinomycetes
The unicellular, filamentous microorganism that lies in a group of Gram-positive bacteria and is also designated as “ray fungi” is found in almost all ecosystems around the globe. They exhibit similar properties to both bacteria and fungi (Kumari et al., 2020). They produce a broad range of clinically useful antibiotics and secondary metabolites. Actinomycetes are abundant in natural environments that facilitate the production of antibiotics as well as secondary metabolites. Their limitless ability to produce bioactive compounds has led to their use for producing metal ion NPs (Bedlovi?ová, 2022). Mechanism of AgNPs bio fabrication inside the cell explained by Sunitha et al. (2014). They suggested that silver ions trap on the outer surface of actinomycete cells through bioactive molecules with electrostatic attractions. Enzymes present in the cell wall may reduce trapped ions to the formation of metal nuclei which then increase due to further reduction of Ag+ ions and accumulation on these nuclei. Shah et al. (2012) and Waghmare et al. (2014) also proposed an almost similar mechanism for AuNPs’ synthesis. nicotinamide-adenine-dinucleotid (NADH)-dependent reductase catalyzes the electron transfer from NADH to gold ions that may cause initiation of gold ions reduction. After that reduced gold ions change to metallic gold (Au0) and then form AuNPs. They conclude that the biosynthesis of metal NPs using actinomycetes was extracellular. Additionally, Wypij et al. (2018) examine Streptomyces xinghaiensis as a source of AgNPs’ synthesis. Synthesized AgNPs were efficient in cytotoxic studies on mouse fibroblasts (3T3) and HeLa cell lines. Synthesis of Ag and Au NPs by both supernatant and cell extract of Streptomyces sp. for antibacterial activities done by Sk?adanowski et al. (2017). Synthesized AgNPs exhibit considerable antimicrobial activity against pathogenic bacteria. A range of prokaryotic microbes has been utilized for the biosynthesis of NPs described in various previous investigations listed in Table 1.
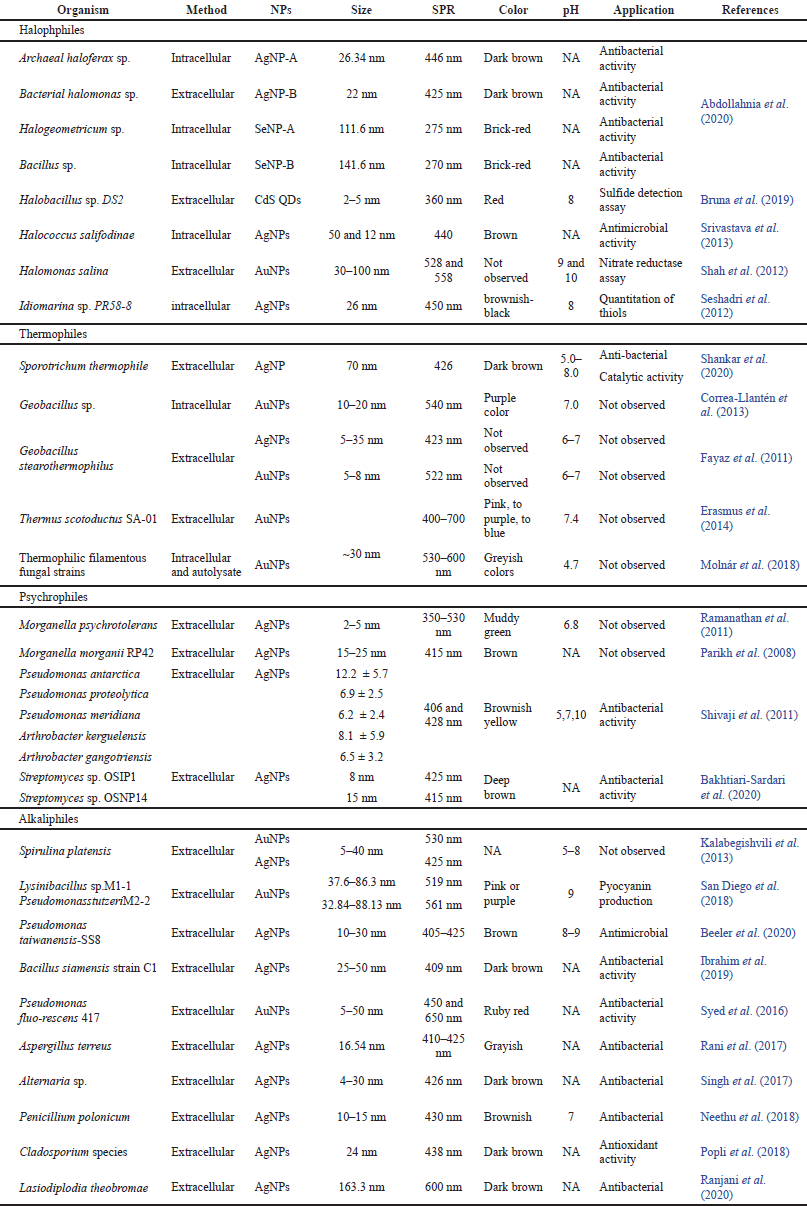 | Table 1. Description of NP synthesis using different bacteria, cyanobacteria and archaebacteria. [Click here to view] |
NP synthesis from archaebacteria
Archaebacteria are a group of microorganisms considered to be the oldest form of life on glob. Archaebacteria evolved separately from the bacteria and blue-green algae, be a possession to the kingdom monera and are classified as bacteria but they are quite distinct in morphology and physiology. However, they share slightly common characteristics with the eukaryotes. These can easily survive under extreme conditions such as very high and low temperature, pH, or anoxic thus known as extremophiles. Halophilic archaebacteria can detoxify heavy metals through various mechanisms and this detoxification process can change metallic ions to NPs. Nowadays researchers are interested to heavy metal remediation by haloarchaea but the capabilities of these microorganisms for the fabrication of NPs have remained understudied. Few reports are available in this context one of which is silver and selenium nanoparticles (SeNPS) synthesis by Abdollahnia et al. (2020). They have isolated halophilic archaebacteria from a solar saltern pool. After synthesis, NPs were identified and compared with previously reported work. Recently, in another study, Haloferax sp strain NRS1 was purified from a solar saltern of the Red Sea, Saudi Arabia. Isolated strain was tested to fabrication of AgNPs by Tag et al. (2021). With the aim of characterization of synthesized AgNPs different findings are listed in Table 1. In a research, Kashefi et al. (2001) explored extremophilic bacteria and archaea for the synthesis of AuNPs by precipitation method. Synthesis of AuNPs or reduction of Au(III) to Au(0) is reported due to the presence of H2 as an electron donor at 100°C. Microbial cells were not able to reduce Au(III) in the absence of H2 or incubation at 30°C. Srivastava et al. (2013) have explained the mechanism of AgNPs’ synthesis based on NADH-dependent nitrate reductase, detailed explanation is available in the section on halophiles.
NPs’ synthesis from extremophiles
Microorganisms surviving in extreme abiotic conditions are unique life forms on this planet. The cellular physiology and adaptability of extremophiles revealed their unique abundance around the globe. The environmental niches for different types of extremophiles differ from mesophilic microbes (Tiquia-Arashiro and Rodrigues, 2016). This unique group of microbes has a unique type of physiology and cellular adaptation mechanism and obviously imparts uniqueness in the biofabrication of NPs too. The synthesis of NPs from different extremophilic microbes is listed in Table 2.
Halophiles in the synthesis of NPs
Microbes surviving in extremely high salt conditions such as marine environments, sea water, salterns, and lakes are known as halophiles. Halophiles consist of several prokaryotes and eukaryotes, having the capability to maintain the osmotic pressure of the environment in the adverse concentration of salts. Halophilic archaea are the predominant class of thalassohaline locations where salinity reaches up to 300 g/l. Halophilic archaea and bacteria both were examined for intracellular/extracellular fabrication of AgNPs and SeNPs by Abdollahnia et al. (2020). AgNPs were synthesized intracellularly using archaeal Haloferax sp. whereas extracellularly using bacterial Halomonas sp. and SeNPs were synthesized intracellularly by the archaeal Halogeometricum sp. while extracellularly by the bacterial Bacillus sp. Another extremophile strain Halobacillus sp. DS2 was isolated from the Dead Sea, and tested for the formation of carbon quantum dots (CQDts) by Bruna et al. (2019). Halobacillus sp. DS2 was resistant to CdCl2 (1.375 mM) at a higher level of NaCl concentrations (3%–22%) and was able to grow in a broad range of pHs (pH 1–9). The formation of CQDts was confirmed by the color change of fluorescence emission that was red in place of blue after 31 hours. Halophilic archaea like Halococcus salifodinae BK3, were reported for the intracellular synthesis of AgNPs (Srivastava et al., 2013). Synthesized NPs were stable and spherical with a size of 50.3, 12 nm. In the formation of AgNPs NADH-dependent nitrate reductase played an important role in reduction and metal-dependent enzyme activity enhancement. In another research, the halophilic proteobacteria Halomonas salina was explored for the synthesis of AuNPs anisotropic in acidic and spherical in alkaline conditions. Halomonas salina has been studied as a producer of nitrate reductase, which may have a critical role in scaffolding or nucleation for the bio-reduction of Au+3 to Au0 and form AuNPs (Shah et al., 2012). Idiomarina sp. PR 58–8 bacterium showed excessive tolerance for silver, so this property of bacterium was explored for the fabrication of intracellular crystalline AgNPs (Seshadri et al., 2012). Extremely halophilic Fe3+-reducing archaea and bacteria isolated from a solar saltern were investigated for the reduction of gold by Kashefi et al. (2001). Studies exhibit that these strains of halophilic archaea and bacteria are potent sources of AuNPs’ synthesis. These research works conclude that halophilic prokaryotes play a significant role as models for the formation of metal NPs in both hydrothermal and cooler halophilic environments.
NP synthesis from thermophiles
Thermophiles are organisms that reach optimum growth at high temperatures. Thermophilic bacteria can live at a much higher temperatures from 50°C to 121°C while other bacteria would not. Shankar et al. (2020) optimized a unique approach “one variable at a time” for the extracellular synthesis of AgNPs using the thermophilic mold Sporotrichum thermophile BJTLRMDU7. The researcher tested the effect of different variables such as pH, temperature, light and dark conditions, and different amounts of culture filtrates with various concentrations of AgNO3 at different times. Molnár et al. (2018) investigate the large number of thermophilic filamentous fungous for the biofabrication of AuNPs. Based on the investigation, they have concluded that protein molecule can stabilize NPs because it has a molecular weight of more than 3 kDa, so proteins can be bound on the surface of NPs by the formation of Van der Waals interactions. These interactions take place due to the availability of conducting electrons of nitrogen and sulfur toms. The first time reported the potential of the thermophilic bacterium Thermus scotoductus to synthesize AuNPs and find out the impact of the reaction conditions on particle formation. Purified protein from this bacterium reduced HAuCl4 for the synthesis of elemental NPs extracellularly. Erasmus et al. (2014) identified the purified protein by N-terminal sequencing as an ABC transporter. A stain of Geobacillus sp., tested by Correa-Llantén et al. (2013), for NADH dependent enzymatic biosynthesis of AuNPs. Fourier transform infrared spectroscopy (FTIR) of synthesized AuNPs after 16 hours of reaction revealed a peak in 1,302 cm−1 which means the aromatic amine group containing molecule working as a capping agent. The results of this study strengthen the idea that protein-type molecules have the potential to stabilize biosynthesized NPs. Fayaz et al. (2011) reported the biogenic synthesis of AgNPs and AuNPs using Geobacillus stearothermophilus. In the presence of G. Stearothermophilus, cell-free extract metal salts lead to the formation of Ag and Au NPs. High stability of NPs can achieve by the secretion of precise capping proteins from the bacterium in the reaction mixture.
NP synthesis from psychrophiles
Microorganisms that can survive at very low temperatures are known as psychrophiles. They not only tolerate but also show maximum growth in this specific condition. Extremely low temperatures around 75% of the Earth’s biosphere is permanently cold so the existence of psychrophiles is predominant in this environment. Many psychrophiles have the capability to reduce metal ions and change into nanoforms so these microorganisms can be one of the best possibilities for NP synthesis (Tiquia-Arashiro and Rodrigues, 2016). In this scenario, Bakhtiari-Sardari et al. (2020), have explored two strains of Streptomyces sp. To the comparative study of AgNPs biosynthesis. Synthesized AgNPs were remarkably effective against the growth of several pathogenic bacteria with the combination of antibiotics and itself as well. Ramanathan et al. (2011) reported that the morphology of AgNPs was achieved by silver resistant Morganella psychrotolerans, a species of psychrophilic bacterium. This is considering that the shape and size of NPs regulate by controlling the growth kinetics of bacterium. Similarly, in another research, Shivaji et al. (2011) examined the bioformation and stability of AgNPs using cell-free culture supernatant of five species of psychrophiles, based on temperature, pH or the species of bacteria. He concludes that the fabrication of NPs varies from bacterial species to species at similar temperature and pH. To understand biochemical mechanisms of silver-resistant bacteria Morganella morganiistrain, the extracellular synthesis of crystalline AgNPs was reported by Parikh et al. (2008). Analysis of biomolecules that are associated with the outer surface of NPs by FTIR spectroscopy revealed the presence of a protein that was responsible for the reduction of silver-to-AgNPs.
NP synthesis from alkaliphiles
Alkaliphiles are a type of extremophile that can grow optimally at alkaline pH above 9. Alkaliphiles can be prokaryotic, eukaryotic, and archaea. Alkaliphiles had explored by a few researchers for the synthesis of NPs. In this context, Pseudomonas taiwanensis-SS8 were isolated from root nodules of clover plants and is explored to develop stable and eco-friendly AgNPs. FTIR analysis reviled that the biological molecules present in culture broth perform the dual functions of synthesis and stabilization of AgNPs. Moreover, antibacterial activity is also tested which revealed that synthesized AgNPs have an excellent antibacterial property (Beeler et al., 2020). In addition, AuNPs were fabricated using two alkaliphilic bacterial species, Lysinibacillus and Pseudomonas. By different characterization evident the AuNPs size of 37.6–86.3 nm and 32.84–88.13 nm by Lysinibacillus sp. and Pseudomonas respectively. Biosynthesized AuNPs exhibit potential in biomedical applications by inhibiting pyocyanin production San Diego et al. (2018). Spirulina platensis is a strain of filamentous cyanobacterium. Kalabegishvili et al. (2013)explored S. platensis effectively to the extracellular production of AuNPs and AgNPs. Fabricated Au and AgNPs using S. platensis biomass were crystal in morphology. According to Kalabegishvili et al. (2013), gold and silver metal ions were rapidly adsorbed on the outer surface of bacterium and then slowly transported into the cells, and NPs were accumulated there.
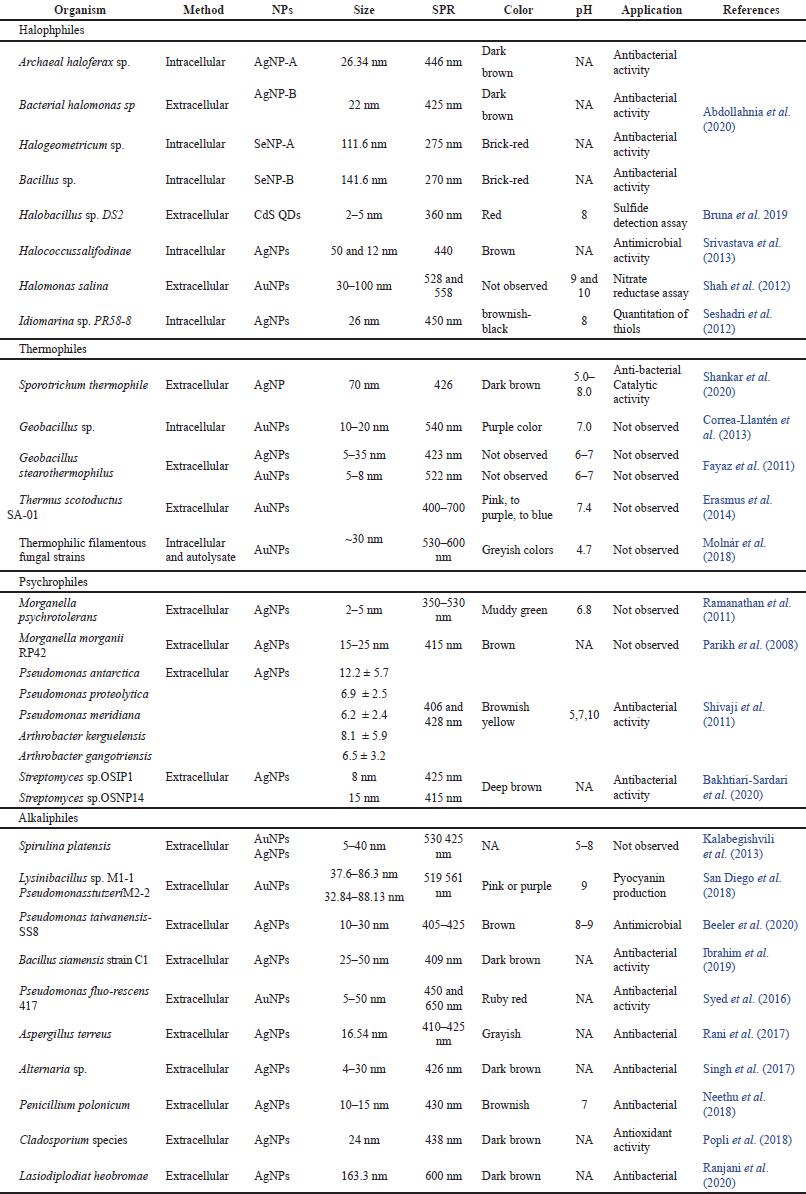 | Table 2. Extremophilic microbes mediated NPs and their applications in different fields. [Click here to view] |
NP synthesis from acidophiles
Acidophiles are acid-loving extremophiles that can survive at a very low pH value. Acidophiles grow vigorously in sites of the acidic environment such as acid mine drainage, sulfuric acid containing fields, acidothermal hot springs, and bioreactors. Acidophiles can maintain pH homeostasis by controlling proton movement by the cell membrane. (Tiquia-Arashiro and Rodrigues, 2016). Acidithiobacillus ferrooxidans BY-3 explored the biosynthesis of Fe3O4 NPs to understand biocompatibility. Synthesized Fe3O4 NPs were yielded by combining ultracentrifugation and magnetic separation. Cellular metabolic activity test, micronucleus test, and fragile red blood cells detection assay were carried out to estimate in-vitro cytotoxicity, blood toxicity, and genotoxicity of magnetosomes, respectively. Results of these assays revealed that Fe3O4 NPs were not cytotoxic, or genotoxic, and had no hemolytic effects up to 4.0 mg/ml. These tests indicated that synthesized NPs have good biocompatibility (Malhotra et al., 2020).
The biofabricated Fe3O4 NPs were showing ferrimagnetism and membrane-bound properties. Such a type of characterization made magnetosomes promising for numerous applications in the field of biomedicine and biotechnology. They appear to be favorable immobilization of various metabolites, biomolecules, and bioactive substances. Moreover, the description of several extremophilic microbes utilized in the formation and application of different NPs is explained in Table 2.
NP SYNTHESIS FROM ENDOPHYTES
Endophytes are microorganisms that present almost in all types of plants, intracellularly or extracellularly, in a symbiotic relationship without causing any harm or deterioration to the plant cells. Endophytes can be present in different parts of a plant like roots, leaves, stems, flowers, and seeds. Endophytes are very resilient and adaptive according to the nature of their host plants. Under symbiotic association inside the plant tissues, they secrete and form several compounds helpful in plant growth and do not making any adverse effect on plant tissues. Few reports focus on endophytic fungi that can produce bioactive compounds inside the plant and they can be considerable alternate source of bioactive compounds. These bioactive compounds have different antioxidant, anticancer, immune-modulating, and antimicrobial properties (Rahman et al., 2019). To detect plant growth promotion and antibacterial ability, AgNPs were synthesized from endophytic bacterium Bacillus siamensis strain (Ibrahim et al., 2019). The biosynthesized AgNPs were further characterized by various characterization methods. In analysis of UV-visible spectroscopy AgNPs showed maximum absorbance at 409 nm and TEM analysis revealed the size of NPs that was 25–50 nm. Plant growth promotion study showed that the synthesized AgNPs significantly enhance the growth of rice seedlings. Furthermore, results of antibacterial assay exhibit that AgNPs at different concentrations of 5, 10, 15, and 20 mg ml−1 have a strong antimicrobial effect against Xoo strain LND0005 and Ao strain RS-1. The inhibitory effect of AgNPs was increasing when increasing the concentration of NPs. In a report, a strain of endophytic bacteria, Pseudomonas fluorescens 417, obtained from the Coffea Arabica showed the potential to synthesize AuNPs . The NPs fabricated from endophytes have given significant microbial activity against several clinical pathogens (Syed et al., 2016). Therefore, numerous researchers are synthesizing AgNPs from plant endophytes to be used in the medical field further.
NPS’ SYNTHESIS FROM EUKARYOTIC MICROBES
Many microbial cells have evolved to utilize metal ions as cofactors for optimum activity of different enzymes and proteins. Thus, the cells have adopted to change elemental metals to metal NPs. Indeed, several algae, yeast, and fungi are able to be reducing metal ions through metallo-regulatory mechanism. Different NPs synthesized using eukaryotic microorganisms listed in Table 3.
NPs biosynthesis from micro-algae
Algae have long been used in food and pharmaceutical industries but now a days algae are exploited widely in agriculture, food packaging, preservation, cosmetics, fertilizers, and agents for bioremediation. Moreover, algal communities are utilized tremendously for the biosynthesis of metal NPs too. In a recent branch of science called phyco-nanotechnology, no toxic reducing agents are required for synthesis of NPs and it is therefore a low-cost green synthesis (Chaudhary et al., 2020). Within this framework, biosynthesis of Ag and CuNPs using green alga Botryococcus braunii was done by Arya et al. (2018), TEM analysis showed the size of CuNPs and AgNPs 10–70 nm and 40–100 nm respectively. FTIR of synthesized NPs reveals the peak 1,637.82 cm−1 for N–H bending vibrations in amide of alga protein as a capping molecule. Biogenic NPs exhibited excellent antibacterial and antifungal activity against pathogenic bacteria and fungus both. In a study by Ramakrishna et al. (2016), brown algae Turbinaria conoides and Sargassum tenerrimum were utilized for the synthesis of AuNPs .
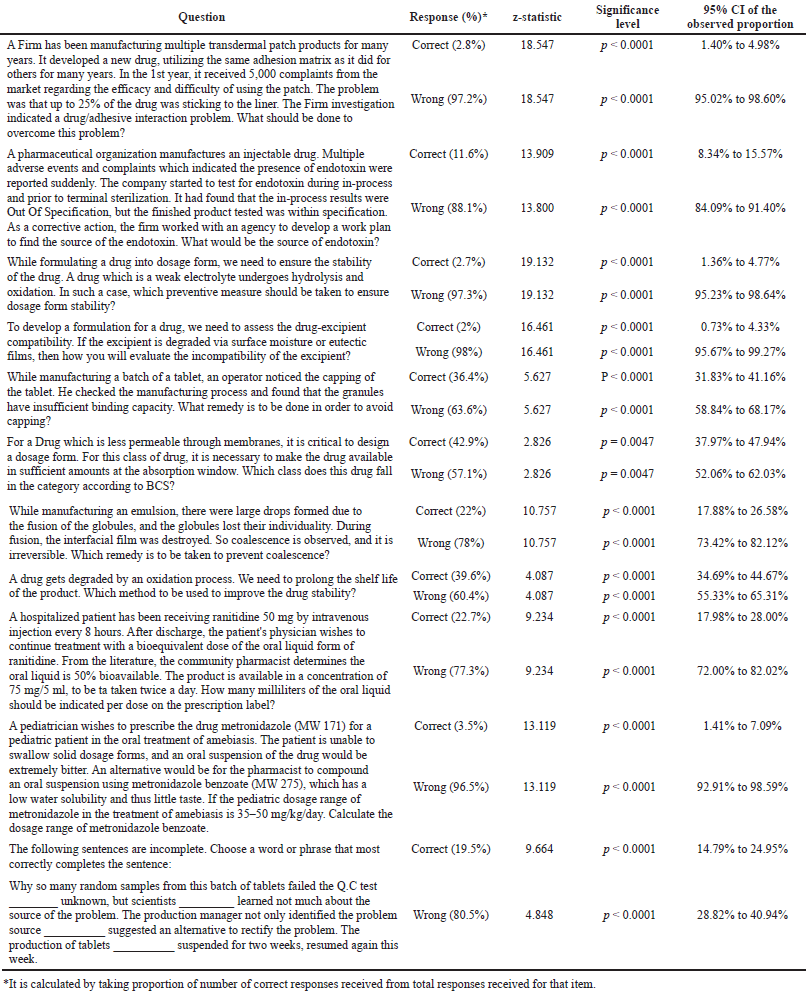 | Table 3. Synthesis of different nanomaterials using eukaryotic microorganisms. [Click here to view] |
This was observed that the biosynthesis of AuNPs is facilitated by the presence of OH group in the algal extract into the spherical elemental AuNPs . This predicted that the hydroxyl group present in the algal extract works as the capping and reducing agent to convert Au (III) into elemental AuNPs. Pithophora oedogoni was explored successfully for fast and easy fabrication of AgNPs. The aqueous extract of algal cell was used in the reduction of silver nitrate solution into AgNPs. Synthesized AgNPs showed antimicrobial activity against seven tested pathogenic bacteria (Sinha et al., 2015). Ecofriendly approach as an alternate to the more complicated chemical procedures for synthesis of transition metals using Caulerpa serrulata has evolved by Aboelfetoh et al. (2017). Caulerpa serrulata extract can reduce silver ion to stable colloidal AgNPs of 10 ± 2 nm. They optimized concentration of extract, contact time, pH values, and temperature. Catalytic and antibacterial activity examined by synthesized NPs. Selvam and Sivakumar, (2015), explained the simple and sustainable biosynthesis of AgNPs using the aqueous extract of red alga Hypnea musciformis. Surface plasmon resonance of synthesized NPs occurred at 440 nm. Surface morphology and size of the Ag NPs were analyzed by atomic force microscopy. The 2D and 3D images of the NPs revealed the well-separated spherical and sizes of the particles were in the range of 2–55.8 nm. Formed AgNPs were able to degradation of dyes in the presence of visible light and pave the attention for ecological safety or bioremediation. Subramaniyam et al. (2015) examined the possibility of synthesis of FeNPs by soil microalga, Chlorococcum sp. MM1. The obtained NPs were spherical shaped and 20–50 nm in size. In the energy dispersive X-ray analysis spectra of synthesized NPs, oxygen peak was lower than the iron oxide so it was the evidence that FeNPs has synthesized. On the basis of obtained peaks researchers conclude that oxygen and carbon dioxide is present of oxygen as well as carbon peak in the spectra may be due to polysaccharides and glycoproteins, indicating the involvement of biomolecules in capping of the FeNPs. FTIR analysis revealed the changes in functional groups of carbohydrates and proteins due to participation in reduction and capping process.
NP synthesis from fungi
Role of fungi as effective nanofactories is attracting attention of researchers worldwide. Mycogenic route for NPs’ synthesis has been well recognized because this totipotent eukaryotic microorganism has several remarkable characteristics such as excellent secretor of protein, easy to isolate and culture, extracellular synthesis of NP, better manipulation, high yields, and lesser harm of the residues. Moreover, microbial biosynthesis of NPs obtained from fungus triggers the coating of biomolecules over the surface of NPs that seems to improve the stability and biological activity that is well documented. Fungi is a great source of various extracellular enzymes that influences NPs’ synthesis (Guilger-Casagrande and Lima, 2019; Saxena et al., 2014). One of the investigations done by Fayaz et al. (2010) explained the utilization of a fungal strain Trichoderma viride for the extracellular bio-fabrication of AgNPs using silver nitrate solution. Numerous biomolecules such as enzymes, proteins, and polysaccharides present in the extracellular extract reduce the transition metal ions into nano-entities by fusion of biomolecules. This is used worldwide to get stable and easily synthesized metal NPs. In this scenario, Gudikandula et al. (2017) explored the Trametes liubarski and Ganoderma enigmaticum, white rot fungus for fabrication of AgNP. The characterization of AgNPs showed the size 15–25 nm and absorbance at ~ 420 nm. The interaction between capping agents present in cell-free extracts and AgNPs was better achieved by FTIR analysis. A significant antimicrobial outcome was obtained against pathogenic bacterial strains too.
Fungus secretes secondary metabolites in their surrounding that can reduced the metal ions this approach used by Bagur et al. (2019), for the biosynthesis of AgNPs using the endophyte fungal extract of Exserohilum rostrata, isolated from the leaf of Ocimum tenuiflorum. The biogenically formed AgNPs with a mean size of 16.2 nm used secondary metabolite such as polyphenol that is present in the extract for reducing and stabilizing (capping) agent. Synthesized AgNPs enhanced various biomedical applications like antimicrobial, antioxidants, anti-inflammatory, and anticancer activity. The synthesis of ZnONPs using endophytic fungal strain Alternaria tenuissima was described for the first time because ZnONPs had high absorption rate, lower toxicity, good bioavailability, and biocompatibility compared to their conventional Zinc sources. The synthesized NPs were characterized by various techniques like UV spectroscopy, XRD, TEM and FTIR, zeta potential. Fabricated NPs showed UV absorbance at 369 nm and zeta potential value was -23.92 mV which means the synthesized NPs have high stability. ZnONPs were evaluated for the antioxidant activity by 2,2′-diphenyle picrylhydrazyl radical scavenging test and photocatalytic potential for the degradation of methylene blue (Abdelhakim et al., 2019). Apart from this, Tyagi et al. (2019) and Clarance et al. (2020) also discussed about biosynthesis of metal NPs from fungal strains and the details of their findings is documented in Table 3.
NP synthesis from yeast
Single-celled eukaryotic microbes, yeasts are best model for study of microbial synthesis of metal NPs. About 1,500 different yeast species have been identified. Several studies have been describing well the successful fabrication of NPs using yeast. Due to easy, controlled, and rapid cultivation of yeast in laboratories, the easier mass production of NPs was achieved in numerous investigations (Moghaddam et al., 2015; Yurkov et al., 2011).
The Baker’s yeast extract based AgNPs were utilized for their antimicrobial potential in a report. Synthesized AgNPs were found to be 13.8 nm in size, well dispersed and very stable because reductive biomolecules of yeast extract such as amino acids, alpha-linolenic acid, and carbohydrates were found over the surface of AgNPs. The net charge on the NPs surface was negative, triggering the electrostatic repulsions along with interactions in an alkaline solution (Shu et al., 2020). In another investigation, a unique magnetic mesoporous Fe3O4 structure have been synthesized by Zhou et al. (2009a) using yeast cells via co-precipitation method. The mean particles size of Fe3O4 calculated by TEM was 17.4 nm. FTIR analysis reveals the peak around 3,376 cm−1 relates to the OH group and at 1,643 and 1,538 cm−1 are determine to amide I and amide II, the characteristic infra red absorption of protein which could be one of the important components of yeast cells. Obtained bands at 636 and 561 cm−1 are contributed to the high crystallinity of Fe3O4 NPs. Semiconductors quantum crystallite are of specific interest in solid state physics so for this aim, Candida glabrata and Schizosaccharomyces pombe, were utilized by Dameron et al. (1989) for fabrication of CdS quantum crystallite structure with a size of 20 Å. In this context, fabrication of an ideal diode attempt by Kowshik et al. (2002) biosynthesized CdS NPs using S. pomb species of yeast. Synthesized CdSNPs were 1–1.5 nm in diameter.
In an investigation, extremophilic yeast isolated and purified from Portuguese acid mine drainage was used for the synthesis of Ag and AuNPs. Bio-fabricated AgNPs and AuNPs were found to be in 20 nm and 30–100 nm in size, respectively. The open circuit potential data illustrates well about the role of yeast cell wall in the biosynthesis of metal NPs (Mourato et al., 2011). In addition, a simple precipitation method is used for the biosynthesis of Iron phosphate nanopowders with instant yeast. A different characterization approach confirmed that pure and homogenous FePO4 crystals were synthesized at 543°C (Zhou et al., 2009b).
NP synthesis from protozoans
Conventional methods of NPs’ synthesis may affect environment, so researchers are examining different biological systems with exceptional biocompatibility for fabrication of nanocomposites. In the way of biological synthesis of NPs, many microbes have been tested but the ability of protozoa has not been extensively explored. Being the source and owing to the animal-like characters, the protozoa can yield better biocompatible NPs (Khan, 2022). Few studies are reported about the capability of protozoa in synthesizing metallic NPs. For instance, bio-fabrication of conventional Au and AgNPs was also done using Leishmania sp. (Ramezani et al., 2012). Results that obtained from the characterization of Ag and AuNPs are discussed in Table 3. In addition, extracellular and intracellular synthesis of SeNPs was done using a protozoan sp. Tetrahymena thermophila (Cui et al., 2016). They explain the importance of glutathione in reduction of metal to form SeNPS. Currently, John et al. (2020) has explored psychrophilic protozoon E. focardii associated Pseudomonas as source of Ag NPs’ synthesis. Biosynthetically fabricated AgNPs were compared with chemically synthesized AgNPs. Antimicrobial activity results revealed that biologically synthesized AgNPs were more effective against pathogenic microorganisms. CdS1-XSeX Quantum dots bio-fabrication using Tetrahymena pyriformis (an aquatic protozoa) reported by Cui et al. (2019).
APPLICATIONS OF MICROBE-BASED NPS
In recent years, the nanotechnology community has moved towards a more comprehensive integration of biotechnology, biomedical engineering, agriculture sector, food sector, environmental remediation, pharmaceutical industry, etc. Nanomaterials are at the center of academic and industrial attention owing to their numerous potential applications (Fig. 3). Synthesis of nanomaterials and control of their characteristics and properties have been explored for diverse applications such as antibacterial and antifungal (Arya et al., 2018), catalysis (Husain et al., 2019), photocatalytic activity (Keskin et al., 2016), cytotoxic activity (Hamida et al., 2020), and anticancer activity (Clarance et al., 2020).
Antimicrobial activity
The emerging resistance in bacteria to traditional antibacterial agents has increased the need to develop novel antibacterial agents against both Gram-positive and Gram-negative bacteria. Gram-negative bacteria are more sensitive to antibacterial NPs due to the thinner bacterial cell wall whereas Gram-positive bacteria are more resistance to various antibacterial agents due to the presence of peptidoglycan layer in the cell wall of Gram-positive bacteria. Gram-positive organisms are responsible for various pathogenic diseases require strong inhibitory agents for its control. In this context, biofabrication of AuNPs performed by Suganya et al. (2015). The antibacterial activity of protein conserved AuNPs has been tested against Gram-positive organisms Bacillus subtilis and S. aureus. The bactericidal activity of AuNPs against B. subtilis and S. aureus showed a maximum of 97.4% and 80.5% reduction in colonies with 150 μg/ml, respectively. In another research biosynthesized AgNPs using Pseudomonas strain showed a larger inhibition zone (Ø 19.0 mm) against E. coli and the smallest inhibition zone (Ø 14.0 mm) against Pseudomonas aeruginosa and Serratia marcescens. Among Gram-positive bacteria, the highest zone of 15 mm was formed against S. aureus (John et al., 2020). In recent research, Abdollahnia et al. (2020), synthesized Ag and SeNPS using archaebacterial and bacterial strains. The intracellular synthesis of AgNP was done from archaeal Haloferax sp. and extracellular synthesis of AgNP from bacterial Halomonas sp. Moreover, intracellular synthesis of SeNPS was done by archaeal Halogeometricum sp. and extracellular by bacterial Bacillus sp. Using these synthesized NPs, significant antibacterial activity was observed against different disease-causing bacterial strains. The minimum inhibitory concentration required to inhibit the growth of 50% of organisms (MIC50) of AgNP-A and Ag-NP-B were found to be 10–40 ppm while MIC50 of Se NP-A and Se- NP-B were 50–200 ppm against E. coli, S. aureus, P. aeruginosa, and B. subtilis.
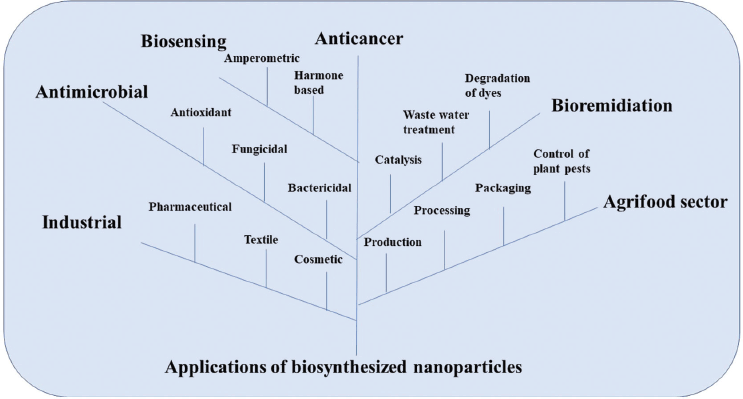 | Figure 3. Microbes based NPs showing their wide application in various fields such as pharmaceutical, agriculture, industrial and allied sectors. [Click here to view] |
Due to well-described antimicrobial mechanism against pathogenic bacteria, AgNPs have been proposed as alternative over traditional antibiotics to overcome bacterial resistance. Recently many researchers synthesized AgNPs from different sources, for example, Hamouda et al. (2019), synthesized AgNPs using cyanobacterium O. limnetica cell extract. Hamida et al. (2020) used an extract of Desertifilum IPPASV1220, a strain of cyanobacteria to obtain functionalized AgNPs. Shankar et al. (2020) used thermophilic mold S. thermophile BJTLRMDU7 for bio-fabrication of AgNPs. Bakhtiari-Sardari et al. (2020)obtained AgNPs from Streptomyces Psychrophiles. Beeler et al. (2020) biofabricated AgNPs form Pseudomonas (antibacterial mechanism). A plant pathogen Lasiodiplodia theobromae was also used by Ranjani et al. (2020) for the biosynthesis of AgNPs.
Fungicidal activity
Currently, fungal infections have become a widespread ignitious public health issue because there are fewer options as antifungal drugs and fungi develop resistance against these drugs continuously. AgNPs have excellent fungicidal activity and therefore may be a new option for the treatment of infections caused by fungal strains. AgNPs were found to be the most active antifungal agents synthesized from different sources. In a previous investigation, the synthesis of AgNPs from Pseudomonas strain, which have been investigated for their antimicrobial activities at different concentrations against several pathogenic bacterial strain among C. albicans a fungal strain. Results showed the larger zone of inhibition against C. albicans was (Ø 15.0 mm) (John et al., 2020). In addition, ZnO NPs fabricated form endophytic fungus Alternaria tenuissima evaluated their effect against C. albicans ATCC 10231 and three different plant pathogenic fungi (Alternaria solani, Aspergillus niger and Fusarium oxysporum). Inhibition zone of C. albicans was 01.87 + 0.06 mm at a minimum concentration of 50 µg ml−1ZnONPs. The results clearly indicate that the biosynthesized ZnONPs have formidable antifungal potential against fungal species (Abdelhakim et al., 2019). In one of the investigations, CuNPs were biosynthesized by Hassan et al. (2018) utilizing endophytic bacterium isolated from a healthy and very useful medicinal plant C. arvensis (L.). The application of these synthesized CuNPs represented a very good antimicrobial activity against disease-causing microbes, phytopathogenic fungal strains and can be used as insecticides too. This can be a future replacement for plant-threatening pesticides and other hazardous chemicals too.
The green synthesis of AuNPs using green algae has also been investigated for their antimicrobial activity and fewer studies have been reported yet. In a recent study, AuNPs and AgNPs were synthesized using extracellular cell extract of microalga Neodesmus pupukensis. Synthesized NPs were subjected to observe their antifungal activity. Evaluation showed the antifungal potency of AuNPs with mycelial inhibition of 79.4%, 44.3%, 75.4%, 54.9%, and 66.4% against A. niger, Aspergillus fumigatus, Aspergillus flavus, Fusarium solani and C. albicans, respectively, while AgNPs had 80.6%, 57.1%, 79.4%, 65.4%, and 69.8% against A. niger, A. fumigatus, A. flavus, F. solani, and C. albicans respectively (Omomowo et al., 2020).
Anti-cancerous activity
The development of nano-biotechnology is leading to the application of NPs in cancer therapy and targeted drug delivery (anticancer drugs). Several studies on anti-cancer activities of green synthesized NPs have been reported in recent decades. To study the anticancer activity of NPs, MTT colorimetric assay is most widely used. MTT access cell proliferation and viability for cultured eukaryotic cells based on the reduction of colorless tetrazolium salts to strongly colored formazans, which are then quantified by absorbance. Reduction of tetrazolium salts based on the activity of NAD(P)H coenzyme and dehydrogenases from metabolically active cells. In a recent research first time reported the anticancer activity of AgNPs obtained using Desertifilum a novel cyanobacterial strain. The cytotoxicity of AgNPs was observed against CaCo-2, MCF-7, and HepG2 cell lines by MTT assay. The result of the observations concluded that biosynthesized AgNPs significantly reduced the growth of above used cancer cell lines. Study revealed the dose-dependent reduction in the proliferation of the cancer cell lines. The half-maximal inhibitory concentration (IC50) values of AgNPs against MCF-7, HepG2, and Caco-2 cells were 58, 32, and 90 μg/ml, respectively (Hamida et al., 2020). In a similar way cytotoxicity of biogenic AgNPs’ synthesized by Nostoc sp. was screened against Caco-2 cells. The observations showed that biogenic AgNPs have significant dose-dependent cytotoxic activity against human colon cancer cells with an IC50 of 150 μg/ml (Bin-Meferij and Hamida, 2019). In addition, aqueous extract of O. limnetica, a cyanobacterial strain was used for the synthesis of AgNPs (3–18 nm). Bio-fabricated AgNPs showed significant anti-cancerous activity against both human breast (MCF-7) cell line and human colon cancer (HCT-116) cell line. The result of cytotoxic assay revealed IC50 6.147 μg/ml for MCF-7 human breast cell line and IC50 5.369 μg/ml for human colon cancer cell line HCT -116 (Hamouda et al., 2019). Apart from these, there has been much important research in which the anticancer potential of NPs has been tested, for example, Balaraman et al. (2020) evaluated cytotoxic potential of AgNPs fabricated form Sargassum myriocystum against the HeLa cell line, AgNPs anticancer capability against breast cancer MCF-7 cell line was tested by Gopu et al. (2020), AuNPs manufactured utilizing endophytic fungal strain F. solani ATLOY – 8. AuNPs showed excellent anticancer efficiency against human breast cancer cells (MCF-7) and cervical cancer cells (He La) (Clarance et al., 2020).
Nano-bioremediation
NPs offer a great potential for the remediation of various contaminants in the environment, because of their high surface area and enhanced reactivity. Significantly, the small size and associated capacity for subsurface transport provide opportunities for in situ treatment of contaminated sites (Seqqat et al., 2019). For the past two decades, researchers have attempted to produce, characterize, and apply various nanomaterials to treat organic and inorganic contaminants in water and soil. For example, Husain et al. (2019), has attempted the green and economic synthesis of AgNPs using cyanobacteria (Microchaete NCCU-342) for determination of decolorization of azo dye (MR). In this comparative study, dye decolorization potential of the synthesized AgNPs was evaluated in contrast with cell extract of Microchaete against azo dye MR. The decolorization rate of MR (50 mg/l) within 2 hours was 84.60% with AgNPs (1 μg/ml) and 49.80% from the extract (80 μg/ml), respectively. While no change in color of control (50 mg/l MR and 1 mM AgNO3). Methylene blue (azo dye) quite toxic for environment due to its high scale use and untreated discharge in water bodies. To reduce its toxicity and its contaminating effect on humans through polluted water, researchers have tried to find different ways and material ability to reduction or decolorization of dye. In this context, Keskin et al. (2016) studied the dye decolorization ability of the biosynthesized AgNPs from cyanobacterial strains against organic dye methylene blue. Results revealed approximately 18% degradations of methylene blue within 4 hours another azo dye is methyl orange also have an accountable toxic effect on environment so for the reduction of methyl orange an appreciable work done by Selvam and Sivakumar, (2015). In this research, natural light was used for catalysis and this was its special feature. Researcher used red alga, H. musciformis for the extracellular synthesis of AgNPs. The author concluded that biogenic AgNPs were able to degrade dyes in the presence of visible light.
Similarly nondegradable Congo red (an azo dye), is an organic compound, of the sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl) bis (4-aminonaphthalene-1-sulfonic acid) is a major pollutant. It is used world widely as colorant in numerous areas. It can cause cancer as well as gene mutation so remediation of congo red becomes very important. To the consideration of congo red reduction green synthesis of AgNPs using green algae (C. serrulata) has attempted. Observations showed that synthesized AgNPs were stable and have great catalytic activity against congo red (Aboelfetoh et al., 2017). 4-Nitrophenol is an organic compound used in many industrial processes. Its extensive use makes 4-NP a common pollutant in soil and surface and groundwater. It has serious environmental impacts due to its toxicity and mutagenic potential to humans and other living organisms (Serrà et al., 2020). In this regard, an eco-friendly method employed for the synthesis of AgNPs by a thermophilic mold S. thermophile. Further, catalytic activities of the synthesized AgNPs have been explored. AgNPs successfully reduced p-nitrophenol to p-aminophenol. Reduction of 4-nitrophenol as a viable alternative (Shankar et al., 2020).
Biosensing
In the field of diagnosis, the biosensors based on nanomaterials are on priority due to low cost and rapid outcome along with better selectivity and sensitivity. Researchers are trying to design new nanomaterial-based biosensors, which can enhance the potential of field-deployable microfabricated devices (Su et al., 2017).
In one of the recent investigations, the colorimetric sensing of hydrogen peroxide for AgNPs (synthesized using Noctiluca scintillans) was evaluated and that is used as an antiseptic used in various dermal infections. In this result, the decomposition of hydrogen peroxide on AgNPs was observed in which this was concluded that catalytic surface was temperature, time, and pH-dependent (Elgamouz et al., 2020). In addition, Platinum NPs synthesized from S. myriocystumare were utilized as a biosensor in the detection of adrenaline level in the body. Adrenaline-based drug is generally used to treat asthma, heart attacks, and allergies (Sharma et al., 2019). In another study, amperometric biosensor fabricated for the laccase- and alcohol oxidase based on lead NPs were synthesized using extracellular extract of thermotolerant yeast Ogataea polymorpha. Out of several green NPs synthesized in the study, PdNPs were found to be most effective and were further utilized in the construction of alcohol oxidase and laccase-based amperometric biosensors. Therefore, it appears that gPdNPs might be a promising candidate with better efficiency, efficacy, and affinities to their substrates (Gayda et al., 2019).
The slow exposure of heavy metals discharged into the aquatic environment can cause deteriorated conditions. Therefore, remediation of heavy metals has become very crucial, so researchers are trying to develop selective and sensitive biosensors for heavy metals monitoring. In this context, the efficiency of FeNPs was analyzed for the reduction in the amount of Cr(VI), a toxic environmental pollutant (Prema et al., 2022). Moreover, FeNPs were synthesized using microalga, Chlorococcum sp. MM11 and results showed that nano-iron was found to be more efficient in removing toxic Cr(VI) than bulk iron (Subramaniyam et al., 2015).
Agrifood sector
The agricultural sector is currently facing many problems to provide food without the influence the environment with the increasing population due to the excessive and continuous release of chemical fertilizers and pesticides, accumulation of toxic substances, and deficiency of water holding capacity of the soil, resulting decrease of soil fertility, on the other hand, plant diseases of agricultural farms are the major biotic constraints which eventually affect crop production and food shortages. The contamination of microbes and food spoilage are major problems in food packaging that directly affect public health due to food-borne diseases (Mustapha et al., 2022). Therefore, there is a need to change traditional methods of food production, processing, and replace them with the latest technologies like green nanotechnology (Bahrulolum et al., 2021). In this direction, Ahmed et al. (2020) designed bioactive silver from natively isolated Bacillus cereus strain SZT1 to control bacterial leaf blight disease of rice that is mainly caused by Xanthomonas oryzae. To control this pathogen, antibacterial assay showed that biosynthesized AgNPs can inhibit bacterial growth to a great extent. The inhibitory effect of AgNPs was found to be dose-dependent and the largest antibacterial activity was observed in AgNPs suspension of 20 μg ml−1, which gives a maximum zone of inhibition (25.11 ± 0.35 mm). Further, the pot experiment explained that AgNPs were found to be effective weapons for bacterial leaf blight disease by enhancing the plant biomass and by decreasing the concentration of ROS inside the cells along with a significant increase in the antioxidant enzyme activity. Additionally, Amin (2020) synthesized AgNPs as a safe and cost-effective pesticide using Ulva lactuca, a green alga. Biosynthesized AgNPs were effective against different microbes causing diseases in agricultural crops. Besides this, Cryptococcus laurentii and Rhodotorula glutinis, two yeast strains were also used for biogenic AgNPs by Fernández et al. (2016). The antifungal activity of the developed AgNPs was evaluated on the phytopathogenic fungi such as B. cinerea, P. expansum, A. niger, Alternaria sp. and Rhizopus sp., the common producers of postharvest diseases in pome fruits. This was observed that biofabricated NPs were more effective in controlling and diminishing the growth of pathogenic fungi than the chemically synthesized NPs.
Drug delivery
Microbe-assisted NPs’ synthesis and their application in drug delivery and biomedical sector have drawn attention worldwide. Nanomaterial fabricated using microbes has higher toxic metal tolerance and therefore provides a suitable application in the field of biomineralization and bioremediation. Moreover, application revealed the disease diagnosis and a better therapeutic agent. Numerous primary and secondary metabolites like enzymes, organic acids, flavonoids, polysaccharides, and amino acids are produced because of microbial growth, that facilitate the fabrication of nanomaterials as a capping/reducing agent. Due to the above-mentioned properties, microbe-mediated eco-friendly green synthesis of NPs may pave the way for biocompatible and bioavailable effective sources of drug delivery system in pharmaceutical sectors (Sachin and Karn, 2021). Another research describes that metal NPs are enhancing the drug index by limiting multidrug resistance through site specificity and efficient delivery of therapeutic agents. Moreover, metal NPs are better utilized in the development of diagnostics and the production of better nutraceuticals and other improved biocompatible materials. The green synthesis of metal NPs is used in increasing the half-life and stability of drug carrier in circulation, better drug targeting into the suitable target site as well as an increase in required biodistribution (Chandrakala et al., 2022). Several previous investigations describe the use of metal NPs in regenerative medicine and tissue engineering also. The replacements of organs and functional tissues require a higher control over biological consequences and that requires delivery of bioactive agents like growth factors, cytokines, inhibitors, and chemokines. NP-based systems facilitate the controlled delivery of bioactive agents and growth factors (Fathi-Achachelouei et al., 2019).
As researchers, we cannot ignore the toxic effects of metal NPs in the environment either synthesized by a chemical or biological method. There is bioaccumulation and release of numerous NPs in the environment that leads to toxic effects on humans, animals, other communities, and their surroundings. Therefore, it is very crucial and important to study the interaction of different types of metal NPs with biological systems and their level of toxicity for human and animal health. A recent investigation illuminates this issue in the name of nanotoxicology (Sarkar et al., 2014). As for as the level of toxicity is concerned, the green synthesized metal NPs has a significantly lower adverse effect on the environment.
The excessive synthesis and enhanced production of metal/non-metal-based NPs results in higher exposure to the environment and humans that ultimately leads to increase in metal toxicity. This was observed that NPs are being accumulated in different body parts such as liver, heart, kidney, and spleen due to inhalation, skin contact, and indirect ingestion. This was also investigated that NP exposure induces the release of ROS as free radicals, causing symptoms like inflammation, oxidative stress, and damage to protein, DNA, and cell membranes (Sengul and Asmatulu, 2020). In another investigation the impact of NP exposure was obtained in terms of loss of tubular architecture, degenerative changes in the glomeruli, interrupted tubular basal laminae, and loss of brush border too after histopathological examination (Nosrati et al., 2021). This review therefore illuminates the issue of application and exposure of NPs also either synthesized by green synthesis or chemical method.
CONCLUSION
The purpose of this review is not only a compilation of investigations and results but also insights into the efficiency and execution of biosynthesis of NPs from microbial cells, biomass, cell extract, supernatant, and other associated microbial parts. For example, a very restricted or no report is associated with the NP biosynthesis from cyanobacterial media supernatant. Moreover, NPs are being used extensively in every field, so it is very necessary to develop a method for NP synthesis which has minimum impact on environment and organisms. In this context, the green method of NP synthesis is proving to be very useful as no harmful chemicals are used as stabilizing and reducing agents in this method. The synthesis of microorganisms NPs is being explored by many researchers under green synthesis. Several research works have shown that prokaryotic and eukaryotic microorganisms are better choices for manufacturing nanomaterials in place of chemical or physical synthesis. In this review, we have attempted to enlighten several research works related to the synthesis of microbe-based NPs and their applications in various fields along with the future necessity. This review may be helpful to peer researchers who have an interest in green synthesis of NP or wish to conduct research into the applicability of NPs. There are many species of prokaryotic and eukaryotic microorganisms such as acidophiles, alkaliphiles, firmicutes, helminths, and protozoans, which have not received much work in the field of NP synthesis, so there is great potential to explore them. At the same time, there are many aspects like pH, temperature, concentration of reactants, and extensive analysis of capping agents that need to be studied with elaborations. Synthesis of AgNPs by green root has been studied repeatedly but the study of other metal NPs is also important in favor of environment and human health.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelhakim HK, El-Sayed ER, Rashidi FB. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J Appl Microbiol, 2019; 128:1634–46.
Abdollahnia M, Makhdoumi A, Mashreghi M, Eshghi H. Exploring the potentials of halophilic prokaryotes from a solar saltern for synthesizing nanoparticles: the case of silver and selenium. PLOS ONE, 2020; 15(3):e0229886.
Aboelfetoh EF, El-Shenody RA, Ghobara MM. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess, 2017; 189:349.
Ahmed T, Shahid M, Noman M, Niazi MBK, Mahmood F, Manzoor I, Zhang Y, Li B, Yang Y, Yan C, Chen J. Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens, 2020; 9(3):160.
Amin HH. Biosynthesized silver nanoparticles using Ulva lactuca as a safe synthetic pesticide (In vitro). Open Agric, 2020; 5:291–9.
Arya A, Gupta K, Chundawat TS, Vaya D. Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg Chem Appl, 2018; 2018:9; https://doi.org/10.1155/2018/7879403
Azizi S, Ahmad MB, Namvar F, Mohamad R. Green biosynthesis and characterization of zincoxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett, 2014; 116:275–7.
Bagur H, Poojari CC, Melappa G, Rangappa R, Chandrasekhar N, Somu P. Biogenically synthesized silver nanoparticles using endophyte fungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J Clust Sci, 2019; 31:1241–55.
Bahrulolum H, Nooraei S, Javanshir N, Tarrahimofrad H, Mirbagheri VS, Easton AJ, Ahmadian G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J Nanobiotechnol, 2021; 19:86; https://doi.org/10.1186/s12951-021-00834-3.
Bakhtiari-Sardari A, Mashreghi M, Eshghi H, Behnam-Rasouli F, Lashani E, Shahnavaz B. Comparative evaluation of silver nanoparticles biosynthesis by two cold-tolerant Streptomyces strains and their biological activities. Biotechnol Lett, 2020; 42(10):1985–99.
Balaraman P, Balasubramanian B, Kaliannan D, Durai M, Kamyab H, Park S, Chelliapan S, Lee CT, Maluventhen V, Maruthupandian A. Phyco-synthesis of silver nanoparticles mediated from marine slgae Sargassum myriocystum and its potential biological and environmental applications. Waste Biomass Valorization, 2020; 11:5255–71.
Bedlovi?ová Z. Green synthesis of silver nanoparticles using actinomycetes, nanobiotechnology for plant protection. In: Abd-Elsalam K (ed.). Green synthesis of silver nanomaterials, 1st edition, pp 547–69, 2022.
Beeler E, Choy N, Franks J, Mulcahy F, Singh OV. Extracellular synthesis and characterization of silver nanoparticles from alkaliphilic Pseudomonas sp. J Nanosci Nanotechnol, 2020; 20:1567–77.
Bin-Meferij MM, Hamida RS. Biofabrication and antitumor activity of silver nanoparticles utilizing novel Nostoc sp. Bahar M. Int J Nanomed, 2019; 14:9019–29.
Brayner R, Yepremian C, Djediat C, Coradin T, Herbst F, Livage J, Fievet F, Coute A. Photosynthetic microorganism-mediated synthesis of akaganeite (β-FeOOH) nanorods. Langmuir, 2009; 25(17):10062–67.
Bruna N, Collao B, Tello A, Caravantes P, Díaz-Silva N, Monrás JP, Órdenes-Aenishanslins N, Flores M, Espinoza-Gonzalez R, Bravo D, Pérez-Donoso JM. Synthesis of salt-stable fluorescent nanoparticles (quantum dots) by polyextremophile halophilic bacteria. Sci Rep, 2019; 9:1953.
Cao G. Nanostructures and nanomaterials. synthesis, properties, and applications. Imperial College Press, London, UK, 2004; doi: 10.1142/9781860945960.
Cao G, Wang Y. Nanostructures and nanomaterials-synthesis, properties, and applications. Imperial College Press, London, UK, 2011.
Castro L, Blázquez ML, Muñoz JA, González F, Ballester A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol, 2013; 7(3):109–16
Cele T. Preparation of nanoparticles. In: Avramescu SM, Akhtar K, Fierascu I, Khan SB, Ali F, Asiri AM (eds.). Engineered nanomaterials, Intech Open, 2020; DOI: http://dx.doi.org/10.5772/intechopen.90771.
Cepoi L, Rudi L, Chiriac T, Valuta A, Zinicovscaia I, Duca G, Kirkesali E, Frontasyeva M, Culicov O, Pavlov S, Bobrikov I. Biochemical changes in cyanobacteria during the synthesis of silver nanoparticles. Can J Microbiol, 2014; 61:13–21.
Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emerg Mater, 2022; 5:1593–615; https://doi.org/10.1007/s42247-021-00335-x
Charinpanitkul T, Faungnawakij K, Tanthapanichakoon W. Review of recent research on nanoparticle production in Thailand. Adv Powder Technol, 2008; 19:443–57.
Chaudhary R, Nawaz K, Khan AK, Hano C, Abbasi BH, Anjum S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules, 2020; 10:1498; doi:10.3390/biom10111498.
Clarance P, Luvankar B, Sales J, Khusro A, Agastian P, Tack JC, Khulaifi MMA, AL-Shwaiman HA, Elgorban AM, Syed A, Kim HJ. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer andbiomedical applications. Saudi J Biol Sci, 2020; 27:706–12.
Correa-Llantén DN, Muñoz-Ibacache SA, Castro ME, Muñoz PA, Blamey JM. Gold nanoparticles synthesized by Geobacillus sp. strain ID17 a thermophilic bacterium isolated from Deception Island, Antarctica. Microb Cell Factories, 2013; 12:75.
Cui YH, Li LL, Tian LJ, Zhou NQ, Liu DF, Lam PKS. Synthesis of Cd1-XSeX quantum dots in a protozoa Tetrahymena pytiformis. Environ Biotechnol, 2019; 103:973–80; doi: 10.1007/s00253-018-9499-y
Cui YH, Li LL, Zhou NQ, Liu JH, Huang Q, Wang HJ. In vivo synthesis of nano-selenium by Tetrahymena thermophila SB210. Enzym Microb Technol, 2016; 95:185–91; doi: 10.1016/j. enzmictec.2016.08.017
Dameron CT, Reese RN, Mehra RK, Kortan AR, Carroll PJ, Steigerwald ML, Brus LE, Winge DR. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature, 1989; 338:596–7.
de Duve C. The birth of complex cells. Sci Am, 1996; 274(4):50–7; doi: 10.1038/scientificamerican0496-50.
Elgamouz A, Idriss H, Nassab C, Bihi A, Bajou K, Hasan K, Abu Haija M, Patole SP. Green synthesis, characterization, antimicrobial, anti-cancer, and optimization of colorimetric sensing of hydrogen peroxide of algae extract capped silver nanoparticles. Nanomaterials, 2020; 10:1861.
El-Naggar NEA, Hussein MH, El-Sawah AA. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci Rep, 2017; 7:10844.
Eltarahony M, Zaki S, Abd-El-Haleem D. Concurrent synthesis of zero- and one-dimensional, spherical, rod-, needle-, and wire-shaped CuO nanoparticles by Proteus mirabilis 10B. J Nanomater, 2018; 2018:14; https://doi.org/10.1155/2018/1849616.
Erasmus M, Cason ED, Marwijk JV, Botes E, Gericke M, Heerden EV. Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull, 2014; 47:245–53.
Fathi-Achachelouei M, Knopf-Marques H, Ribeiro da Silva CE, Barthès J, Bat E, Tezcaner A, Vrana NE. Use of nanoparticles in tissue engineering and regenerative medicine. Front Bioeng Biotechnol, 2019; 7:113; doi: 10.3389/fbioe.2019.00113
Fayaz MA, Balaji K, Girilal M, Kalaichelvan PT, Venkatesan R. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J Agric Food Chem, 2009; 57(14):6246–52.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria. Nanomed Nanotechnol Biol Med, 2010; 6:103–9.
Fayaz AM, Girilal M, Rahman M, Venkatesan R, Kalaichelvan PT. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochem, 2011; 46:1958–62.
Fernández JG, Fernández-Baldo MA, Berni E, Camí G, Durán N, Raba J, Sanz MI. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem, 2016; 51:1306–13.
Gayda GZ, Demkiv OM, Stasyuk NY, Serkiz RY, Lootsik MD, Errachid A, Gonchar MV, Nisnevitch M. Metallic nanoparticles obtained via “green” synthesis as a platform for biosensor construction. Appl Sci, 2019; 9:720.
Gopu M, Kumar P, Selvankumar T, Senthilkumar B, Sudhakar C, Govarthanan M, Kumar RS, Selvam K. Green biomimetic silver nanoparticles utilizing the red algae Amphiroarigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioprocess Biosyst Eng, 2020; 44(2):217–23.
Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape control in gold nanoparticle synthesis. Chem Soc Rev, 2008; 37(9):1783–91.
Gudikandula K, Vadapally P, SingaraCharya MA. Biogenic synthesis of silver nanoparticles from white rot fungi: their characterization and antibacterial studies. OpenNano, 2017; 2:64–78.
Guilger-Casagrande M, Lima R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol, 2019; 7:287.
Hamida RS, Abdelmeguid NE, Ali MA, Bin-Meferij MM, Khalil MI. Synthesis of silver nanoparticles using a novel cyanobacteria desertifilum sp. extract: their antibacterial and cytotoxicity effects. Int J Nanomed, 2020; 15:49–63.
Hamouda RA, Hussein MH, Abo-elmagd RA, Bawazir SS. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep, 2019; 9:13071.
Hassan SELD, Salem SS, Fouda A, Awad MA, Mamdouh SEG, Abdullah MA. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Radiat Res Appl Sci, 2018; 11:262e270.
Husain S, Afreen S, Hemlata, Yasin D, Afzal B, Fatma T. Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J Microbiol Methods, 2019; 162:77–82.
Ibrahim E, Fouad H, Zhang M, Zhang Y, Qiu W, Yan C, Li B, Moc J, Chen J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv, 2019; 9:29293–9.
Iqtedar M, Aslam M, Akhyar M, Shehzaad A, Abdullah R, Kaleem A. Extracellular biosynthesis, characterization, optimization of silver nanoparticles (AgNPs) using Bacillus mojavensis BTCB15 and its antimicrobial activity against multidrug resistant pathogens. Prep Biochem Biotechnol, 2019; 49(2):136–42.
Jha AK, Prasad K, Prasad K. A green low-cost biosynthesis of Sb2O3 nanoparticlesc. Biochem Eng J, 2009; 43:303–6.
John MS, Nagoth JA, Ramasamy KP, Mancini A, Giuli G, Natalello A, Ballarini P, Miceli C, Pucciarelli S. Synthesis of bioactive silver nanoparticles by a Pseudomonas strain associated with the antarctic psychrophilic protozoon Euplotes focardii. Mar Drugs, 2020; 18:38.
Kalabegishvili T, Murusidze I, Kirkesali E, Rcheulishvili A, Ginturi E, Kuchava N, Bagdavadze N, Gelagutashvili E, Frontasyeva MV, Zinicovscaia I, Pavlov SS, Dmitriev AY. Gold and silver nanoparticles in Spirulina platensis: biomass for medical application. Ecol Chem Eng S, 2013; 20:621–31.
Kashefi K, Jason MT, Kelly PN, Derek RL. Reductive precipitation of gold by dissimilatory Fe(III)-reducing bacteria and archaea. Appl Environ Microbiol, 2001; 67(7):3275–9.
Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci, 2015; 499–504; DOI 10.1007/s13204-014-0341-2.
Keskin NOS, KoçberberK?l?ç N, Dönmez G, Tekinay T. Green synthesis of silver nanoparticles using cyanobacteria and evaluation of their photocatalytic and antimicrobial activity. J Nano Res, 2016; 40:120–7.
Khan YA. Protozoa: as emerging candidates for the synthesis of NPs. Microb Nanotechnol Green Synth Appl, 2022; 135–51; DOI:10.1007/978-981-16-1923-6_8.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem, 2017; https://doi.org/10.1016/j.arabjc..05.011.
Korbekandi H, Mohseni S, Jouneghani RM, Pourhossein M, Iravani S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol, 2014; 1–5; doi: 10.3109/21691401.2014.937870.
Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology, 2003; 14:95–100.
Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng, 2002; 78(5):583–8.
Kumari S, Tehri N, Gahlaut A, Hooda V. Actinomycetes mediated synthesis, characterization, and applications of metallic nanoparticles. Inorg Nano-Met Chem, 2020; 51(10):1386–95; https://doi.org/10.1080/24701556.2020.1835978.
Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I) – thiosulfate and gold (III) – chloride complexes. Langmuir, 2006a; 22:2780–7.
Lengke MF, Fleet ME, Southam G. Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV)-chloride complex. Langmuir, 2006b; 22:7318–23.
Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir, 2007; 23(5):2695.
Mahdavi M, Namvar F, Ahmad MB, Mohamad R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules, 2013; 18:5954–64.
Majeed S, Danish M, Ibrahim MNM, Sekeri SH, Ansari MT, Nanda A, Ahmad G. Bacteria mediated synthesis of iron oxide nanoparticles and their antibacterial, antioxidant, cytocompatibility properties. J Clust Sci, 2021; 32:1083–94.
Majumder A, Ramrakhiani A, Mukherjee D, Mishra U, Halder A, Mandal AK, Ghosh S. Green synthesis of iron oxide nanoparticles for arsenic remediation in water and sludge utilization. Clean Technol Environ Policy, 2019; 21:795–813.
Malhotra N, Lee JS, Liman RAD, Ruallo JMS, Villaflores OB, Ger TR, Hsiao CD. Potential toxicity of iron oxide magnetic nanoparticles: a review. Molecules, 2020; 25(14):3159; doi: 10.3390/molecules25143159.
Mayr E. Two empires or three? Proc Natl Acad Sci U S A, 1998; 95(17):9720–3; doi:10.1073/pnas.95.17.9720
Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta KC, Nautiyal CS. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour Technol, 2014; 166:235–42.
Moghaddam AB, Namvar F, Moniri M, Tahir PM, Azizi S, Mohamad R. Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules, 2015; 20:16540–65.
Molnár Z, Bódai V, Szakacs G, Erdélyi B, Fogarassy Z, Sáfrán G, Varga T, Kónya Z, Tóth-Szeles E, Sz?cs R, Lagzi I. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep, 2018; 8:3943.
Mourato A, Gadanho M, Lino AR, Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl, 2011; 2011:546074.
Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int J Environ Res Public Health, 2022; 19:674.
Naito M, Hosokawa M, Yokoyama T, Nogi K. Nanoparticle technology handbook. Elsevier, Amsterdam, The Netherlands, 2007.
Neethu S, Midhun SJ, Sunil M, Soumya S, Radhakrishnan E, Jyothis M. Efficient visible light induced synthesis of silver nanoparticles by Penicillium polonicum ARA 10 isolated from Chetomorpha antennina and its antibacterial efficacy against Salmonella enteric serovar typhimurium. J Photochem Photobiol B, 2018; 180:175–85.
Nosrati H, Hamzepoor M, Sohrabi M, Saidijam M, Assari MJ, Shabab N, Mahmoudian ZG, Alizadeh Z. The potential renal toxicity of silver nanoparticles after repeated oral exposure and its underlying mechanisms. BMC Nephrol, 2021; 22:228; https://doi.org/10.1186/s12882-021-02428-5
Omomowo I, Adenigba V, Ogunsona S, Adeyinka G, Oluyide O, Adedayo A, Fatukasi B. Antimicrobial and antioxidant activities of algal-mediated silver and gold nanoparticles. IOP Conf Ser Mater Sci Eng, 2020; 805:012010.
Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci, 2018; 19(12):4100.
Parial D, Patra HK, Dasgupta AKR, Pal R. Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol, 2012; 47:22–9.
Parikh RY, Singh S, Prasad BLV, Patole MS, Sastry M, Shouche YS. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: towards understanding biochemical synthesis mechanism. ChemBioChem, 2008; 9:1415–22.
Patel A, Enman J, Gulkova A, Guntoro PI, Dutkiewicz A, Ghorbani Y, Rova U, Christakopoulos P, Matsakas L. Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. Chemosphere, 2021; 263:128306.
Popli D, Anil V, Subramanyam AB, Rao SN, Rai RV, Govindappa M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif Cells Nanomed Biotechnol, 2018; 5:1–8.
Prema P, Nguyen VH, Venkatachalam K, Ali JMMHM, Salem MZM, Ravindran B, Balaji P. Hexavalent chromium removal from aqueous solutions using biogenic iron nanoparticles: kinetics and equilibrium study. Environ Res, 2022; 205:112477.
Rahman A, Ismail A, Jumbianti D, Magdalena S, Sudrajat H. Synthesis of copper oxide nanoparticles by using Phormidium cyanobacterium. Indo J Chem, 2009; 9(3):355–60.
Rahman S, Rahman L, Khalil AT, Ali N, Zia D, Ali M, Shinwari ZK. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl Microbiol Biotechnol, 2019; 103:2551–69.
Ramakrishna M, Babu DR, Gengan RM, Rao SCGN. Phytoremediation using wetland plants: an ecosustainable approach. Int J Phytoremed, 2016; 10:133–60.
Ramanathan R, Mullane AP, Parikh RY, Smooker PM, Bhargava SK, Bansal V. Bacterial kinetics-controlled shape-directed biosynthesis of silver nanoplates using Morganella psychrotolerans. Langmuir, 2011; 27(2):714–9.
Ramezani F, Jebali A, Kazemi B. A green approach for synthesis of gold and silver nanoparticles by Leishmania sp. Appl Biochem Biotechnol, 2012; 168:1549–55.
Ramírez-Rodríguez GB, Sasso GD, Carmona FJ, Miguel-Rojas C, Pe?rez-de-Luque A, Masciocchi N, Guagliardi A, Delgado-Lo?pez JM. Engineering biomimetic calcium phosphate nanoparticles: a green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl Bio Mater, 2020; 3:1344−53.
Rani R, Sharma D, Chaturvedi M, Yadav JP. Green synthesis, characterization and antibacterial activity of silver nanoparticles of endophytic fungi Aspergillus terreus. J Nanomed Nanotechnol, 2017; 8:1–8.
Ranjani S, Shariq Ahmed M, Mohd Adnan, Senthil Kumar N, Ruckmani K, Hemalatha S. Synthesis, characterization and applications of endophytic fungal nanoparticles. Inorg Nano-Met Chem, 2020; 51(2):280–7.
Roychoudhury P, Gopal PK, Paul S, Pal R. Cyanobacteria assisted biosynthesis of silver nanoparticles a potential antileukemic agent. J Appl Phycol, 2016; 28:3387–94.
Sachin K, Karn SK. Microbial fabricated nanosystems: applications in drug delivery and targeting. Front Chem, 2021; 9:617353; doi: 10.3389/fchem.2021.617353.
Salem S, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res, 2020; 199(1):344–70.
San Diego KDG, Alindayu JIA, Baculi QR. Biosynthesis of gold nanoparticles by bacteria from hyperalkaline spring and evaluation of their inhibitory activity against pyocyanin production. J Microbiol Biotech Food Sci, 2018; 8(2):781–7.
Sarkar A, Ghosh M, Sil PC. Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol, 2014; 14(1):730–3; https://doi.org/10.1166/jnn.2014.8752.
Saxena J, Sharmaa MM, Guptab S, Singh A. Emerging role of fungi in nanoparticle synthesis and their applications. World J Pharm Pharm Sci, 2014; 3(9):1586–613.
Selvam GG, Sivakumar K. Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) J.V. Lamouroux. App Nanosci, 2015; 5:617–22.
Selvarajan E, Srinivasan MV. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater Lett, 2013; 112:180–2.
Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett, 2020; 18:1659–83; https://doi.org/10.1007/s10311-020-01033-6.
Seqqat R, Blaney L, Quesada D, Kumar B, Cumbal L. Nanoparticles for environment, engineering, and nanomedicine. J Nanotechnol, 2019; 2019:2850723; https://doi.org/10.1155/2019/2850723
Serrà A, Artal R, Pozo M, Garcia-Amorós J, Gómez E. Simple environmentally-friendly reduction of 4-nitrophenol. Catalysts, 2020; 10:458.
Seshadri S, Prakash A, Kowshik M. Biosynthesis of silver nanoparticles by marinebacterium, Idiomarina sp. p R58–8. Bull Mater Sci, 2012; 35:1201–5.
Shah R, Oza G, Pandey S, Sharon M. Biogenic fabrication of gold nanoparticles using Halomonassalina. J Microbiol Biotechnol Res, 2012; 2:485–92.
Shankar A, Kumar V, Kaushik NK, Kumar A, Malik V, Singh D, Singh B. Sporotrichum thermophile culture extract-mediated greener synthesis of silver nanoparticles: eco-friendly functional group transformation and anti-bacterial study. Curr Res Green Sustain Chem, 2020; 3:100029.
Sharma D, Kanchi S, Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab J Chem, 2019; 12(8):3576–600.
Shebl A, Hassan AA, Salama DM, El-Aziz MEA, Abd Elwahed MSA. Green synthesis of nanofertilizers and their application as a Foliar for Cucurbita pepo L. J Nanomater, 2019; 4:1–11.
Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem, 2011; 46:1800–7.
Shu M, He F, Li Z, Zhu X, Ma Y, Zhou Z, Yang Z, Gao F, Zeng M. Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res Lett, 2020; 15:14.
Singh G, Babele PK, Kumar A, Srivastava A, Sinha RP, Tyagi MB. Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. J Photochem Photobiol B Biol, 2014; 138:55–62.
Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol, 2017: 15(1):31–9.
Sinha SN, Paul D, Halder N, Sengupta D, Patra SK. Green synthesis of silver nanoparticles using fresh water green alga Pithophoraoedogonia (Mont.). Appl Nanosci, 2015; 5:703–9.
Sk?adanowski M, Wypij M, Laskowski D, Golin´ska P, Dahm H, Rai M. Silver and gold nanoparticles synthesized from Streptomyces sp. isolated from acid forest soil with special reference to its antibacterial activity against pathogens. J Clust Sci, 2017; 28:59–79.
Srivastava P, Braganc J, Ramanan SR, Kowshik M. Synthesis of silver nanoparticles using haloarchaeal isolate Halococcussalifodinae BK3. Extremophiles, 2013; 17(5):821–31.
Stanier RY, van Niel CB. The concept of a bacterium. Arch Mikrobiol, 1962; 42:17–35.
Su H, Li S, Jin Y, Xian Z, Yang D, Zhou W, Mangaran F, Leung F, Sithamparanathan G, Kerman K. Nanomaterial-based biosensors for biological detections. Adv Health Care Technol, 2017; 3:19–29.
Subramaniam Ramachandran V, Radhakrishnan M, Balaraman Ravindrran M, Alagarsamy V, Palanisamy GS. Functionalized nanoparticles: a paradigm shift in regenerative endodontic procedures. Cureus, 2022; 14(12):e32678; doi:10.7759/cureus.32678.
Subramaniyam V, Ramraj S, Bose S, Thavamani P, Megharaj M, Chen Z, Naidu R. Chlorococcum sp. MM11- a novel phyco-nanofactory for the synthesis of iron NP. J Appl Phycol, 2015; 275:1861–9.
Suganya KU, Govindaraju K, Kumar VG, Dhas TS, Karthick V, Singaravelu G, Elanchezhiyan M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Grampositive organisms. Mater Sci Eng, 2015; 47:351–6.
Sunitha A, Geo S, Sukanya S, Praseetha PK, Dhanya RP. Biosynthesis of silver nanoparticles from actinomycetes for therapeutic applications. Int J Nano Dimens, 2014; 5:155–62.
Syed B, Prasad NM, Satish S. Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity. J Microsc Ultrastruct, 2016; 4:162–6.
Tag HM, Saddiq AA, Alkinani M, Hagagy N. Biosynthesis of silver nanoparticles using Haloferax sp. NRS1: image analysis, characterization, in vitro thrombolysis and cytotoxicity. AMB Expr, 2021; 11:75.
Tiquia-Arashiro S, Rodrigues D. Extremophiles: applications in nanotechnology. Springer International Publishing AG, Cham, Switzerland, 2016; DOI 10.1007/978-3-319-45215-9_1.
Tyagi S, Tyagi PK, Gola D, Chauhan N, Bharti RK. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: characterization and antibacterial potential. SN Appl Sci, 2019; 1:1545.
Usman AI, Aziz AA, Noqta OA. Green sonochemical synthesis of gold nanoparticles using palm oil leaves extracts. Mater Today Proc, 2019; 7:803–7.
Waghmare SS, Deshmukh AM, Sadowski Z. Biosynthesis, optimization, purification and characterization of gold nanoparticles. Afr J Microbiol Res, 2014; 8:138–46.
Wypij M, Czarnecka J, ?wiecimska M, Dahm H, Rai M, Golinska P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J Microbiol Biotechnol, 2018; 34:23.
Yamaguchi M, Mori Y, Kozuka Y, Okada H, Uematsu K, Tame A, Furukawa H, Maruyama T, Worman C, Yokoyama K. Prokaryote or eukaryote? A unique microorganism from the deep sea. J Electron Microsc, 2012; 61(6):423–31.
Yurkov AM, Kemler M, Begerow D. Species accumulation curves and incidence-based species richness estimators to appraise the diversity of cultivable yeasts from beech forest soils. PLoS ONE, 2011; 6(8):e23671.
Zhang X, Yan S, Tyagi RD, Surampalli RY. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere, 2011; 82(4):489–94.
Zhou W, He W, Zhang X, Yan S, Sun X, Tian X, Han X. Biosynthesis of iron phosphate nanopowders. Powder Technol, 2009b; 194:106–8.
Zhou W, He W, Zhong S, Wang Y, Zhao H, Li Z, Yan S. Biosynthesis and magnetic properties of mesoporous Fe3O4 composites. J Magn Magn Mat, 2009a; 321:1025–8.