INTRODUCTION
Communicable ailments are significant sources of morbidity and death universally, notably in developing countries, accounting for about 50% of diseases in countries with low healthcare facilities and as much as 20% of mortality rates in industrialized countries (Khalil et al., 2020; Motsumi et al., 2020; Mtunzi et al., 2017a; Ntshanka et al., 2020). Despite the high level of innovation and antibiotic application in microbiology, the intermittent occurrences of epidemics caused by drug-resistant microbes and the emergence of unknown disease-causing microorganisms pose a significant threat to healthcare (Abubakar, 2010; Nguedia and Shey, 2014; Silber et al., 2016).
The development of resistant strains to some antibiotics has complicated the management of infectious diseases, given that drugs are only effective against one-third of existing ailments (Fankam et al., 2015; Sahu et al., 2014). Microbial drug resistance became persistent because of drug abuse and misuse of antibiotics. Most drugs are ineffective against diseases for which they had previously been misused. This results in resistant pathogens becoming virulent, increasing the risk of complications and death (Fankam et al., 2015; Nguedia and Shey, 2014; Sampedro et al., 2009). Most drugs are discovered from biological natural resources, and natural yields from microbes have been the major cradle of antibiotic delivery. With the cumulative approval of herbal medicine as a substitute form of healthcare, the selection of herbal plants for bioactive constituents has become a very imperative aspect of the health system because they serve as a favorable cradle of innovative antibiotic exemplars (Khan et al., 2022; Sabo and Knezevic, 2019; Serralheiro et al., 2020). Antibiotics derived from fungi or living organisms are produced industrially using a fermentative process (Alfadil et al., 2014; Silber et al., 2016; Wright et al., 2014).
The predominance of other diseases like hematological and autoimmune disorders, human immunodeficiency virus (HIV) infection, cancer, and important immune system dysfunction may cause symbiotic microbes to change to pathogens under definite circumstances, typically called opportunistic contamination. Opportunistic pathogens consist of fungi, viruses, protozoa, and bacteria, taking advantage of immunocompromised patients and displaying new health challenges worldwide. Opportunistic diseases involve diminishing host defenses, occurring because of genetic deficiencies, introduction to antibiotics, and immunosuppressive substances or due to communicable diseases possessing immunosuppressive properties (Nagata et al., 2011; Ntshanka et al., 2020; Ryan and Ray, 2004; Yang et al., 2013).
Some chemotherapeutic mediators presently used are noxious with accompanying antagonistic side effects. Hence, there is a general necessity for novel chemotherapeutic mediators against several disease pathoaetiologies that are exceedingly resourceful, have low toxicity, and exhibit minor ecofriendly impacts. Herbal medications have various traditional claims, such as managing infectious sources. Several extracts of plant species were established against hundreds of microbial strains via various in vitro models and some had good action pharmacological consequences (Bhat, 2014; Fankam et al., 2015; Khumalo et al., 2018; Luís et al., 2016; Motsumi et al., 2020; Nguedia and Shey, 2014). However, a limited number of these herbal plant extracts have been screened in animal or human studies to regulate safety and efficiency. Natural products and their byproducts characterize about 50% of all drugs in clinical use (Cragg and Newman, 2013; Fankam et al., 2015; Khumalo et al., 2018; Lahlou, 2013).
Natural products from natural cradles like plants, animals, and microorganisms, dated before human antiquity, perhaps thousands of years (Ji et al., 2016; Khan, 2018). These products can be categorized into four diverse groups according to their biosynthetic derivation, polyketides, alkaloids, terpenoids, and phenylpropanoids (Bisht et al., 2021; Guo, 2017), and continue to offer novel chemical structures with high levels of biological activity (Guo, 2017; Khan, 2018; Moloney, 2016). The mechanisms underlying many biological properties have been ascribed to numerous types of propolis, including antitumor, anti-inflammatory, wound healing, antioxidant, antimicrobial, and immunomodulatory activities (Shaikh et al., 2016). Plants do produce potentially toxic substances aside production of beneficial phytochemicals; therefore, toxicity assays incorporation in the bioactivity evaluation of medicinal plants is very important in understanding their therapeutic effects (Alam et al., 2018; Araújo et al., 2013; Cundell, 2014; Luís et al., 2016; Ntshanka et al., 2020; Shah et al., 2010; Verma, 2016). The Chinese traditional medicine community is the world’s largest medicinal plant user is, with more than 5,000 plants and plant products registered in their pharmacopeia (Ji et al., 2016).
Traditional medicine in South Africa supports using abundant plant species for the treatment or management as prophylaxis against several kinds of ailment (infectious and noninfectious) (Masoko et al., 2010, 2012; Mtunzi et al., 2017a, 2017b; Street and Prinsloo, 2013). In South Africa, medicinal species are being traded for usage in local medicines since most are from ethnopharmacological guides (De Wet et al., 2013; Mabona and Van Vuuren, 2013; Street and Prinsloo, 2013). The sustainable use and control of medicinal plants are of a significant contest to all shareholders. Parts of many medicinal plants, like the stem, bark, and roots, are being harvested and merchandized in an unmanageable routine that may lead to the augmented death of the tree that is the source of medication. Assessment and authentication of leaf extract bioactivity as a promising substitute for stem, roots, and bark use to afford a viable opportunity for safeguarding medicinal plants (De Wet et al., 2013; Street and Prinsloo, 2013). Herbal medicine is the most significant medicine for most people on planet earth, specifically those who do not have access to modern and expensive drugs. Interestingly, it has formed the foundation of every medicine, the mother of all remedies in modern days. The exploitation of medicinal plants as herbal medicine alongside their curative perspective is well documented (Alam et al., 2018; Bhat, 2014; Cundell, 2014; Motsumi et al., 2020; Mtunzi et al., 2017a, 2017b; Sabo and Knezevic, 2019; Street and Prinsloo, 2013). The World Health Organization estimates that populace about 80% residing in developing nations exclusively practice traditional medicine (Eloff, 1998; Motsumi et al., 2020; Mtunzi et al., 2017a).
Medicinal plant therapies have also been featured conspicuously in the ailments treatment of production and domestic animals, and ethnoveterinary therapeutic practices remain an imperative aspect of animal healthcare in unindustrialized countries (Ji et al., 2016; Khan, 2018). Combretum species is featured conspicuously among the utilized medicinal plants in South African traditional medicine as agents for handling communicable diseases like diarrhea (Combretum imberbe Wawra, Combretum vendee A.E.van Wyk), malaria (Combretum ghasalense), stomach disorders (Combretum molle R. Br. ex G. Don.), and coughs [C. molle R. Br. ex G. Don., C. imberbe Wawra, Combretum erythrophyllum (Burch.) Sond.] (Eloff et al., 2008; Mtunzi et al., 2017b; Ntshanka et al., 2020). Combretum erythrophyllum is a member of the Combretaceae family, generally used for venereal disease management (Van Wyk and Gericke, 2000). Root parts are used as a laxative, while dried and pulverized gum is applied to blisters (Venter and Venter, 1996).
The roots and bark decoctions of C. erythrophyllum are utilized to treat cough and unproductiveness and as an aphrodisiac (Ahmed et al., 2014; Mtunzi et al., 2017b). The leaves are used to treat cough and stomach pains, while the seeds, which have been reported to be poisonous, are used to remove intestinal worms in dogs (Van Wyk et al., 2009). Combretum erythrophyllum is commonly scattered in the Southern Africa region, most commonly found in South Africa along the coast in the Eastern Province, namely Zimbabwe, KwaZulu-Natal, Northern South Africa (Mpumalanga, Gauteng, Limpopo, and the eastern parts of Northwest regions), Swaziland, and Mozambique, and marginally into the eastern parts of Botswana (Silén et al., 2023).
Martini et al. (2004a) isolated seven different flavonoids from leave extract C. erythrophyllum (Burch.) collected from a tree within the Pretoria National botanic gardens, South Africa, known to be antibacterial phenolic compounds which include four flavonols: rhamnocitrin (1), rhamnazin (2), 5,7,4′-trihydroxyflavonol (kaempferol) (3), and 7,4′-dihydroxy-5,3′-dimethoxyflavonol (quercetin-5,3′-dimethylether) (4); three flavones: 5,7,4′-trihydroxyfavone (5), 5,4′-dihydroxy-7-methoxyflavone (6), and 5-hydroxy-7,4′-dimethoxyflavone (7) (Fig. 1). All compounds possessed good activity against Enterococcus faecalis and Vibrio cholera, with the minimal inhibitory concentration (MIC) value <100 µg/ml. Rhamnocitrin and quercetin-5,3′-dimethyl ether inhibited Shigella sonnei and Micrococcus luteus with a MIC value of 25 µg/ml (Martini et al., 2004a, 2004b; Mawoza and Ndove, 2015).
In literature, medicinal plants have presented interesting ethnopharmacological potentials as chemotherapeutic agents. The Combretum species has great prospects for the management of various infectious diseases (Eloff et al., 2008) and will have a vital relevance with economic benefit to the perfumery industry (Alam et al., 2018; Barrales-Cureno et al., 2021; Crovadore et al., 2012; Mohaddese, 2016; Sabo and Knezevic, 2019). Nevertheless, the tangible potential of Combretum has not been exploited to the fullest. Hence, this review has made an effort to present a comprehensive overview of the summary of earlier research data regarding ethnopharmacological properties, antimicrobial, antifungal, antioxidant, cytotoxicity activities, and other noteworthy effects of Combretum species as alternative medicine.
METHODS AND LITERATURE QUEST
An epistemological paradigm grounded in a qualitative research approach was utilized for this study. The study seeks to explain, clarify, define, elucidate, and expand more on the understanding of the ethnopharmacological potentials of medicinal plants concerning Combretum species as chemotherapeutic agents for drug discovery.
Available reports and references on the medicinal plant species were accessed from published scientific peer-reviewed journals, books, short communications, reports from national, regional, and international organizations and institutions, theses, conference papers, and other materials. International online databases, including ISI Web of Science, SCOPUS, EBSCO, MEDLINE (National Library of Medicine), chemical abstracts service, Science Direct, SCIMAGO, ProQuest, EMBASE, and Google Scholar, were utilized for literature search using precise search terms. Selected keywords were used but not limited to Combretum species, ethnopharmacological promises, properties of the Combretum genus, phytochemicals, pharmacological, antibiotics, medicinal plants, biological assays, chemical constituents, chemotherapeutic agents, traditional medicine, and traditional uses of medicinal plants of about 600 studies and research articles consulted, articles from 1970 to 2022.
Snowball sampling technique was used in this study, followed by content and semantic analysis of data collected from the literature for systematic documentation of the biological, pharmacological, and traditional uses of medicinal plants: Combretum species around Southern Africa region as alternative medicine.
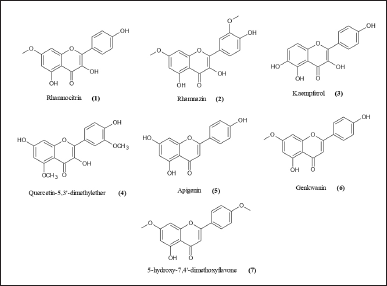 | Figure 1. Chemical structures of flavonoids found in Combretum erythrophyllum (Martini et al., 2004a). [Click here to view] |
RESULTS AND DISCUSSION
Combretaceae family
Combretaceae hosts more than 600 species (Komape et al., 2014; Zhang et al., 2020). Combretum is among the most frequently occurring genera of Combretaceae in tropical and subtropical areas of Africa and Asia. Due to their ethnopharmacological properties, some of these genera are widely used in African traditional medicine (Chukwujekwu and van Staden, 2016; Gumisiriza et al., 2021). The different fragments of the Combretum species are broadly used to treat numerous diseases (Ares et al., 2006; Eloff et al., 2008; Mtunzi et al., 2017b). The species of Combretum, generally known as the forest bushwillow tree (C. kraussii Hochst.), is medium to large in size and is found in the eastern part of South Africa, Swaziland, and Southern Mozambique (Chukwujekwu and van Staden, 2016; Zhang et al., 2020). Combretum kraussii Hochst. is often used as herbal medicine to treat eye infections and wounds and serves as a blood tonic and an appetite stimulant. It can also act as antiseptic and antidiuretic agent, (Chukwujekwu and van Staden, 2016; Quattrocchi, 2012).
Therapeutic potentials of Combretum species
Combretum species as an antioxidant agent
An important development that produces free radicals in living systems, substances, and even in food is referred to as oxidation (Barku et al., 2013). Oxidation is also the chemical reaction involving electron transfer from the electron-rich to the electron-deficient entity (Poljsak et al., 2021). The electron-scarce molecule is labeled an oxidizer or oxidizing agent. Enzymes such as hydroperoxidase and catalase translate hydroperoxides and hydrogen peroxide (H2O2) to nonradical forms and perform natural antioxidants’ role in the human body (Ofoedu et al., 2021). The prescribed oxidation state refers to the postulated charge an atom has if all bonds to other atoms of different elements are completely ionic. It is generally epitomized by integers that can either be zero, positive, or negative (Norman and Pringle, 2022). Free radicals are reactive species containing unpaired electron that attacks macromolecules, including protein, lipid, and DNA. Free radicals are the products of natural human metabolism. Varieties of endogenous free radicals destroying antioxidants exist in the body, while others are obtained from dietary sources like vegetables, fruits, and teas. At present, accessible synthetic antioxidants like gallic esters, butylated hydroxyl toluene, butylated hydroxyl anisole, and tertiary butylated hydroquinone are assumed to bring about or hasty negative health consequences (Mongalo et al., 2012).
Antioxidants preclude oxidative impairment of cells, biomolecules, and reactive oxidative species oxidative species (ROS)-induced illnesses by reacting with free radicals, destroying free radicals, and chelating free catalytic metals (Pizzino et al., 2017). Antioxidant consumption possesses numerous health benefits, including oxidative damage associated with free radical damage and its role in diseases (Ejidike et al., 2019). Antioxidant nutrients, either endogenous or exogenous, natural or synthetic, can search for free radicals in the system and neutralize them before they further damage the body cells (Mandal et al., 2022; Medrano-Macías et al., 2022; Poljsak et al., 2021). Antioxidants are important constituents in the human body that safeguards it from impairment caused by oxidative stress induced by free radicals (Ejidike and Ajibade, 2015; Poljsak et al., 2021). There is emerging interest in the exploration of the antioxidant activities of secondary metabolites from medicinal species to compounds with greater potency and lower toxicities than the presently accessible synthetic ones (Medrano-Macías et al., 2022; Motsumi et al., 2020; Mtunzi et al., 2017a; Ntshanka et al., 2020; Poljsak et al., 2021).
Recent epidemiological evaluations have revealed that many antioxidant compounds possess antibacterial, anticarcinogenic, anti-inflammatory, antiviral, antitumor, antiatherosclerotic, or antimutagenic activities to a bigger or smaller extent (Owen et al., 2000; Verma, 2016). The antioxidant perspective of natural plant products is attributable to several compounds such as phenols and flavonoids, which have a distinct mechanism of action. Consequently, one antioxidant compound was sequestered from C. erythrophyllum and is 5-hydroxy-7,4′-dimethoxyflavone but exhibited the weakest activity (Martini et al. 2004a, 2004b). Oxidative stress is the inequality between the generation and eradication of ROS or reactive nitrogen species (RNS) in support of ROS (Ejidike and Ajibade, 2015; Poljsak et al., 2021; Zhang et al., 2009). Oxidative stress is proficient in triggering cells to lose their function and structure and ultimately cause cell dysfunction. ROS/RNS can be produced within the airway epithelial cells in answer to proinflammatory cytokines like tumor necrosis factor-alpha (TNF-α) (Ejidike and Ajibade, 2015; Lü et al., 2010; Mandal et al., 2022; Medrano-Macías et al., 2022; Poljsak et al., 2021).
ROS and RNS perform various functions, including regulation of gene expression (Mandal et al., 2022) and stimulation of apoptosis (Huang and Zhou, 2021). The manufacture of ROS/RNS might have very detrimental effects and is neutralized by the antioxidant fortifications under standard circumstances in healthy people (Mandal et al., 2022; Medrano-Macías et al., 2022). Oxidative stress arises when there is a variation of balance in support of ROS/RNS and may happen in several situations, like in infection or malnutrition with deficient micronutrients to achieve the requirement for antioxidant defenses (Ejidike and Ajibade, 2015; Mandal et al., 2022; Medrano-Macías et al., 2022). It has been recognized that oxidative stress is among the chief contributory elements of various chronic and deteriorating ailments initiators comprising cancer, ischemic heart disease, atherosclerosis, diabetes mellitus, ageing, immunosuppressant, and neurodegenerative illnesses (Ejidike and Ajibade, 2015; Malekmohammad et al., 2019; Poljsak et al., 2021).
Combretum species as an antimicrobial and antiviral agent
Antimicrobial-resistant strains are the major causes of copious clinical problems (Fennel et al., 2004; Gangoué-Piéboji et al., 2009) and have increased the world’s mortality rate (Ejidike and Ajibade, 2015; Motsumi et al., 2020; Mtunzi et al., 2017a, 2017b; Ntshanka et al., 2020). The resistance built by pathogenic against antibiotics has brought about great interest and the quest for novel antimicrobial drugs/agents from nature (Bouzidi et al., 2016; Dorman and Deans, 2000; Ejidike and Ajibade, 2015; Liouane et al., 2010). The unethical usage of antibiotics has brought about their resistance to bacterial strains (Martini and Eloff, 1998). Plants are an imperative basis for the growth of new chemotherapeutic agents, given that they are made up of potentially useful constituents (Barku et al., 2013). Since time immemorial, traditional plants have been used to prepare drugs, thus acting as a good source of medicine. Moreover, Combretum species have been shown to exhibit potential activities as antibacterial and antiviral agents (Adamu et al., 2005; Filho et al., 2015; Fyhrquist et al., 2006; Katerere et al., 2003; Maregesi et al., 2007; Martini et al., 2004a, 2004b; Martini and Eloff, 1998; Masika and Afolayan, 2002; Masoko et al., 2007, 2010; Mawoza and Ndove, 2015; McGaw et al., 2000; Ntshanka et al., 2020; Olukoya et al., 1993).
Different components of the Combretum plants have been utilized in the native system of medicine for the management of several human ailments ranging from ulcers, wounds, cholera, and snakebite to abdominal disorders (Begum et al., 2002; Maregesi et al., 2007; Masoko et al., 2010; Mawoza and Ndove, 2015). The leaves, stems, roots, and flower parts of Combretum species have been used traditionally for the treatment of neuralgia, throat contagions, wounds, eruptions, and a varied range of skin diseases like rashes, ringworm, and ulcers (Eloff et al., 2008; Masoko et al., 2007, 2010); treatment of related bacterial infections and diseases including pneumonia, chest infections, syphilis, diarrhea, coughs, and colds (Ahmed, 2012; Ahmed et al., 2014; Dawe et al., 2013; Fyhrquist et al., 2006; Gathirwa et al., 2011; Komape et al., 2014; McGaw et al., 2001; Ntshanka et al., 2020); treatment of digestive infections, bleeding, and throat contagions (Dimitrova et al., 2015; Hsouna and Hamdi, 2012); and also menopausal and menstrual complications, breast congestion, cellulite, and fluid retention (Pietrovski et al., 2006; Saraswathi et al., 2011). The leaf extracts have also been reported to treat childhood diseases like measles, chickenpox, and mumps (Brendler and Van Wyk, 2008). The following species have prominently featured as agents for treating contagious diseases: C. imberbe, C. vendee against diarrhea; C. ghasalense Engl. & Diels against malaria; C. molle against stomach disorders; C. molle, C. imberbe, and C. erythrophyllum against coughs (Eloff et al., 2008).
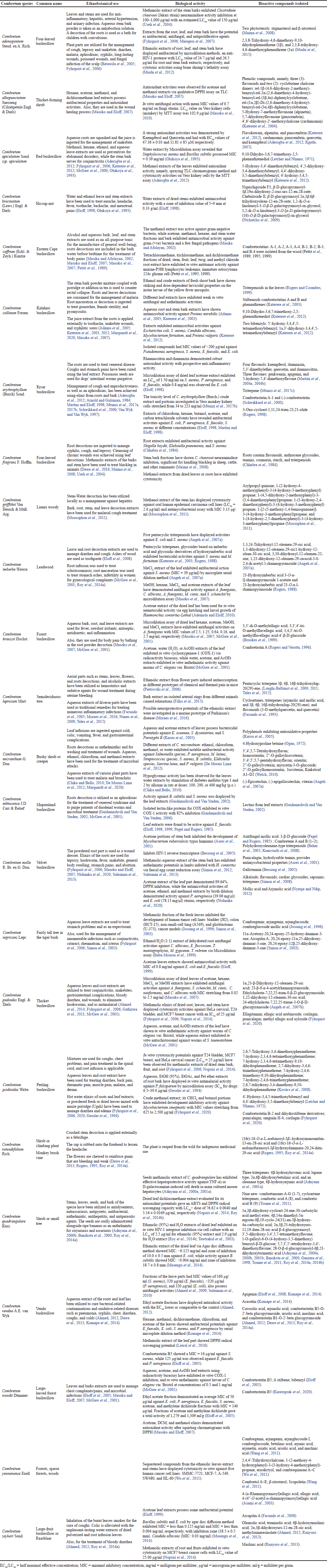 | Table 1. Antimicrobial activities and uses of constitutes sequestered from the Combretum species in traditional medicine. [Click here to view] |
The medicinal benefits of Combretum species lie in some vital chemical constituents responsible for certain physiological exploit on the human body (Edeoga et al., 2005; Filho et al., 2015; Masoko et al., 2007; Masoko and Eloff, 2007; Nagata et al., 2011; Uzor and Osadebe, 2016) and are significant in pharmacological research and drug development (Ademola and Eloff, 2010; Moraes et al., 2016; Roy et al., 2014a, 2014b). Combretum species possess extractable organic substances in quantities sufficient to exhibit antimicrobial activities (Adamu et al., 2005; Katerere et al., 2003; Masika and Afolayan, 2002; Olukoya et al., 1993). Chemicals constituents from Combretum species hold complex arrangements that are not obtainable in synthetic compound collections; hundreds of chemical constituents have been consequential for use as antibacterial agents and other drugs (Aderogba et al., 2012; Facundo et al., 1993; Katerere et al., 2012; Kgatle, 2007; Longhi-Balbinot et al., 2009, 2011; Sabo and Knezevic, 2019; Welch, 2010). The species of Combretum has featured conspicuously as an agent for handling infectious diseases as exemplified in Table 1.
Evidence for medical efficacy of Combretum species
Phytochemical investigations to determine the medical efficacy of Combretum, the most widespread genus of Combretaceae, has paved the way several constituents comprising flavonoids, triterpenoids, phenanthrenes and their derivatives, diarylpropanes, and stilbenoids and their derivatives. Isolates or extracts from this class of species have shown several bioactivities, including antibacterial, antiradical, antifungal, antidiabetic, antihyperglycemic, cytotoxicity, and inhibitory activities, against different human tumor cell lines, anti-inflammatory, antimalarial, anti-snake venom, and anti-HIV/AIDS properties. Also, they have been used for the management of diverse infirmities and diseases (Aderogba et al., 2012; Ares et al., 2006; Belkaid and Hand, 2014; Chika and Bello, 2010; Dawe et al., 2013; De Morais Lima et al., 2012; Kemvoufo et al., 2008; Khumalo et al., 2018; Masoko et al., 2007; Motsumi et al., 2020; Nagata et al., 2011; Uzor and Osadebe, 2016). Antidiabetic activity through adenosine monophosphate-activated protein kinase activation by quercetin from flower extracts of C. lanceolatum has been reported (Dechandt et al., 2013). Anti-candidiasis agents from African Tanzanian plant: C. zeyheri (Runyoro et al., 2013), while lignin derivative from C. alfredii (Bai et al., 2016). Combretum species have also shown great potential as a source of various secondary metabolites. Metabolites and their related endophytic fungus Nigrospora oryzae as proof of a metabolic conglomerate from C. dolichopetalum have been reported by Uzor et al. (2015).
Studies on the antioxidant, antibacterial, cytotoxicity, and antifungal potentials of solvent-to-solvent fractionations of C. erythrophyllum (Burch.) leave elixirs revealed that Combretum species are nontoxic for usage in traditional medicine for the management of infectious and stress-related diseases (Mtunzi et al., 2017b). Methanolic extract of the C. adenogonium Steud. ex A. Rich stem barks inhibited C. chauvoei (Jakari strain) neuraminidase activity as reported by Useh et al. (2004) at 100–1,000 μg/ml with an estimated LC50 value of 150 μg/ml. Extracts from the stem bark, root, and leaf have the potential as antibacterial, antifungal, and antiproliferative agents (Fyhrquist et al., 2006; Maregesi et al., 2007). Ethanolic stem bark, root, and leaf elixirs have displayed antibacterial by microdilution methods, an anti-HIV-1 protease with LC50 value of 24.7 and 26.5 μg/ml for root and stem bark extracts, respectively, and cytotoxic activities using brine shrimp’s lethality assay (Mushi et al., 2012).
Acetone elixir of Combretum mole stem bark had inhibited the evolution of Mycobacterium Tuberculosis typus humanus (ATCC 27294) (Asres et al., 2001), inhibits HIV-1 reverse transcriptase (Bessong et al., 2005). Aqueous-methanol stem bark elixir of C. mole has exhibited anthelmintic activity in infected lambs with H. contortus via faecal egg count reduction test (Simon et al., 2012; Suleiman et al., 2013). Interestingly, powdered and decoctions of C. mole root part have been used as a wound dresser for treatments of leprosy, fever, snake bite, stomach pains, all-purpose body swelling, hookworm, and abortion, While the activities of this C. mole associated with bioactive compounds such as hydrolysable tannin and punicalagin demonstrated antimycobacterial properties (Asres et al., 2001). Compounds such as maslinic acid, ursolic acid; combretastatin B5-O-2′-beta glucopyranoside, corosolic acid, arjunolic acid, combretastatin B1-O-2′-beta glucopyranoside (Ahmed, 2012) isolated from C. vendee A.E. van Wyk have exhibited antimicrobial and antifungal activities (Ahmed et al., 2009; Suleiman et al., 2010); antiradical activity with the EC50 lesser or analogous to the control (Ahmed, 2012).
CONCLUSION
The reports detailed in this review advocate using medicinal plants as alternative medicine. Combretaceae species has displayed a broad spectrum of ethnopharmacological potentials for treating infectious diseases, exhibits significant antimicrobial and antifungal potentials against varieties of bacterial and fungal species, respectively, and also exhibit good antioxidant, anti-inflammatory, antimalarial, antituberculosis, antidiarrhoea, cytotoxicity, anthelmintic, antischistosomal, COX-1 inhibition, and HIV-1 integrase inhibition. Phytochemical constituents of the species are great prospective agents for averting and treating many related oxidative stress diseases. Even though the oils from some of these species have not been harnessed as a fragrance in the perfumery, food, and beverage industry; the oils and active compounds may also possess great potential for protecting food and cosmetics from microbial spoilage. Hence, medicinal plants can be seen as an alternative to medicine if properly used as prescribed or as a precursor for synthesizing chemotherapeutic agents for disease control. Concerning the above investigation, it is evident that Combretaceae species contain bioactive compounds such as triterpenoids, glycosylated triterpenes, and phytochemical constituents of biological importance. Given these outstanding values, few pharmacological and phytochemical analyses have been conducted. Hence, it will greatly benefit the health sector and medicinal chemistry if further research is encouraged and carried out toward identifying bioactive compounds and corroborating their medicinal and pharmacological properties. Areas of research in the economy, domestication, and proliferation, as well as quality control and procedures for sustainable utilization of these plant species as future potential antibiotic and chemotherapeutic agents, should be prioritized. This should be a priority for researchers and stakeholders as these plants can increase the well-being of the populace who finds solace in them.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Directorate of Research, Vaal University of Technology, South Africa, The University of Winnipeg, Winnipeg, Canada, and Anchor University, Lagos, Nigeria, for the support provided.
AUTHOR CONTRIBUTIONS
Involved authors contributed substantially from the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting and revising the article. Authors are eligible to be an author as per the International Committee of Medical Journal Editors requirements/guidelines.
FINANCIAL SUPPORT
This project was supported by the National Research Foundation (Grant No. 120790) and the Directorate of Research, Vaal University of Technology, South Africa, South Africa.
CONFLICTS OF INTEREST
The authors proclaim that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abubakar EMM. Antibacterial potential of crude leaf extracts of Eucalyptus camaldulensis against some pathogenic bacteria. Afr J Plant Sci, 2010; 4:202–9.
Adamu HM, Abayeh OJ, Agho MO, Abdullahi AL, Uba A, Dukku HU, Wufem BM. An ethnobotanical survey of Bauchi State herbal plants and their antimicrobial activity. J Ethnopharmacol, 2005; 99(1):1–4. CrossRef
Ademola IO, Eloff JN. In vitro anthelmintic activity of Combretum molle (R. Br. ex G. Don) (Combretaceae) against Haemonchus contortus ova and larvae. Vet Parasitol, 2010; 169:198–203. CrossRef
Aderogba MA, Kgatle DT, McGaw LJ, Eloff JN. Isolation of antioxidant constituents from Combretum apiculatum subsp. apiculatum. S Afr J Bot, 2012; 79:125–31. CrossRef
Adnyana IK, Tezuka Y, Awale S, Banskota AH, Tran KQ, Kadota S. Quadranosides VI—XI, six new triterpene glucosides from the seeds of Combretum quadrangulare. Chem Pharm Bull, 2000b; 48(8):1114–20. CrossRef
Adnyana IK, Tezuka Y, Awale S, Banskota AH, Tran KQ, Kadota S. 1-O-galloyl-6-O-(4-hydroxy-3,5-dimethoxy) benzoyl- betα-D-glucose, a new hepatoprotective constituent from Combretum quadrangulare. Planta Med, 2001b; 67(4):370–1. CrossRef
Adnyana IK, Tezuka Y, Banskota AH, Tran KQ, Kadota S. Three new triterpenes from the seeds of Combretum quadrangulare and their hepatoprotective activity. J Nat Prod, 2001a; 64(3):360–3. CrossRef
Adnyana IK, Tezuka Y, Banskota AH, Xiong Q, Tran QX, Kadota S. Quadronosides I-IV, new triterpene glucosides from Combretum quadrangulare. J Nat Prod, 2000a; 63(4):496–500. CrossRef
Ahmed AS, 2012. Biological activities of extracts and isolated compounds from Bauhinia galpinii (Fabaceae) and Combretum vendae (Combretaceae) as potential antidiarrhoeal agents. PhD Thesis, Department of paraclinical sciences, Faculty of veterinary, University of Pretotia, Pretotia, South Africa, pp 129–31.
Ahmed AS, Igwe CC, Eloff JN. Preliminary studies of the antibacterial activities of Combretum vendae leave extract. Afr J Tradit Complement Altern Med, 2009; 5:366–7.
Ahmed AS, McGaw LJ, Elgorashi EE, Naidoo V, Eloff JN. Polarity of extracts and fractions of four Combretum (Combretaceae) species used to treat infections and gastrointestinal disorders in southern African traditional medicine has a major effect on different relevant in vitro activities. J Ethnopharmacol, 2014; 154(2):339–50. CrossRef
Alam RTM, Fawzi EM, Alkhalf MI, Alansari WS, Aleya L, Abdel-Daim MM. Anti-inflammatory, immunomodulatory, and antioxidant activities of allicin, norfloxacin, or their combination against Pasteurella multocida infection in male New Zealand rabbits. Oxid Med Cell Longev, 2018; 2018:10. CrossRef
Alfadil MA, Abdallah M, Ibrahim HM. Isolation and screening of streptomyces from local area in Sudan in the presence of amphotericin A and B. AJST, 2014; 1(5):288–92.
Angeh JE, Huang X, Sattler I, Swan GE, Dahse H, Härtl A, Eloff JN. Antimicrobial and anti-inflammatory activity of four known and one new triterpenoids from Combretum imberbe (Combretaceae). J Ethnopharmacol, 2007a; 110(1):56–60. CrossRef
Angeh JE, Huang X, Swan GE, Möllman U, Sattler I, Eloff JN. Novel antibacterial triterpenoids from Combretum padoides (Combretaceae). Arkivoc (IX), 2007b; 2007(9):113–20. CrossRef
Araújo LCJ, Silva VC, Dall’oglio EL, Sousa Jr PT. Flavonoids from Combretum lanceolatum Pohl. Biochem Syst Ecol, 2013; 49:37–8. CrossRef
Ares K, Mazumder A, Bucar F. Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop Med J, 2006; 44(3):269–77.
Arnold HJ, Gulimian M. Pharmacopeia of traditional medicine in Venda. J Ethnopharmacol, 1984; 121:35–74. CrossRef
Asami Y, Ogura T, Otake N, Nishimura T, Xinsheng Y, Sakurai T, Nagasawa H, Sakuda S, Tatsuta K. Isolation and synthesis of a new bioactive ellagic acid derivative from Combretum yunnanensis. J Nat Prod, 2003; 66(5):729–31. CrossRef
Asres K, Bucar F, Edelsbrunner S, Kartnig T, Höger G, Thiel W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother Res, 2001; 15:613–7. CrossRef
Baba-Moussa F, Akpagana K, Bouchet P. Antifungal activities of seven West African Combretaceae used in traditional medicine. J Ethnopharmacol, 1999; 66(3):335–8. CrossRef
Bahar A, Tawfeq AAH, Passreiter CM, Jaber SM. Combretene A and B, two new triterpenes from Combretum molle. Pharm Biol, 2004; 42(2):109–13. CrossRef
Bai M, WU LJ, Cai Y, Wu SY, Song XP, Chen GY, Zheng CJ, Han CR. One new lignin derivative from the Combretum alfredii. Nat Prod Res, 2016; 31(9):1–6. CrossRef
Banskota AH, Tezuka Y, Kim QT, Tanaka K, Saiki I, Kadota S. Thirteen novel cycloartanes-type triterpenes from Combretum quadrangulare. J Nat Prod, 2000; 63:57–64. CrossRef
Barku VYA, Opoku-Boahen Y, Owusu-Ansah E, Mensah EF. Antioxidant activity and the estimation of total phenolic and flavonoid contents of the root extract of Amaranthus spinosus. Asian J Plant Sci Res, 2013; 3(1):69–74.
Barrales-Cureno HJ, Salgado-Garciglia R, Lopez-Valdez LG, Reynoso-Lopez R, Herrera-Cabrera BE, Lucho-Constantino GG, Zaragoza- Martinez F, Reyes-Reyes C, Aftab T, 2021. Use of secondary metabolites from medicinal and aromatic plants in the fragrance industry. In: Aftab T, Hakeem KR (eds.). Medicinal and aromatic plants, Springer, Cham, Switzerland, pp 669–90. CrossRef
Batawila K, Kokou K, Koumaglo K, Gbe’assor M, Foucault D, Bouchet P, Akpagana K. Antifungal activities of five Combretaceae used in togolese traditional medicine. Fitoterapia, 2005; 76:264–8. CrossRef
Begum S, Hassan SI, Siddiqui BS. Two new triperpenoids from the fresh leaves of Psidium guajava. Planta Med, 2002; 68:1149–52. CrossRef
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell, 2014; 157(1):121–41. CrossRef
Bessong PO, Obi CL, Andreola ML, Rojas LB, Pouysegu L, Igumbor E, Meyer JJ, Quideau S, Litvak S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J Ethnopharmacol, 2005; 99(1):83–91. CrossRef
Bhat RB. Medicinal plants and traditional practices of Xhosa people in the Transkei region of Eastern Cape, South Africa. Indian J Tradit Knowl, 2014; 13(2):292–8.
Bisht R, Bhattacharyya A, Shrivastava A, Saxena P. An overview of the medicinally important plant type III PKS derived polyketides. Front Plant Sci, 2021; 12:746908. CrossRef
Bouzidi A, Benzarti A, El Arem A, Mahfoudhi A, Hammami S, Gorcii M, Mastouri M, Hellal AN, Zine Mighri Z. Chemical composition, antioxidant, and antimicrobial effects of Tunisian Limoniastrum guyonianum Durieu ex Boiss extracts. Pak J Pharm Sci, 2016; 29(4):1299–305.
Brendler J, Van Wyk BE. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J Ethnopharmacol, 2008; 119:420–33. CrossRef
Brookes KB, Doudoukina OV, Katsoulis LC, Veale DJH. Uteroactive constituents from Combretum kraussii. S Afr J Chem, 1999; 52:127–32.
Chhabra SC, Uiso FC, Mshiu EN. Phytochemical screening of Tanzanian medicinal plants. J Ethnopharmacol, 1984; 11(2):157–9. CrossRef
Chika A, Bello SO. Antihyperglycaemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J Ethnopharmacol, 2010; 129:34–7. CrossRef
Chukwujekwu JC, van Staden J. In vitro antibacterial activity of Combretum edwardsii, Combretum krausii, and Maytenus nemorosa and their synergistic effects in combination with antibiotics. Front Pharmacol, 2016; 7:208. CrossRef
Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta, 2013; 1830(6):3670–95. CrossRef
Crovadore J, Schalk M, Lefort F. Selection and mass production of Santalum Album L. Calli for induction of sesquiterpenes. Biotechnol Biotechnol Equip, 2012; 26(2):2870–4. CrossRef
Cundell CR. Herbal phytochemicals as immunomodulators. Curr Immunol Rev, 2014; 10:010–8. CrossRef
Dawe A, Kapche GDWF, Bankeu JJK, Fawai Y, Ali MS, Ngadjui BT. Combrestatins A and B, new cycloartane-type from Combretum fragrans. Helv Quim Acta, 2016; 99(8):617–20. CrossRef
Dawe A, Pierre S, Tsala DE, Habtemariam S. Phytochemical constituents of Combretum loefl. (Combretaceae). Pharm Crop, 2013; 4:38–59. CrossRef
Dechandt CRP, Siqueira JT, Souza DLP, Araujo LCJ, Silva VC, Sousa Jr PT, Andrade CMB, Kawashita NH, Baviera AM. Combretum lanceolatum flowers extract shows antidiabetic activity through activation of AMPK by quercetin. Braz J Pharmacogn, 2013; 23(2):291–300. CrossRef
De Morais Lima GR, De Sales IRP, Caldas Filho MRD, De Jesus NZT, De Sousa Falcão H, Barbosa-Filho JM, Cabral AGS, Souto AL, Tavares JF, Batista LM. Bioactivities of the genus Combretum (Combretaceae): a review. Molecules, 2012; 17:9142–206. CrossRef
De Wet H, Nciki S, Van Vuuren SF. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J Ethnobiol Ethnomed, 2013; 9:51. CrossRef
Dimitrova M, Mihaylova D, Popova A, Alexieva J, Sapundzhieva T, Fidan H. Phenolic profile, antibacterial and antioxidant activity of Pelargonium graveolens leaves’ extracts. Sci Bull Series F Biotechnol, 2015; XIX:130–5.
Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol, 2000; 88:308–16. CrossRef
Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol, 2005; 4:685–8. CrossRef
Ejidike IP, Ajibade PA. Transition metal complexes of symmetrical and asymmetrical Schiff bases as antibacterial, antifungal, antioxidant, and anticancer agents: progress and prospects. Rev Inorg Chem, 2015; 35(4):191–224. CrossRef
Ejidike IP, Mtunzi FM, Klink MJ, 2019. Spectroscopic, XRD, in vitro anti-oxidant, antifungal and antibacterial studies of heterocyclic Schiff base nickel(II) complexes bearing anions. In: Ramasami P, Gupta BM, Jhaumeer LS, Li KWH (eds.). Chemistry for a clean and healthy plane, ICPAC-2018, Springer, Cham, Switzerland, pp 283–305. CrossRef
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med, 1998; 64:711–3. CrossRef
Eloff JN. The antibacterial activity of 27 Southern African members of the Combretaceae. S Afr J Sci, 1999; 95:148–52.
Eloff JN, Famakin JO, Katerere DR. Isolation of an antibacterial stilbene from Combretum woodii (Combretaceae) leaves. Afr J Biotechnol, 2005; 4(10):1166–71.
Eloff JN, Katerere DR, McGaw LJ. The biological activity and chemistry of the Southern African Combretaceae. J Ethnopharmacol, 2008; 119:686–99. CrossRef
Facundo VA, Andrade CHS, Silveira ER, Braz-Fihlo R, Hufford CD. Triterpenes and flavonoids from Combretum leprosum. Phytochemistry, 1993; 32:411–5. CrossRef
Facundo VA, Rios KA, Medeiros CM, Militao JSLT, Miranda ALP, Epifanio RD, Carvalho MP, Andrade AT, Pinto AC, Rezende CM. Arjunolic acid in the ethanolic extract of Combretum leprosum root and its use as a potential multi-functional phytomedicine and drug for neurodegenerative disorders: anti-inflammatory and anticholinesterasic activities. J Braz Chem Soc, 2005; 16:1309–12. CrossRef
Facundo VA, Rios KA, Moreira LS, Militão JSLT, Stabelli RG, Braz-Filho R, Silveira ER. Two new cycloartanes from Combretum leprosum MART. (Combretaceae). Rev Latinoam Quím, 2008; 36(3):76–82.
Fankam AG, Kuiate JR, Kuete V. Antibacterial and antibiotic resistance modifying activity of the extracts from Allanblackia gabonensis, Combretum molle and Gladiolus quartinianus against gram-negative bacteria including multi-drug resistant phenotypes. BMC Complement Altern Med, 2015; 15(1):206. CrossRef
Fennel CW, Lindsey KL, McGaw LJ, Sprag LG, Stfford GI, Elgorash EE, Grace OM, Van Staden J. Assessing African medicinal plants for efficiency and safety, pharmacological screening and toxicology. J Ethnopharmacol, 2004; 94:205–17. CrossRef
Filho FCA, Cavalcanti PMS, Passaglia RCAT, Ballejo G. Long-lasting endothelium-dependent relaxation of isolated arteries caused by an extract from the bark of Combretum leprosum. Einstein, 2015; 13(3):395–403. CrossRef
Fyhrquist P, Mwasumbi L, Vuorela P, Vuorela H, Hiltunen R, Murphy C, Adlercreutz H. Preliminary antiproliferative effects of some species of Terminalia, Combretum and Pteleopsis collected in Tanzania on some human cancer cell lines. Fitoterapia, 2006; 77:358–66. CrossRef
Fyhrquist P, Salih EYA, Helenius S, Laakso I, Julkunen-Tiitto R. HPLC-DAD and UHPLC/QTOF-MS analysis of polyphenols in extracts of the African species Combretum padoides, C. zeyheri and C. psidioides related to their antimycobacterial activity. Antibiotics, 2020; 9:459. CrossRef
Gaidamashvili M, Van Staden J. Interaction of lectin-like proteins of South African medicinal plants with staphylococcus aureus and Bacillus subtilis. J Ethnopharmacol, 2002; 80:131–5. CrossRef
Gaidamashvili M, Van Staden J. Prostaglandin inhibitory activity by lectin-like proteins from South African medicinal plants. S Afr J Bot, 2006; 72(4):661–3. CrossRef
Gangoué-Piéboji J, Eze N, Djintchui AN, Ngameni B, Tsabang N, Pegnyemb DE, Biyiti L, Ngassam P, Koulla-Shiro S, Galleni M. The in-vitro antimicrobial activity of some traditionally used medicinal plants against beta-lactam-resistant bacteria. J Infect Dev Ctries, 2009; 3(9):671–80.
Ganzera M, Ellmerer-Müller EP, Stuppner H. Cycloartane triterpenes from Combretum quadrangulare. Phytochemistry, 1998; 49(3):835–8. CrossRef
Gathirwa JW, Rukunga GM, Mwitari PG, Mwikwabe NM, Kimani CW, Muthaura CN, Kiboi DM, Nyangachaa RM, Omar SA. Traditional herbal antimalarial therapy in Kilifi district, Kenya. J Ethnopharmacol, 2011; 134(2):434–42. CrossRef
Gessler MC, Nkunyak MHH, Mwasumbi LB, Heinrich M, Tanner M. Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop, 1994; 56:65–77. CrossRef
Gumisiriza H, Sesaazi CD, Olet EA, Kembabazi O, Birungi G. Medicinal plants used to treat “African” diseases by the local communities of Bwambara sub-county in Rukungiri district, Western Uganda. J Ethnopharmacol, 2021; 268:113578. CrossRef
Guo Z. The modification of natural products for medical use. Acta Pharm Sin B, 2017; 7(2):119–36. CrossRef
Hsouna AB, Hamdi N. Phytochemical composition and antimicrobial activities of the essential oils and organic extracts from pelargonium graveolens growing in Tunisia. Lipids Health Dis, 2012; 11:167. CrossRef
Huang R, Zhou PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Sig Transduct Target Ther, 2021; 6:254. CrossRef
Ji LI, Larregieu CA, Benet LZ. Classification of natural products as sources of drugs according to the biopharmaceutics drug disposition classification system (BDDCS). Chin J Nat Med, 2016; 14(12):0888–97. CrossRef
Jossang A, Seuleiman M, Maidou E, Bodo B. Pentacyclic triterpenes from Combretum nigricans. Phytochemistry, 1996; 41(2):591–4. CrossRef
Karatoprak G?, Küpeli Akkol E, Genç Y, Bardakci H, Yücel Ç, Sobarzo-Sánchez E. Combretastatins: an overview of structure, probable mechanisms of action and potential applications. Molecules, 2020; 25(11):2560. CrossRef
Karou D, Dicko MH, Sanon S, Simpore J, Traore AS. Antioxidants and antibacterial activities of polyphenols from ethnomedicinal plants in Burkina Faso. Afr J Biotechnol, 2005; 4(8):823–8.
Katerere DR, Gray AI, Kennedy AR, Nash RJ, Waigh RD. Cyclobutanes from Combretum albopunctatum. Phytochemistry, 2004; 65(4):433–8. CrossRef
Katerere DR, Gray AI, Kennedy AR, Nash RJ, Waigh RD. Phytochemical, and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia, 2012; 83(5):932–40. CrossRef
Katerere DR, Gray AI, Nash RJ, Waigh RD. Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry, 2003; 63:81–8. CrossRef
Kemvoufo PB, Barboni L, Teponno RB, Mbiantcha M, Nguelefack TB, Hee-Juhn P, Kyung-Tae L, Tapondjou LA. Polyhydroxyoleanane-type triterpenoids from Combretum molle and their anti-inflammatory activity. Phytochem Lett, 2008; 1(4):183–7. CrossRef
Kgatle DT. Isolation and characterization of antioxidant compounds from Combretum apiculatum (Sond.) subsp apiculatum leaf extracts. MSc Thesis, University of Pretoria, Pretoria, South Africa, 2007.
Khalil N, Bishr M, Desouky S, Salama O. Ammi Visnaga L., a potential medicinal plant: a review. Molecules, 2020; 25(2):301. CrossRef
Khan RA. Natural products chemistry: the emerging trends and prospective goals. Saudi Pharm J, 2018; 26(5):739–53. CrossRef
Khan Z, Nath N, Rauf A, Emran TB, Mitra S, Islam F, Chandran D, Barua J, Khandaker MU, Idris AM, Wilairatana P, Thiruvengadam M. Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications. Chem Biol Interact, 2022; 365:110117. CrossRef
Khumalo BM, Qwebani-Ogunleye T, Ejidike IP, Mtunzi FM, Pinkoane M. Evaluation of immune booster formulation by traditional health practitioners: phytochemical, antioxidant, and mineral elements studies. Int J Pharma Bio Sci, 2018; 9(2):29–37. CrossRef
Komape NPM, Aderogba M, Bagla VP, Masoko P, Eloff JN. Anti-bacterial and anti-oxidant activities of leaf extracts of Combretum vendae (Combretecacea) and the isolation of an anti-bacterial compound. Afr J Tradit Complement Altern Med, 2014; 11(5):73–7. CrossRef
Kovács A, Vasas A, Hohmann J. Natural phenanthrenes and their biological activity. Phytochemistry, 2008; 69(5):1084–110. CrossRef
Lahlou M. The success of natural products in drug discovery. Pharmacol Pharm, 2013; 4:17–31. CrossRef
Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clin Phytosci, 2016; 2:23. CrossRef
Letcher RM, Nhamo LRM. Chemical constituents of the combretaceae. Part I. substituted phenanthrenes and 9,10-dihydrophenanthrenes from the heartwood of Combretum apiculatum. J Chem Soc C Org, 1971; 3070–6. CrossRef
Letcher RM, Nhamo LRM. Chemical constituents of the combretaceae. Part III. substituted phenanthrenes, 9,10-dihydrophenanthrenes, and bibenzyls from the heartwood of Combretum psidioides. J Chem Soc Perkin Trans, 1972; 1:2941–6. CrossRef
Liouane K, Saïdana D, Edziri H, Ammar S, Chriaa J, Mahjoub MA, Said K, Mighri Z. Chemical composition and antimicrobial activity of extracts from Gliocladium sp. growing wild in Tunisia. Med Chem Res, 2010; 19:743–56. CrossRef
Longhi-Balbinot DT, Martins DF, Lanznaster D, Silva MD, Facundo VA, Santos ARS. Further analyses of mechanisms underlying the antinociceptive effect of the triterpene 3β, 6β, 16β-trihydroxylup-20(29)-ene in mice. Eur J Pharmacol, 2011; 653:32–40. CrossRef
Longhi-Balbinot DT, Pietrovski E, Gadotti VM, Martins DF, Facundo VA, Santos ARS. Spinal antinociception evoked by the triterpene 3β, 6β, 16β-trihydroxylup-20(29)-ene in mice: evidence for the involvement of the glutamatergic system via NMDA and metabotropic glutamate receptors. Eur J Pharmacol, 2009; 623:30–6. CrossRef
Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med, 2010; 14(4):840–60. CrossRef
Luís Â, Duarte A, Gominho J, Domingues F, Duarte AP. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind Crop Prod, 2016; 79:274–82. CrossRef
Mabona U, Van Vuuren SF. Southern African medicinal plants used to treat skin diseases. South Afr J Botany, 2013; 87:175–93. CrossRef
Maima AO, Thoithi GN, Ndwigah SN, Kamau FN, Kibwage IO. Phytosterols from the stem bark of Combretum fragrans F. Hoffm. East Central Afr J Pharm Sci, 2008; 11:52–5. CrossRef
Malekmohammad K, Sewell RDE, Rafieian-Kopaei M. Antioxidants and atherosclerosis: mechanistic aspects. Biomolecules, 2019; 9(8):301. CrossRef
Mandal M, Sarkar M, Khan A, Biswas M, Masi A, Rakwal R, Agrawal GK, Srivastava A, Sarkar A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants– maintenance of structural individuality and functional blend. Adv Redox Res, 2022; 5:100039. CrossRef
Maregesi SM, Ngassapa OD, Pieters L, Vlietinck AJ. Ethnopharmacological survey of the bunda district, Tanzania: plants used to treat infectious diseases. J Ethnopharmacol, 2007; 113:457–70. CrossRef
Marquardt P, Seide R, Vissiennon C, Schubert A, Birkemeyer C, Ahyi V, Fester K. Phytochemical characterization and in vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules, 2020; 25(2):288. CrossRef
Martini ND, Eloff JN. The preliminary isolation of several antibacterial compounds from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol, 1998; 62:255–63. CrossRef
Martini ND, Katerere DRP, Eloff JN. Seven flavonoids with antibacterial activity isolated from Combretum erythrophyllum. S Afr J Botany, 2004a; 70(2):310–2. CrossRef
Martini N, Katerere DRP, Eloff JN. Biological activity of five antibacterial flavonoids isolated from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol, 2004b; 93:207–12. CrossRef
Masengu C, Zimba F, Mangoyi R, Mukanganyama S. Inhibitory activity of Combretum zeyheri and its S9 metabolites against Escherichia coli, Bacillus subtilis and Candida albicans. J Microb Biochem Technol, 2014; 6(4):228–35. CrossRef
Masika PJ, Afolayan AJ. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J Ethnopharmacol, 2002; 83:129–34. CrossRef
Masoko P, Eloff JN. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr J Trad CAM, 2007; 4(2):231–9. CrossRef
Masoko MA, Kgatle DT, McGaw LJ, Eloff JN. Isolation of antioxidant constituents from Combretum apiculatum subsp. apiculatum. S Afr J Bot, 2012; 79:125–31. CrossRef
Masoko P, Picard J, Eloff JN. The antifungal activity of twenty-four Southern African Combretum species (Combretaceae). S Afr J Bot, 2007; 73:173–83. CrossRef
Masoko P, Picard J, Howard RL, Mampuru LJ, Eloff JN. In vivo antifungal effect of Combretum and Terminalia species extracts on cutaneous wound healing in immunosuppressed rats. Pharm Biol, 2010; 48(6):621–32. CrossRef
Mawoza T, Ndove T. Combretum erythrophyllum (Burch.) Sond. (Combretaceae): a review of its ethnomedicinal uses, phytochemistry and pharmacology. Global J Biol Agric Health Sci, 2015; 4(1):105–9.
McGaw LJ, Jager AK, Staden JV. Antibacterial, anthelmintic, and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol, 2000; 72:247–63. CrossRef
McGaw LJ, Rabe T, Sparg SG, Jager AK, Eloff JN, Van Staden J. An investigation on the biological activity of Combretum species. J Ethnopharmacol, 2001; 75:45–50. CrossRef
Medrano-Macías J, Flores-Gallegos AC, Nava-Reyna E, Morales I, Tortella G, Solís-Gaona S, Benavides-Mendoza A. Reactive oxygen, nitrogen, and sulfur species (RONSS) as a metabolic cluster for signaling and biostimulation of plants: an overview. Plants, 2022; 11:3203. CrossRef
Mohaddese M. Rosa damascene as holy ancient herb with novel applications. J Tradit Complement Med, 2016; 6:10–6. CrossRef
Moloney MG. Natural products as a source for novel antibiotics. Trends Pharmacol Sci, 2016; 37(8):689–701. CrossRef
Mongalo NI, Opoku AR, Zobolo AM. Antibacterial and antioxidant activity of the extracts of Waltheria indica Linn. collected from Capricorn district, Limpopo Province, South Africa. J Med Plants Res, 2012; 6(43):5593–8.
Moosophon P, Kanokmedhakul S, Kanokmedhakul K. Diarylpropanes and an arylpropyl quinone from Combretum griffithii. J Nat Prod, 2011; 74(10):2216–8. CrossRef
Moraes LS, Rohor BZ, Areal LB, Pereira EV, Santos AMC, Facundo VA, Santos ARS, Pires RGW, Martins-Silva C. Medicinal plant Combretum leprosum mart ameliorates motor, biochemical and molecular alterations in a Parkinson’s disease model induced by MPTP. J Ethnopharmacol, 2016; 185:68–76. CrossRef
Motsumi PT, Qwebani-Ogunleye T, Ejidike IP, Mtunzi FM, Nate Z. Teedia lucida root extracts by ultrasonication and maceration techniques: phytochemical screening, antimicrobial and antioxidant activity. Rasayan J Chem, 2020; 13(1):423–33. CrossRef
Mtunzi FM, Ejidike IP, Ledwaba I, Ahmed A, Pakade VE, Klink MJ, Modise SJ. Solvent–solvent fractionations of Combretum erythrophyllum (Burch.) leave extract: studies of their antibacterial, antifungal, antioxidant, and cytotoxicity potentials. Asian Pac J Trop Biomed, 2017b; 10(7):670–9. CrossRef
Mtunzi FM, Ejidike IP, Matamela T, Dikio ED, Klink MJ. Phytochemical profiling, antioxidant and antibacterial activities of leaf extracts from Rhus leptodictya. Int J Pharmacogn Phytochem Res, 2017a; 9(8):1090–9. CrossRef
Mtunzi F, Ledwaba I, Klink M, Dikio E, Ejidike P, Pakade V. Antibacterial activity of a triterpene isolated from Combretum erythrophyllum ethyl acetate fraction. Org Med Chem Int J, 2017c; 4(3):555640. CrossRef
Mushi NF, Innocent E, Kidukul AW. Cytotoxic and antimicrobial activities of substituted phenanthrenes from the roots of Combretum adenogonium Steud Ex A. Rich (Combretaceae). J Intercult Ethnopharmacol, 2015; 4(1):52–6. CrossRef
Mushi NF, Mbwambo ZH, Innocent E, Tewtrakul S. Antibacterial, anti-HIV-1 protease and cytotoxic activities of aqueous ethanolic extracts from Combretum adenogonium Steud. Ex A. Rich (Combretaceae). BMC Complement Altern Med, 2012; 12:163. CrossRef
Nagata JM, Jew AR, Kimeu JM, Salmen CR, Bukusi EA, Cohen CR. Medical pluralism on Mfangano Island: use of medicinal plants among persons living with HIV/AIDS in Suba district, Kenya. J Ethnopharmacol, 2011; 135(2):501–9. CrossRef
Nguedia AJC, Shey ND. African medicinal plant derived products as therapeutic arsenals against multidrug resistant microorganisms. J Pharmacogn Phytother, 2014; 6(5):59–69.
Nopsiri W, Chansakaow S, Putiyanan S, Natakankitkul, S, Santiarworn D. Antioxidant and anticancer activities from leaf extracts of four Combretum species from Northern Thailand. CMU J Nat Sci, 2014; 13(2):195–205. CrossRef
Norman NC, Pringle PG. In defence of oxidation states. Dalton Trans, 2022; 51(2):400–10. CrossRef
Ntchatcho G, Verotta L, Finzi PV, Zanoni G, Vidari G. A new beta-D-glucopyranosyl 2-oxo-urs-12-en-28-oate from the Cameroonian plant Combretum bracteatum. Nat Prod Commun, 2009; 4(12):1631–6. CrossRef
Ntshanka NM, Ejidike IP, Mtunzi FM, Moloto MJ, Mubiayi KP. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed Pharmacol J, 2020; 13(4):1683–94. CrossRef
Nunes PHM, Cavalcanti PMS, Galvão SMP, Martins MCC. Antiulcerogenic activity of Combretum leprosum. Pharmazie, 2009; 64:58–62.
Nyenje ME, Ndip RN. Bioactivity of the acetone extract of the stem bark of Combretum molle on selected bacterial pathogens: preliminary phytochemical screening. J Med Plants Res, 2012; 6(8):1476–81. CrossRef
Ofoedu CE, You L, Osuji CM, Iwouno JO, Kabuo NO, Ojukwu M, Agunwah IM, Chacha JS, Muobike OP, Agunbiade AO, Sardo G, Bono G, Okpala COR, Korzeniowska M. Hydrogen peroxide effects on natural-sourced polysacchrides: free radical formation/production, degradation process, and reaction mechanism-A critical synopsis. Foods, 2021; 10(4):699. CrossRef
Ogan AU. The alkaloids in the leaves of Combretum micranthum. Studies on West African medicinal plants. VII. Planta Med, 1972; 21(2):210–7. CrossRef
Olukoya DK, Idika N, Odugbemi T. Antibacterial activity of some medicinal plants from Nigeria. J Ethnopharmacol, 1993; 39(1):69–72. CrossRef
Owen RW, Giacosa A, Hull WE, Haubner R, Spigelhalder B, Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer, 2000; 36(10):1235–47. CrossRef
Pegel KA, Rogers CB. The characterization of mollic acid-3β-D-xyloside and its genuine aglycon mollic acid, two novel 1α-hydroxycycloartenoids from Combretum molle. J Chem Soc Perkin Trans, 1985; 1:1711–5. CrossRef
Pettit GR, Singh SB, Boyd MR, Hamel E, Pettit RK, Schmidt JM, Hogan F. Antineoplastic agents. 291. Isolation and synthesis of combretastatin A-4, A-5 and A-6. J Med Chem, 1995; 38(10):1666–72. CrossRef
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia, 1989; 45(2):209–11. CrossRef
Pettit GR, Toki BE, Herald DL, Boyd MR, Hamel E, Pettit RK, Chapuis JC. Antineoplastic agents. 410. Asymmetric hydroxylation of trans-combretastatin A-4. J Med Chem, 1999; 42(8):1459–65. CrossRef
Pietrovski EF, Rosa KA, Facundo VA, Rios K, Marques MC, Santos ARS. Antinociceptive properties of the ethanolic extract and of the triterpene 3β, 6β, 16β-trihydroxylup-20(29)-ene obtained from flowers of Combretum leprosum in mice. Pharmacol Biochem Behav, 2006; 83:90–9. CrossRef
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev, 2017; 2017:8416763. CrossRef
Poljsak B, Kova? V, Milisav I. Antioxidants, food processing and health. Antioxidants (Basel), 2021; 10(3):433. CrossRef
Quattrocchi U, 2012. CRC World dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology (5 Volume Set). Taylor & Francis Group; CRC Press, Boca Raton, FL, pp 1070–81.
Rogers CB. Pentacyclic triterpenoids from rhamnosides Combretum imberbe leaves. Phytochemistry, 1988; 27(10):3217–20. CrossRef
Rogers CB. Acidic dammarane arabinofuranosides from Combretum rotundifolium. Phytochemistry, 1995; 40(3):833–6. CrossRef
Rogers CB. Cycloartenoid dienone acids and lactones from Combretum erythrophyllum. Phytochemistry, 1998; 49(7):2069–76. CrossRef
Rogers CB, Coombes PH. Acidic triterpenes glycosides in trichome secretions differentiate subspecies of Combretum collinum in South Africa. Biochem Syst Ecol, 1999; 27:321–3. CrossRef
Rogers CB, Verotta L. Chemistry and biological properties of the African Combretaceae. In: Hostettman K, Chinyanganga F, Maillard M, Wolfender JL (eds.). Chemistry, biological and pharmacological properties of African medicinal plants, University of Zimbabwe Publications, Harare, Zimbabwe, 1996.
Roy S, Gorai D, Acharya R, Roy R. Combretum (Combretaceae): biological activity and phytochemistry. Indo Am J Pharm Res, 2014a; 4(11):5266–99.
Roy R, Singh RK, Jash SK, Sarkar A, Gorai D. Combretum quadrangulare (Combretaceae): phytochemical constituents and biological activity. Indo Am J Pharm Res, 2014b; 4(8):3416–30.
Runyoro DKB, Srivastava SK, Darokar MP, Olipa ND, Cosam CJ, Mecky INM. Anticandidiasis agents from a Tanzanian plant, Combretum zeyheri. Med Chem Res, 2013; 22(3):1258–62. CrossRef
Ryan KJ, Ray CG. Sherris medical microbiology: an introduction to infectious diseases. 4th edition, McGraw Hill, New York, NY, pp 484–8, 2004.
Sabo VA, Knezevic P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. Plant extracts and essential oils: a review. Ind Crop Prod, 2019; 132:413–29. CrossRef
Sahu MC, Patnaik R, Padhy RN. In vitro combinational efficacy of ceftriaxone and leaf extract of Combretum albidum G. Don against multidrug-resistant Pseudomonas aeruginosa and host-toxicity testing with lymphocytes from human cord blood. J Acute Med, 2014; 4(1):26–37. CrossRef
Sampedro MF, Piper KE, McDowell A, Patrick S, Mandrekar JN, Rouse MS, Steckelberg JM, Patel, R. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis, 2009; 64(2):138–45. CrossRef
Saraswathi J, Venkatesh K, Baburao N, Hilal MH, Roja Rani A. Phytopharmacological importance of pelargonium species. J Med Plants Res, 2011; 5(13):2587–98.
Schwikkard S, Zhou BN, Glass TE, Sharp JL, Mattern MR, Johnson RK, Kingston DGI. Bioactive compounds from Combretum erythrophyllum. J Nat Prod, 2000; 63:457–60. CrossRef
Serralheiro ML, Guedes R, Fadel SR, Bendif H. Data on identification of primary and secondary metabolites in aqueous extract of Verbascum betonicifolium. Data Brief, 2020; 32:106146. CrossRef
Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera Indica (Mango). Pharmacogn Rev, 2010; 4(7):42–8. CrossRef
Shaikh RU, Pund MM, Gacche RN. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J Tradit Complement Med, 2016; 6(4):355–61. CrossRef
Silber J, Kramer A, Labes A, Tasdemir D. From discovery to production: biotechnology of marine fungi for the production of new antibiotics. Mar Drugs, 2016; 14(7):137. CrossRef
Silén H, Salih EYA, Mgbeahuruike EE, Fyhrqvist P. Ethnopharmacology, antimicrobial potency, and phytochemistry of African Combretum and Pteleopsis species (Combretaceae): a review. Antibiotics, 2023; 12(2):264. CrossRef
Simon MK, Ajanusi OJ, Abubakar MS, Idris AL, Suleiman MM. The anthelmintic effect of aqueous methanol extract of Combretum molle (R. Br. x. G. Don) (Combretaceae) in lambs experimentally infected with Haemonchus contortus. Vet Parasitol, 2012; 187(1–2):280–4. CrossRef
Simon MK, Ajanusi, OJ, George BD, Abubakar MS, Meduna JA. In vivo evaluation of the stem bark of Combretum molle (R. Br. x. G. Don) for anthelmintic properties. Cont J Vet Sci, 2008; 2:1–11.
Simon G, Dewelle J, Nacoulma O, Guissou P, Kiss R, Daloze D, Braekman JC. Cytotoxic pentacyclic triterpenes from Combretum nigricans. Fitoterapia, 2003; 74(4):339–44. CrossRef
Street RA, Prinsloo G. Commercially important medicinal plants of South Africa: a review. J Chem, 2013; 2013:16. CrossRef
Suleiman MM, McGaw LJ, Naidoo V, Eloff JN. Evaluation of several tree species for activity against the animal fungal pathogen Aspergillus fumigatus. S Afr J Bot, 2010; 76:64–71. CrossRef
Suleiman MM, Simon MK, Ajanusi OJ, Idris AL, Abubakar MS. In vitro anthelmintic activity of the stem-bark of Combretum molle R. Br. x. G. Don (Combretaceae) against Haemonchus contortus. J Med Plants Res, 2013; 7(15):952–6.
Teles CBG, Moreira-Dill LS, Silva AA, Facundo VA, Azevedo Jr WFA, Silva LHP, Motta MCM, Stábeli RG, Silva Jardim I. A lupane-triterpene isolated from Combretum leprosum Mart. Fruit extracts that interferes with the intracellular development of Leishmania (L.) amazonensis in vitro. BMC Complement Altern Med, 2015; 15:165. CrossRef
Tewtrakul S, Miyashiro H, Nakamura N, Hattori M, Kawahata T, Otake T, Yoshinaga T, Fujiwara, T, Supavita T, Yuenyongsawad S, Rattanasuwon P, Dej-Adisai S. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother Res, 2003; 17:232–9. CrossRef
Toume K, Nakazawa T, Ohtsuki T, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Cycloartane triterpenes isolated from Combretum quadrangulare in a screening program for death-receptor expression enhancing activity. J Nat Prod, 2011; 74(2):249–55. CrossRef
Useh NM, Nok AJ, Ambali SF, Esievo KAN. The inhibition of Clostridium chauvoei (Jakari strain) neuraminidase activity by methanolic extracts of the stem barks of Tamarindus indicus and Combretum fragrans. J Enzyme Inhib Med Chem, 2004; 19(4):339–42. CrossRef
Uzor PF, Ebrahim W, Osadebe OP, Nwodo JN, Okoye BF, Muller WEG, Lin W, Proksch P. Metabolites from Combretum dolichopetalum and its associated endophytic fungus Nigrospora pryzae. Evidence for a metabolic partnership. Fitoterapia, 2015; 105:147–50. CrossRef
Uzor PF, Osadebe PO. Antidiabetic activity of the chemical constituents of Combretum dolichopetalum root in mice. EXCLI J, 2016; 15:290–6.
Van Wyk, BE, Gericke N. Peoples plants: a guide to useful plants of Southern Africa. Briza Publications, Pretoria, Southern Africa, p 351, 2000.
Van Wyk BE, Van Ouddshoorn B, Gericke N. Medicinal plants of South Africa. Briza Publications, Pretoria, South Africa, 2009.
Van Wyk B, Van Wyk P. Field guide to trees of Southern Africa. Struik Publishers (Pty) Ltd., Cape Town, Southern Africa, p 536, 1997.
Venter F, Venter JA. Making the most of indigenous trees. 1st edition, Briza Publications, Pretoria, Southern Africa, p 304, 1996.
Verma S. Medicinal plants with anti-inflammatory activity. J Phytopharmacol, 2016; 5(4):157–9. CrossRef
Wang LQ, Wu MM, Liu JP, Li Y, Hua Y, Wang YY, Li XY, Chen YG, Wang JH. Five new diarylpropa-1-ols from Combretum yunnanense. Planta Med, 2011; 77:1841–4. CrossRef
Welch CR. Chemistry and pharmacology of kinkéliba (Combretum micranthum), a West African medicinal plant. PhD Thesis, The State University of New Jersey, New Brunswick, NJ, pp 10–62, 2010.
Wright PM, Seiple IB, Myers AG. The evolving role of chemical synthesis in antibacterial drug discovery. Angew Chem Int Ed Engl, 2014; 53(34):8840–69. CrossRef
Wu MM, Wang LQ, Hua Y, Chen YG, Wang YY, Li XY, Li Y, Li T, Yang XY, Tang ZR. New chalcone and dimeric chalcones with 1,4-p-benzoquinone residue from Combretum yunnanense. Planta Med, 2011; 77(5):481–4.
Yang Q, Wu D, Mao W, Liu X, Bao K, Lin Q, Lu F, Zou C, Li C. Chinese medicinal herbs for childhood pneumonia: a systematic review of effectiveness and safety. Evid Based Complement Alternat Med, 2013; 2013:25. CrossRef
Zhang Y, Li HL, Zhong JD, Wang Y, Yuan CC. Chloroplast genome sequences and comparative analyses of Combretaceae mangroves with related species. Biomed Res Int, 2020; 2020:5867673. CrossRef
Zhang WH, Lin-Han BL, Zhang HD. Antioxidant activities of extracts from Areca (Areca catectu L.) flower, husk and seed. Electron J Environ Agric Food Chem, 2009; 8:740–8.