INTRODUCTION
One-third of all marketed drugs are now sold in a single isomeric form and chirality is now a significant factor in the development of new pharmaceuticals with regulatory and therapeutic considerations driving the process (Mukherjee and Bera, 2012). Since chirality is a crucial characteristic of all organisms, including humans and the significance of chirality is currently increasing quickly. It has become abundantly clear that chirality is essential for human health and cannot be disregarded. Significant advancements have been made in the chirality-related research fields of chiral separation, asymmetric catalysis, and chiral recognition (Song et al., 2020). The enantiomers of a chiral drug molecule may behave differently after administration, so the pharmaceutical industry places a high value on chiral resolution. To have a therapeutic effect, a molecule must engage a target receptor when it is administered. Drug molecules that are chiral will only fit into this receptor in one of their enantiomers (the eutomer), producing the desired therapeutic effect. A lesser effect could result from the other enantiomer (distomer), interacting or not with the receptor. The distomer can occasionally interact with different receptors, leading to side effects or even toxicity. In order to distinguish the eutomer from the distomer during drug substance identification and impurity determinations, additional research is needed on the enantiomers of active compounds during the development process. Racemates resolution is still difficult because of their similar characteristics in chiral environments, and work on highly specialized separation techniques is ongoing to resolve individual enantiomers (Liu et al., 2015). In this study, we used an amylose-based column for the chiral resolution (Ates et al., 2013). Imeglimin belongs to the class of dihydro-1, 3, 5-triazine derivatives. As a first-in-class treatment for type-2 diabetes (T2D), imeglimin is a novel oral agent that is currently being studied T2D (Doupis et al., 2021). At the results of a number pivotal phase III trials, there were no reports of severe hypoglycemia, statistically significant glucose reductions, and favorable safety and tolerability profiles. It is available as oral tablet (Twymeeg) of 500 mg. The usual adult dosage is 500 mg of imeglimin Hydrochloride administered orally daily in the morning and evening. It contains 98.8% purity of C6H13N5. Hcl. Imeglimin’s mechanism of action consists of two effects: (a) increased insulin activity, which may have the ability to lower hepatic glucose output, and (b) increased insulin signaling in the liver and skeletal muscle. Imeglimin also maintains beta cell mass while increasing glucose-stimulated insulin secretion (Hallakou-Bozec et al., 2021). Based on literature survey, it was observed that there are some methods used for the estimation of imeglimin in biological matrices (Tomita et al., 2022), chiral resolution in crystallization method applying by magnetic substrates (Bhowmick et al., 2021), and methanol solvent (Wacharine-Antar et al., 2010) for imeglimin drug, but no analytical chiral method has been developed for the determination of imeglimin in its formulation. However, the reported method had less selectivity and sensitivity with long run time. LC-MS/MS has better selectivity and sensitivity when quantifying the targeted compounds, because it has a mass-specific detector that is independent of a specific functional group (Tang et al., 2022). As a result, the objective of this research is to separate and develop a fast and sensitive method for chiral separation and estimation of (+) and (−) enantiomers in its formulation using LC-MS/MS.
MATERIALS AND METHODS
Reagents
Yamamura Chemical (YMC) India private limited gifted pure Imeglimin (+/−) as a working standard and formulation of imeglimin from Sumitomo Dainippon Pharma Co., Ltd. SD fine chemicals and Merck, Mumbai, India supplied the chemicals ammonium acetate and solvents methanol and acetonitrile (LC-MS grade). The Milli Q RO system was used to purify the water (Millipore, Bedford).
Instrumentation
Ultra fast liquid chromatography coupled with tandem triple quadrupole mass spectrometer (Shimadzu LC-MS/MS, 8030, Tokyo, Japan) equipped with interfaced by electrospray ionization (ESI) and solvent delivery system LC-20AD pump, SPD M20 PDA detector, SIL-20AC auto sampler, CTO 20AC column oven, and CMB-20 alite controller. Using LC lab solution software, the data acquisition was performed. For the study, optimized factors include heat block temperature, desolvation line, Nebulizer gas, collision energy, etc., The mass spectrometer was run in positive ionization detection mode (M+H) with an ESI source. The nebulizer pressure was set to 345 kPa, ionization temperature was set to 300°C, the capillary voltage was 5,000 V and gas flow rate was 11 l/minute. The collision cell gas was ultrapure nitrogen and the ionization source gas was nitrogen.
Chromatographic conditions
The enantio-selective separation was achieved using a chiral stationary phase as reverse phase (RP) Chiralpak IG-3 (100 × 4.6 mm, 3 µm) and the isocratic mobile phase composition of methanol and 10 mM ammonium acetate (95:5 v/v ratio) at the flow rate of the detection of analyte was 0.5 ml/minutes. About 10 µl of injection volume of each sample injects into the system and employed at an ambient column temperature. The total run time for the chiral separation was 5 minutes. The resolution targets were detected using a Shimadzu – 8,030 triple quadrupole mass spectrometer with ESI interfaced with the mass analyzer. The mass spectrometer was run in positive ionization detection mode (M+H) with an multiple reaction monitoring (MRM) mode with the following transitions: m/z 156.0 (parent ion) → m/z 113.0 (daughter ion) for (+/−) imeglimin, respectively (Figs. 1 and 2).
Standard solution preparation for (+/−) imeglimin
Working standard of (+/−) Imeglimin 1,000 µg/ml concentration was prepared by dissolving 10 mg of the enantiomeric drug in a 10 ml volumetric flask with methanol and making up the volume with methanol. A working concentration of 1,000 ng/ml was prepared from the above solution. The calibration curve for (+) and (−) Imeglimin of 10–100 ng/ml enantiomeric drug was prepared using the working standard.
Method validation for imeglimin
The optimized LC-MS/MS method was validated in accordance with the International Conference on Harmonization (ICH) guidelines in the aspects of specificity and carry-over, limit of quantification (LOQ), limit of detection (LOD), linearity, accuracy and precision, robustness, etc. (ICH, 1996).
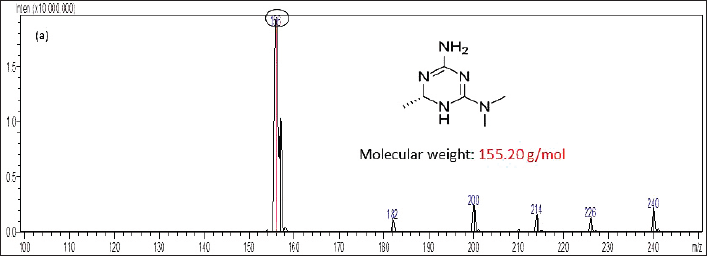 | Figure 1. Molecular spectra for imeglimin enantiomers. [Click here to view] |
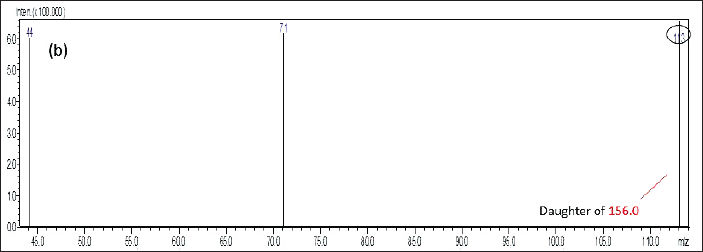 | Figure 2. Daughter ion spectra for imeglimin enantiomers. [Click here to view] |
Accuracy and precision
The recovery of the method was used to define the accuracy of the method. According to ICH guideline, the accuracy of the proposed LC-MS/MS method was evaluated from the three levels of quality control samples by analyzing the six replicates. The recovery of the precision was carried out and the percent relative standard deviation (RSD) was recorded.
Specificity and carry over
The ability to clearly assess the analyte in the presence of components that might be anticipated to be present is known as specificity. Typically, these could be degradants, impurities, etc.
Linearity
The linearity concentrations were used to measure the slope, intercept values, and R2 value using regression equation (Y = mx + C). Six different concentrations of individual enantiomers of imeglimin in the range of 10–100 ng/ml were used to determine the linearity.
Sensitivity
LOD and LOQ were determined by signal to noise ratios (S/Ns) of 3:1 (LOD) and 10:1 (LOQ), respectively, in accordance with ICH guidelines a method is considered sensitive if it can detect incredibly low concentrations.
Robustness
The robustness of the developed method was determined by altering experimental conditions are mobile phase, flow rate and injection volume, etc., was studied.
Estimation of the (+) and (−) enantiomers
Twenty tablets of Twymeeg Tablets (500 mg) were accurately weighed and the average weight was taken and crushed into powder with the equivalent weight to 100 mg of racemic (+/−) imeglimin containing 50 mg each of (+) and (−) enantiomers of imeglimin was accurately weighed and add 50 ml of organic solvent (methanol) was added and kept in a sonicate for 15–20 minutes and the solution was made up to 100 ml with the organic solvent, filtered through 0.45-µm membrane. The solutions were further diluted, and the optimized chromatographic conditions were used to analyze the standard and sample solutions (Table 6).
RESULTS AND DISCUSSION
In present study, RP direct chiral LC-MS/MS technique has used to developed and validated for the chiral resolution of (+/−) Imeglimin. In order to separate the enantiomers, develop a simple, sensitive, and effective LC-MS/MS method for (+/−) imeglimin was carried out to select appropriate and optimal conditions. The resolution factor, theoretical plates, tailing factor, peak area, and peak asymmetry factor were selected as optimized conditions during the screening phase of the enantiomeric drug. RP Chiralpak IG-3 (100 × 4.6 mm, 3 µm) as the chiral stationary phase and methanol and 10 mM ammonium acetate (95:5 v/v ratio) as the isocratic mobile phase with a flow rate of 0.5 ml/minute constitute the optimal chromatographic conditions. The total chromatographic resolution run time was 5 minutes. The (+) enantiomeric retention time (RT) was found to be 2.876 minutes and the (−) enantiomeric RT was found to be 4.325 minutes, respectively (Fig. 3).
Specificity
During the elution time of the individual enantiomers, no potential interference peaks were noticed. As a result, the method was found to be specific and highly sensitive.
Linearity
The calibration curve for (+) and (−) imeglimin was defined at six various concentration levels, and the R2 value was 0.9955 and 0.9938. Individual enantiomeric concentration of working linearity ranges from 10 to 100 ng/ml (Table 1). The results of (+) and (−) regression equations Y = 15,432X − 13,776 and Y = 9,205.7X + 14,387 were found to be linear (Figs. 4 and 5).
Accuracy and precision
Recovery studies were conducted to determine the method accuracy and the recovery percentage of enantiomers ranged between 98.4% and 99.7% (Table 2). The percentage RSD of the individual enantiomers was found to be (1.41% to 0.70%) and (1.86% to 0.71%) for the precision studies (Table 3).
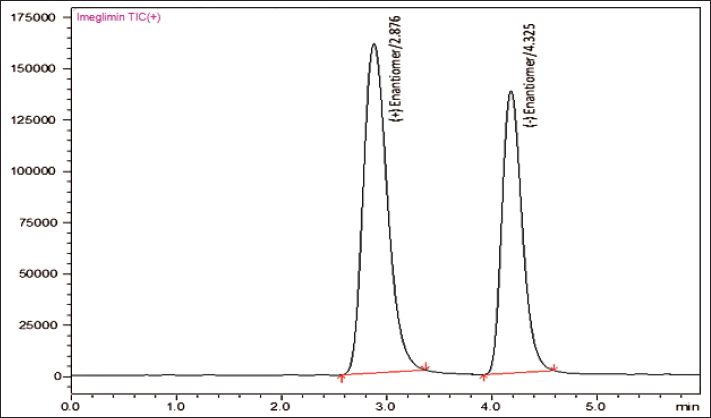 | Figure 3. MRM chromatogram for (+) and (−) enantiomers in formulation. [Click here to view] |
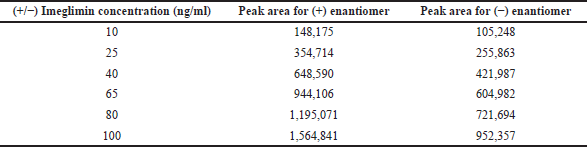 | Table 1. Linearity range for (+) and (−) enantiomers. [Click here to view] |
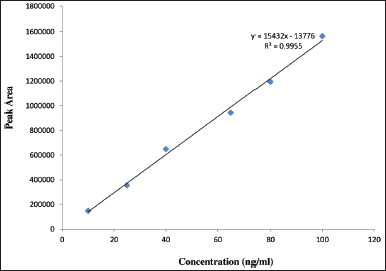 | Figure 4. Calibration curve for (+) enantiomers. [Click here to view] |
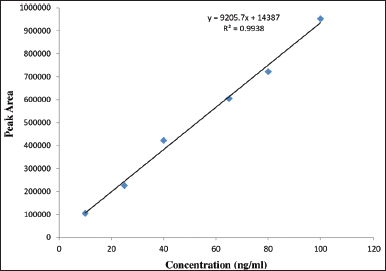 | Figure 5. Calibration curve for (−) enantiomers. [Click here to view] |
 | Table 2. Accuracy studies for (+) and (−) enantiomers. [Click here to view] |
 | Table 3. Precision studied for (+) and (−) enantiomer. [Click here to view] |
 | Table 4. System suitability studies for (+) and (−) enantiomers. [Click here to view] |
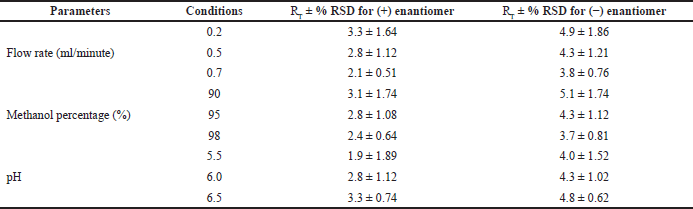 | Table 5. Robustness studies for (+) and (−) enantiomer. [Click here to view] |
 | Table 6. Recovery studies for (+) and (−) enantiomers. [Click here to view] |
LOD and LOQ
Based on the S/N ratio and minimum level of peak area, the LOD and LOQ were obtained at 3.0 and 10.0 ng/ml for the (+) and (−) enantiomers for the developed method.
System suitability
As per ICH guidelines, a system suitability study was conducted to determine the system suitability parameters are resolution factor, RT, tailing factor, and theoretical plates (N). The results were found to be within the limits (Table 4).
Robustness
The conditions followed for the robustness study are flow rate (0.2, 0.5, and 0.7 ml/minutes), pH (5.5, 6.0, and 6.5), and percentage methanol (90%, 95%, and 98%). The Individual percentage RSD for the selected conditions was calculated for pH (1.89%–0.74% and 1.52%–0.62%), flow rate (1.64%–0.51% and 1.86%–0.76%), and methanol percentage (1.74%–0.64% and 1.74%–0.81%) (Table 5).
CONCLUSION
As per ICH guidelines, a sensitive direct chiral RP LC-MS/MS method was developed and validated. The direct chiral RP LC-MS/MS method for chiral resolution was found to be sensitive, fast, accurate, precise, and robust, and it provides good sensitivity and reproducibility. This method will be useful for pharmaceutical, pharmacokinetics, and bioequivalence study.
ACKNOWLEDGMENT
We thank YMC private limited, Hyderabad, India, for providing working standard for our research work and thankful to Dr. K. Nagesh Kumar, P. V. Ratnam, and B. Sandeep for their kind support throughout the research work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ates H, Younes AA, Mangelings D, Vander Heyden Y. Enantioselectivity of polysaccharide-based chiral selectors in polar organic solvents chromatography: implementation of chlorinated selectors in a separation strategy. J Pharm Biomed Anal, 2013; 74:1–3. CrossRef
Bhowmick D, Sang Y, Santra K, Halbauer M, Capua E, Paltiel Y, Naaman R, Tassinari F. Simultaneous high-purity enantiomeric resolution of conglomerates using magnetic substrates. Cryst Growth Des, 2021; 21(5):2925–31. CrossRef
Doupis J, Baris N, Avramidis K. Imeglimin: a new promising and effective weapon in the treatment of type 2 diabetes. TouchREV Endocrinol, 2021; 17(2):88. CrossRef
Hallakou-Bozec S, Vial G, Kergoat M, Fouqueray P, Bolze S, Borel AL, Fontaine E, Moller DE. Mechanism of action of imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab, 2021; 23(3):664–73. CrossRef
ICH. Q3B validation of analytical procedures: methodology. International Conference on Harmonization, November 1996. [ONLINE] Available via https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf (Accessed 20 August 2019).
Liu Y, Tian A, Wang X, Qi J, Wang F, Ma Y, Ito Y, Wei Y. Fabrication of chiral amino acid ionic liquid modified magnetic multifunctional nanospheres for centrifugal chiral chromatography separation of racemates. J Chromatogr A, 2015; 26(1400):40–6. CrossRef
Mukherjee AS, Bera AJ. Importance of chirality and chiral chromatography in pharmaceutical industry: a detailed study. J Chem Pharm Sci, 2012; 2(4):334–46.
Song L, Pan M, Zhao R, Deng J, Wu Y. Recent advances, challenges and perspectives in enantioselective release. J Control Release, 2020; 10(324):156–71. CrossRef
Tang L, Swezey RR, Green CE, Lee MS, Bunin DI, Parman T. A tandem liquid chromatography and tandem mass spectrometry (LC/LC–MS/MS) technique to separate and quantify steroid isomers 11β-methyl-19-nortestosterone and testosterone. J Chromatogr B, 2022; 15(1193):123165. CrossRef
Tomita Y, Hansson E, Mazuir F, Wellhagen GJ, Ooi QX, Mezzalana E, Kitamura A, Nemoto D, Bolze S. Imeglimin population pharmacokinetics and dose adjustment predictions for renal impairment in Japanese and Western patients with type 2 diabetes. Clin Transl Sci, 2022; 15(4):1014–26. CrossRef
Wacharine-Antar S, Levilain G, Dupray V, Coquerel G. Resolution of (±)-imeglimin-2, 4-dichlorophenylacetate methanol solvate by preferential crystallization. Org Process Res Dev, 2010; 14(6):1358–63. CrossRef