INTRODUCTION
Pycnoporus sanguineus, a species of mushroom under the order Polyporales is normally found on decaying hardwood (Fig. 1). It is characterized by its bright red-orange basidiocarp with no distinct stipe. P. sanguineus has various industrial and medicinal uses;including pharmaceutical wastewater treatment (Watanabe et al., 2012), detoxification of textile dye, and degrading a wide variety of organic pollutants (Pointing and Vrijmoed, 2000). Cinnabarin and other compounds present in this mushroom have antioxidant (Tuong et al., 2020), antibacterial (Jaszek et al., 2015), and anticancer properties (Piet et al., 2021). Laccase, a protein produced by this mushroom, exhibited strong cytotoxic activity against colon cancer (Piet et al., 2021). Moreover, its heavy metal absorbance ability is currently being studied as a potential absorber of heavy metals in the bloodstream (Yahaya and Don, 2014). Accordingly, this mushroom contains beneficial bioactive compounds that can be used in various fields. Therefore, there is a need to establish efficient production technology for this mushroom to continually harness its potential.
Various researchers have successfully grown this mushroom in submerged fermentation using both commercial and indigenous materials. Pointing et al. (2000) enhanced its laccase production by modification of the nutrient content of the broth medium. Stirred tank bioreactor with optimized temperature and pH concentrations is also applicable for the efficient production of its biomass (Souza et al., 2021). Mendoza et al. (2020) utilized coconut water as a growth medium for the production of compounds with strong antioxidant activity. However, carbon and nitrogen sources are the major factors that directly influence the production and bioactivity of this mushroom (Eugenio et al., 2009). Aside from the nutrient source, temperature, light intensity, aeration, and agitation influence the successful biomass production of mushrooms in submerged culture (Dulay et al., 2015a). Thus, submerged fermentation is an efficient production technique that can be used to produce mycelial biomass with promising functional activities.
 | Figure 1. Wild fruiting bodies of P. sanguineus growing on forest log. [Click here to view] |
Submerged cultivation can be used as an alternative to the existing mushroom production technology wherein mycelial biomass is produced in liquid media. Flasks, stirred tanks, airlift bioreactors, and fed-batch fermentation are the most common methods for submerged cultivation (Bakratsas et al., 2021). Various species have already been successfully grown in submerged cultures including Pleurotus ostreatus (Velioglu and Uzek, 2015), Lentinula edodes (Garcia-Cruz et al., 2020), and Cordyceps sinensis (Chen et al., 2016). In the Philippines, Dulay et al. (2015a) and Dulay et al. (2015b) were able to optimize liquid culture conditions of different Lentinus species, Pleurotus cystidiosus, Ganoderma lucidum, Schizophyllum commune, and Volvariella volvacea using the conventional shake flask culture. Thus, this technique can be used to produce biomass as other species of mushrooms are not capable of producing fruiting body outside their natural habitat.
Reports regarding the optimum submerged culture conditions of wild Philippine mushrooms are very limited. However, with the aim of utilizing mycelia as a source of functional food and bioactive compounds, the Center for Tropical Mushroom Research and Development in Central Luzon State University (CTMRD-CLSU) is continuously conducting studies to establish biomass production technology of wild mushrooms using indigenous materials. For example, Lentinus swartzii mycelia grown in the optimized liquid culture conditions possess strong antioxidant and antidiabetic activities (Austria et al., 2021). In addition, the antioxidant potential of both Lentinus sajor-caju and Lentinus tigrinus is reported to be influenced by the culture medium (Dulay et al., 2015b). Mycelial biomass of G. lucidum, V. volvacea, S. commune, and P. cystidiosus grown in submerged conditions was found to contain amounts of polar lipids, triglycerides, and free fatty acids (Dulay et al., 2015a). Accordingly, mushroom mycelia demonstrated excellent potential as a source of bioactive compounds that are beneficial to mankind.
This study was conducted in our interest to determine the optimum submerged culture conditions for P. sanguineus with reference to its nutritional and physical growth preferences. The establishment of an efficient cultivation technique for this mushroom allows effective utilization of its biomass. This was the first report in the country regarding the optimum liquid culture conditions for the mycelia biomass production of P. sanguineus. The potential bioactivity of its mycelia biomass was also determined.
MATERIALS AND METHODS
Optimization of submerged culture conditions
Source of culture
Pure culture of P. sanguineus (CTMRD 7137) was obtained from the culture collection of Bioassay Laboratory, Department of Biological Science, College of Science, CLSU, Science City of Muñoz, Nueva Ecija, Philippines.
Influence of nutritional factors
Prior to the evaluation of the nutritional and physical factors, culture inoculants were prepared. Using an inoculating needle, the agar block was transferred into plates containing media. Then, the plates were incubated at room temperature. After full ramification, mycelial discs were prepared using a cork borer.
The influence of four indigenous liquid culture media (potato sucrose broth, corn grit broth, coconut water, and rice bran broth) on the mycelial biomass production of P. sanguineus was determined. The culture broths were prepared following the method of Dulay et al. (2015a). About 30 ml of each media was dispensed in bottles and subjected to sterilization at 121°C, 15 psi for 30 minutes. A 10 mm mycelial disc was aseptically inoculated into each bottle containing a liquid culture medium. The culture bottles were incubated for 7 days at room temperature, then, the mycelia were harvested and the mycelial dry weight was recorded. This was followed by the evaluation of pH. Using 1 M NaOH or 1 M HCl, the pH concentration of the most suitable liquid culture medium was adjusted to 4.0–8.0 with an interval of 1.0. This was followed by sterilization, inoculation of mycelial discs, and incubation for 7 days.
Influence of physical factors
The most suitable liquid culture medium adjusted to the best pH was used to determine the optimum physical factors for the mycelial biomass production of P. sanguineus. These include temperature, illumination, and agitation. To determine the optimum temperature, the culture bottles inoculated with mycelial discs were incubated at refrigerated (8°C), air-conditioned (21°C), and room temperature (28°C). It was followed by the evaluation of the influence of illumination wherein culture bottles with the mycelial disc were incubated at two different illumination conditions, namely, lighted and dark. Lastly, culture bottles were incubated in static and agitated conditions to determine the influence of agitation. The optimum nutritional and physical factors were determined in terms of mycelial dry weight after 7 days of incubation. Each setup was carried out in triplicate.
Determination of bioactivity of mycelial extract
Production of mycelial bBiomass
Using the optimum submerged culture conditions, mycelial biomass was produced. This was done by aseptically inoculating mycelial discs into sterile bottles containing media. Mycelial biomasses were harvested after 7 days of incubation and air-dried until constant weight is achieved.
Ethanolic extraction
Dried mycelia were powdered using a blender. Powdered mycelia were soaked in 95% ethanol with a ratio of 1g:10 ml ethanol for 48 hours prior to filtration. The solvent was removed using a rotary evaporator. The crude extract was placed in a sterile vial with a cover and stored in the refrigerator prior to use.
Source of bacterial cultures
Bacterial cultures of Staphylococcus aureus (UPLB BIOTECH 1582) and Escherichia coli (UPLB BIOTECH 1634) were obtained from the culture collection of Philippine National Collection of Microorganisms, BIOTECH, University of the Philippines, Los Baños, Laguna.
Antibacterial assay
The antibacterial potential of the ethanol mycelial extract was tested against S. aureus and E. coli using the disc diffusion method. Microbial cultures were prepared in a test tube containing nutrient broth and were incubated for 12 to 24 hours. Prior to use, the turbidity of the broth medium was compared to 0.5 McFarland standard. Using a cotton swab, each bacterial culture was swabbed into sterile plates containing media. Then, 6-mm sterile paper discs soaked in the mycelial extract were placed on the surface of the medium. The zone of inhibition formed around the disc was measured after 24 hours of incubation. The test was carried out in triplicate.
Brine shrimp lethality assay
The cytotoxic activity of the extract was assessed using a brine shrimp lethality assay. The eggs were hatched in artificial seawater (25 g rock salt in 1l dH2O) for 24–48 hours. Varying concentrations of P. sanguineus extract were prepared (1, 10, 100, 1,000, and 10,000 μg/ml). Five ml of each extract concentration was placed in vials and ten brine shrimp larvae were exposed into each vial. Artificial seawater served as the control and the test was performed in triplicate. The number of dead larvae was recorded 24 hours after treatment application (hpta). Percentage mortality was computed and the LC50 value was determined using probit analysis. The computed LC50 value was interpreted based on the interpretation established by Bastos et al. (2009), wherein LC50 values greater than one thousand (≥1,000 μg/ml) were considered nontoxic, and greater than 500 but less than 1,000 (≥500 ≤1,000 μg/ml) were weakly toxic, while LC50 values less than 500 (≤500 μg/ml) were regarded as toxic.
Toxicity and teratogenicity assay
The toxic and teratogenic properties of the extract were determined using zebrafish embryo following the method of Dulay et al. (2012b). Five concentrations of the extract were prepared (1 μg/ml, 10 μg/ml, 100 μg/ml, 1,000 μg/ml, and 10,000 μg/ml) in vials and embryo water was used as the control. Each vial containing different extract concentrations was replicated three times. Three embryos at the segmentation phase were introduced into each vial and incubated at room temperature. The observation was done using a microscope 12, 24, and 48 hours after treatment application and the number of dead embryos was recorded. Teratogenic effects and percentage hatchability were determined after 48 hours of exposure. Coagulation, absence of heartbeat, tail not detached, and no somite were considered toxic effects of the extract, while malformation of the head, heart and tail, scoliosis, growth retardation, and yolk deformity were regarded as teratogenic effects (Nagel, 2002).
Statistical analysis
All treatments were laid out in a complete randomized design. ANOVA was used to analyze the data and Tukey’s Honestly Significant Difference was used to compare treatment means at a 5% level of significance. Paired t-test was used to compare the treatment means of illumination and agitation conditions.
RESULTS AND DISCUSSION
Influence of liquid culture media
Liquid culture media is one of the important factors to be considered for the luxurious and rapid growth of mushroom mycelia. Mycelial biomass production of P. sanguineus as influenced by the different liquid culture media after 7 days of incubation was investigated. Among the four media tested, rice bran broth produced the highest mycelial biomass of P. sanguineus with a 100.13 mg/30 ml yield (Table 1). This could be explained by the nutritional composition of rice bran that favors the efficient mycelial growth of this mushroom. According to Kalpanadevi et al. (2018), it contains protein, fat, carbohydrates, fibers, ash, amino acids, minerals, and lipids. Some micronutrients essential for the mycelial growth of fungi like oryzanol, tocopherols, tocotrienols, and phytosterol are also present in rice bran (Nagendra et al., 2011). Therefore, these nutritional components of rice bran make it more favorable for the efficient biomass production of P. sanguineus than the other media used. A similar result was obtained by Quiroz-Castañeda et al. (2011) for this mushroom which showed excellent mycelial growth in a rice husk medium. Marim et al. (2016) concluded that the laccase production of P. sanguineus is greatly influenced by nitrogen concentration in the culture media. According to Narh et al. (2018), rice bran can be used as an efficient alternative source of nitrogen for growing most mushrooms. In this study, rice bran was used as a source of nitrogen and sucrose as a source of carbon. The presence of these two primary nutrient requirements of fungi in the substrate favored the efficient biomass production of this mushroom. The same result was observed by Eugenio et al. (2009) wherein a high amount of laccase was produced in media with sucrose than in other sources of carbon used. Other species of mushrooms which produced optimum mycelial growth in rice bran include Trametes elegans (Dulay et al., 2021b) and L. tigrinus (Dulay et al., 2015b). Pleurotus eryngii also showed excellent mycelial growth in media supplemented with rice bran (Peng et al., 2000). Contrastingly, other mushrooms such as various species of Lentinus, G. lucidum, and A. polytricha showed excellent mycelial growth in coconut water gulaman (Dulay et al., 2021a; Kalaw et al., 2016). Among the four media used, corn grit broth has the lowest biomass yield with 20.02 mg/30ml. Dulay et al. (2012a) gathered similar results for this media which produced the lowest germination of basidiospores of L. tigrinus. On the other hand, corn grit produced very thick mycelia and showed the shortest incubation period of L. strigosus (Dulay et al., 2017).
 | Table 1. Mycelial biomass yield of P. sanguineus grown in liquid culture media after seven days of incubation. [Click here to view] |
Influence of pH
The pH of the media affects the growth and metabolism of most mushrooms. In this study, it was observed that a wide range of pH supports the mycelial biomass production of P. sanguineus (Table 2). Biomass production peaked at pH 7 with 162.77 mg/30 ml. However, the statistical analysis showed that there is no significant difference between the mean biomass yields. According to Shu and Lung (2004), the pH of the medium directly affects cell growth, which could possibly be due to the nutrient intake of the cell membrane. This result is congruent with the findings of Quiroz-Castañeda et al. (2009) for P. sanguineus which showed maximum enzyme activity production at pH 5. Moreover, it was found that these enzymes exhibit strong activity and stability in pH 2 to 8. On the other hand, in the study conducted by Teoh et al. (2011), the highest biomass yield of this mushroom is obtained at pH 4.7. Falkoski et al. (2012) observed that the enzyme activities of this mushroom peaked at 3.5–4.5. Interestingly, this finding is comparable to the results obtained by Dong et al. (2017) for Irpex lacteus which produced optimum biomass at pH 3 to 9. On the other hand, some mushrooms like Cordyceps militaris have a specific pH preference wherein biomass and exobiopolymer production were favored at pH 6 (Park et al., 2001). P. sanguineus is not pH sensitive wherein optimum mycelial biomass can be produced from pH 4–8. These results imply that the pH preferences of mushrooms vary depending on the species wherein beyond optimum pH, the growth rate of some mushrooms is decreased (Basu et al., 2015).
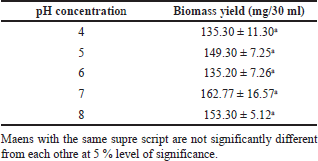 | Table 2. Mycelial biomass yield of P. sanguineus grown in liquid culture media at varying pH levels after seven days of incubation. [Click here to view] |
Influence of temperature
One of the most critical factors to be considered in mushroom cultivation is temperature since growth rate of organism decreases beyond the optimum level (Wang and Zong, 2007). In this study, the influence of temperature on mycelial biomass production of P. sanguineus was determined using the most suitable liquid culture media adjusted to the best pH. Table 3 presents the mean biomass yield after 7 days of incubation. Culture bottles incubated at 28°C recorded the highest biomass yield of 147.20 mg/30 ml. Biomass yield was significantly reduced at lower temperatures (21°C) whereas no biomass was produced at 8°C. A similar result was obtained by Quiroz-Castañeda et al. (2009) wherein 28°C favored laccase production of P. sanguineus in liquid media. These results are comparable with the temperature requirements of Chlorophyllum molybdites (Garcia et al., 2020), P. sanguineus and Pycnoporus cinnabarinus (Sharma and Jaitly, 2017), G. lucidum, P. cystidiosus, V. volvacea, S. commune, L. tigrinus, and L. swartzii (Dulay et al., 2015a; Dulay et al., 2015b). Moreover, Dulay et al. (2021a) found out that room temperature favors luxuriant mycelial growth of various Lentinus species. Contrastingly, Smania et al. (1997), Alberti et al. (2021), and Lee et al. (2004) observed that the optimum mycelial growth and antibiotic production of P. sanguineus, Oudemansiella canarii, and Grifola frondosa were favored under 25°C. According to Lin (2004), the temperature preferences of fungi can be used to classify them into three groups; these are temperate, tropical, or semitemperate. Results gathered in this study suggest that P. sanguineus is a tropical fungus.
Influence of illumination
Illumination or the intensity of light is one of the environmental factors that affect the growth and development of some fungi. Table 3 shows the mean biomass yield of P. sanguineus incubated under lighted and dark conditions. A significantly higher yield was produced in lighted condition with 168.47 mg/30 ml compared to dark (87.6 mg/30 ml). This result is in congruence with the findings of Smania et al. (1997) wherein P. sanguineus showed higher biomass yield under lighted condition. It was reported that light has a positive impact on the fruiting body development of king oysters (Zadrazil, 1978). Poyedinok et al. (2008) conveyed that a certain range of light wavelength causes alteration of the essential composition of mushroom spore, aside from this, disturbances on the important enzymes responsible for the growth processes during the vegetative stage occur. Since P. sanguineus produced a higher yield in lighted conditions, it indicates that the presence of light has a positive impact on the biomass production of this mushroom. However, other mushrooms like Coprinus comatus, L. tigrinus, L. strigosus, and L. swartzii exhibited better mycelial growth in dark conditions (Duarte et al., 2012; Dulay et al., 2020; Mendoza et al., 2020). Meanwhile, Landingin et al. (2020) and Jacob et al. (2015) reported that mycelial growth performances of Cyclocybe cylindracea, Pleurotus citrinolipeatus, Pleurotus djamor, and Pleurotus salmoneostramineus were not directly affected by light. These results indicate that different species of mushrooms have different light preferences.
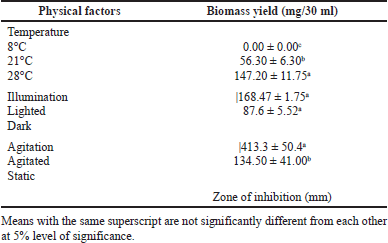 | Table 3. Mycelial biomass yield of P. sanguineus grown in liquid culture media as affected by temperature, illumination and agitation after seven days of incubation. [Click here to view] |
Influence of agitation
Agitation provides better gas supply, mass, and heat transfer to the growing mycelia (Cui et al., 1997). In this study, the culture bottles were incubated under two agitation conditions: agitated (100 rpm) and static. The biomass yield of P. sanguineus was improved under agitated conditions compared to cultures incubated in static conditions (Table 3). A relative increase in biomass yield of this mushroom (413.3 mg/30 ml) can be explained by the shaking condition of the medium. It allows the mycelia to efficiently utilize the nutrients present in the media as there is enough supply of oxygen (Peng et al., 2000). This is also proved by Pointing and Vrijmoed (2000) for the P. sanguineus Thailand strain wherein biomass production increased when incubated in agitated conditions compared to static. Aside from biomass production, the enzymatic activity of this mushroom improved as a result of agitated conditions (Marim et al., 2016). Other mushrooms with the same agitation preference include G. lucidum (Dulay et al., 2015a), Polyporus tricholoma (Vieira et al., 2008), and Pleurotus pulmonarius (Abdullah et al., 2013). However, some species of mushroom such as S. commune, P. cystidiosus, and V. volvacea did not show sensitivity to agitation (Dulay et al., 2015a). In some cases, higher intensity of agitation produced higher yield, so it is also important to evaluate the influence of varying speed of the agitator since only 100 rpm was used in this study. For instance, Aspergillus niger produced higher biomass and glucose oxidase at 700 rpm than at lower rpm (460) (Zetelaki and Vas, 1968). The mycelial biomass production of P. sanguineus in its optimized submerged culture conditions is shown in Figure 1.
Antibacterial activity of mycelial extracts
Mushrooms naturally produce bioactive compounds as part of their defense mechanism in order to survive. In this study, the antibacterial potential of P. sanguineus ethanolic extract was evaluated against E. coli and S. aureus. The mean diameter zone of inhibitions after 24 hours of incubation is presented in Table 4. P. sanguineus ethanol extract showed a 7.47 mm diameter zone of inhibition against S. aureus while no zone of inhibition was observed in E. coli (Fig. 2). This confirms the findings of Rosa et al. (2003) wherein most gram-positive bacteria show more sensitivity to Cinnabarin from P. sanguineus than gram-negative bacteria. Other related studies confirmed that ethanolic, acetonitrile, and hexane extracts of L. tigrinus were found to be active against S. aureus but inactive against E. coli (Dulay et al., 2014; Dulay et al., 2017). Similarly, ethyl acetate extracts of P. sanguineus showed inhibitory activity against Candida krusei, Listeria monocytogenes, and S. aureus, but no zone of inhibition was observed in E. coli (Rosa et al., 2003). However, the ethanolic mycelial extract of this mushroom grown in coconut water demonstrated promising antibacterial potential against both S. aureus and E. coli (Mendoza et al., 2020). The observed differences in the response of bacterial pathogens to the extract can be explained by the findings of Tamboli and Lee (2013) who disclosed that gram-positive and gram-negative bacteria have different cell wall structures. E. coli can resist various foreign materials from entering the cell due to the high level of lipid material present in its cell wall which serves as a barrier (Agatemor, 2009). It is also possible that the amount of the compound responsible for its antibacterial activity is not enough since it is crude. Meanwhile, Mendoza et al. (2020) reported that P. sanguineus mycelia contain novel secondary metabolites including triterpenes, anthrones, tannins, flavonoids, phenols, steroids, alkaloids, anthraquinones, and fatty acids. Among the above-mentioned compounds, triterpenes, anthrones, and alkaloids act as the most effective antimicrobial agents (Asamenew et al., 2011; Chudzik et al., 2015; Saxena et al., 2013). These compounds need to be isolated and purified in order to determine the exact concentration needed to inhibit the growth of bacteria. Thus, based on the results gathered in this study, the ethanolic extract of P. sanguineus is a good source of compounds with antibacterial potential against S. aureus.
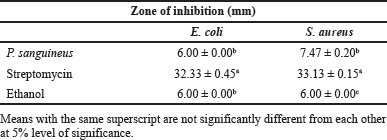 | Table 4. Antibacterial activity of P. sanguineus extract against E. coli and S. aureus. [Click here to view] |
 | Figure 2. Mycelial biomass of P. sanguineus in its optimized submerged culture conditions after seven days of incubation. [Click here to view] |
Cytotoxic activity of mycelial extract
Mushrooms contain agents with anticancer activity. It was reported that P. sanguineus contains a high amount of phenols, carbohydrates, and protein compounds such as laccase that oxidize and degrade cancer-inducing compounds (Piet et al., 2021). In this study, the cytotoxic activity of P. sanguineus mycelia ethanol extract was assessed using a brine shrimp lethality assay. The computed LC50 value is equal to 190.55 μg/ml which indicates that the extract is toxic. Results showed that the mortality rate of brine shrimp larvae is dose-dependent; it can be observed that as the concentration increases, the mortality rate also increases. Similarly, Mendoza et al. (2020) gathered similar results for mycelial extracts of P. sanguineus grown on coconut water wherein an LC50 value of 16.02–154.83 μg/ml was obtained. It produces a valuable amount of triterpene, a secondary compound that is known for its anticancer activity (Chudzik et al., 2015). Moreover, Piet et al. (2021) found that P. sanguineus demonstrated high cytotoxicity toward colon cancer cells which is correlated with the presence of phenolic compounds. The findings of this study clearly suggest that P. sanguineus contain bioactive compounds with the cytotoxic property.
Teratogenic effects of P. sanguineus extract
Teratogenicity is defined as the ability of a certain extract or drug to cause morphological malformations in a developing organism (Elefant et al., 2020). This work demonstrated the teratogenicity of P. sanguineus extracts in zebrafish embryos. Table 5 shows the mean percentage hatchability of embryos exposed to the different concentrations of the extract at 48 hours after treatment application. Figure 4A shows the normal hatched embryo in the control group after 48 hours. A significantly lower hatching rate was observed in embryos exposed to 1 μg/ml, 10 μg/ml, and 100 μg/ml compared to the control group. Accordingly, these results clearly indicate that the hatchability of embryos is influenced by varying concentrations of P. sanguineus extract. It was observed that the hatching rate decreases as the extract concentration increases. Exposure of the embryo to certain chemicals during the early stages of development can cause morphological abnormalities that affect the developmental processes, which further leads to delayed hatching (Dulay et al., 2012b). Growth retardation was found to be the most noticeable teratogenic effect (Figure 4C and G) of the extract after 48 hours. Miao et al. (2022) claimed that the compound responsible for the teratogenicity of mushrooms works by interrupting some biochemical processes, resulting in reduced blood flow and locomotor activity of the embryo. Insufficient amount of glucose, triglyceride, and cholesterol in the bloodstream was also observed since the mushroom extract disrupts the genes responsible for lipid and cholesterol metabolism. Observations in this study indicate that exposure of zebrafish embryos to P. sanguineus extract can cause growth retardation suggesting that it contains substances with teratogenic properties.
Lethal effects of P. sanguineus extract
The toxic or lethal effect of P. sanguineus extract was assessed using zebrafish embryo. Table 6 presents the mean percentage mortality observed after 12, 24, and 48 hours of exposure to different concentrations of the extract. It was found that the lethal effects of P. sanguineus extract were dose and time of exposure dependent. In all observation periods, embryos exposed to lower concentrations of the extract survived whereas high mortality rates were observed in embryos exposed to higher concentrations (10,000 μg/ml and 1,000 μg/ml). Coagulation (Fig. 4H) was the most obvious lethal effect of P. sanguineus extract. Embryos with observed growth retardation caused by 1,000 μg/ml extract showed a complete absence of heartbeat which confirmed their mortality after 48 hours. Aside from P. sanguineus, other species of mushrooms such as P. ostreatus, L. sajor-caju, L. tigrinus, Trichaleurina celebica, and G. lucidum (De Castro and Dulay 2015; Dulay et al., 2012b; Dulay et al., 2014; Sogan et al., 2018) exhibited toxicity to zebrafish embryo. Mendoza et al. (2020) revealed that the mycelia of this mushroom produce triterpenes, phenols, and anthraquinones all of which possess anticancer activity. The presence of these secondary metabolites, therefore, act together causing adverse effects that lead to the death of the embryo. Lima et al. (2012) disclosed that mushrooms contain aromatic hydrazines which are considered carcinogenic. They also observed that patients diagnosed with failures in the renal system can develop cryptogenic encephalopathy when exposed to mushroom compounds with similar characteristics as vitamin D. These results suggest that high concentrations of P. sanguineus can cause lethal effects to zebrafish embryos. Thus, it could be a remarkable resource of toxic substances that can be used for the formulation of effective anticancer drugs.
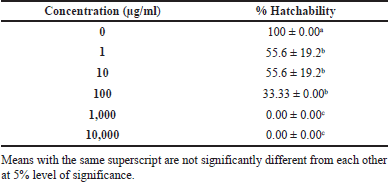 | Table 5. Hatchability rate embryos at different concentrations of P. sanguineus extract after 48 hours. [Click here to view] |
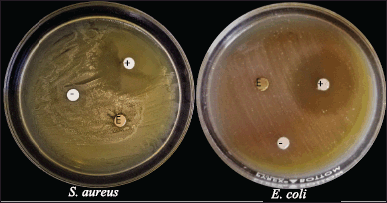 | Figure 3. Antibacterial assay plates showing the zones of inhibition exhibited by the mycelial extract of P. sanguineus against S. aureus and E. coli. (E) mycelial extract, (+) streptomycin and (-) ethanol. [Click here to view] |
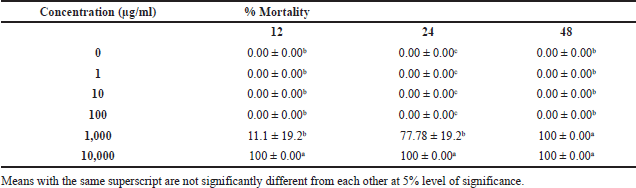 | Table 6. Mortality rate at 12, 24, 48 hpta of embryos at different concentrations of P. sanguineus extract. [Click here to view] |
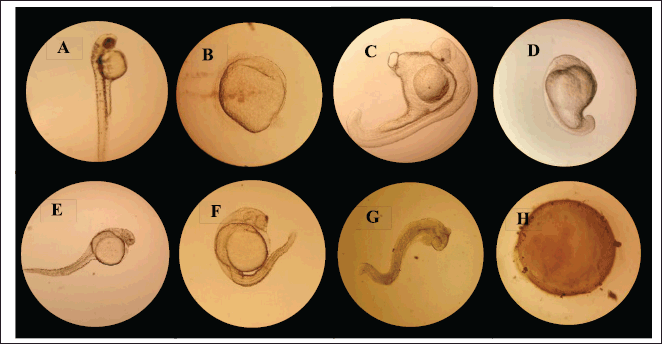 | Figure 4. Teratogenic and toxic effects of P. sanguineus extract on zebrafish embryos at 48 hpta. (A) normal hatched embryo (embryo water). (B-G) Growth retardation at 1 μg/ml to 1,000 μg/ml extract concentration (H) Coagulated embryo incubated at 10,000 μg/ml and 1,000 μg/ml extract concentration. [Click here to view] |
CONCLUSION
Results gathered in this study suggest that a high biomass yield of P. sanguineus in liquid culture can be obtained when provided with the most suitable growth medium and pH. Light, temperature, and agitation are important physical factors that directly improve the mycelia growth of this mushroom during incubation. The observed antibacterial and cytotoxicity of P. sanguineus extract imply that it could be used as a valuable resource of antibacterial and cytotoxic compounds.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was funded by the Academic Research Council of Central Luzon State University.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdullah N, Ismail R, Johari NM, Annuar MS. Production of liquid spawn of an edible grey oyster mushroom, Pleurotus pulmonarius (Fr.) Quel by submerged fermentation and sporophore yield on rubber wood sawdust. Sci Hortic, 2013; 161:65–9. CrossRef
Agatemor C. Antimicrobial activity of aqueous and ethanol extracts of nine Nigerian spices against four food borne bacteria. Elec J Environ Agricu Food Chem, 2009; 8(3):195–200.
Alberti MM, Perez-Chavez AM, Niveiro N, Alberto E. Towards an optimal methodology for basidiomes production of naturally occurring species of the genus Oudemansiella (Basidiomycetes). Curr Microbiol, 2021; 78(4):1256–66. CrossRef
Asamenew G, Bisrat D, Mazumder A, Asres K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytother Res, 2011; 12:1756–60. CrossRef
Austria AB, Dulay RM, Pambid RC. Mycochemicals, antioxidant and anti–diabetic properties of Philippine sawgill mushroom Lentinus swartzii (higher basidiomycetes). Asian J Agricu Biol, 2021; 10 :202006365. CrossRef
Bakratsas G, Polydera A, Katapodis P, Stamatis H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods, 2021; 4:100086. CrossRef
Bastos ML, Lima MR, Conserva LM, Andrade VS, Rocha EM, Lemos RP. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann Clin Microbiol Antimicrob, 2009; 8(1):1–6. CrossRef
Basu S, Bose C, Ojha N, Das N, Das J, Pal M, Khurana S. Evolution of bacterial and fungal growth media. Bioinformation, 2015; 11(4):182. CrossRef
Chen X, Wu JY, Gui X. Production and characterization of exopolysaccharides in mycelial culture of Cordyceps sinensisfungus Cs-HK1 with different carbon sources. Chin J Chem Eng, 2016; 24(1):158–62. CrossRef
Chudzik M, Korzonek-Szlacheta I, Krol W. Triterpenes as potentially cytotoxic compounds. Molecules, 2015; 20:1610–25. CrossRef
Cui YQ, Van der Lans RG, Luyben KC. Effect of agitation intensities on fungal morphology of submerged fermentation. Biotechnol Bioeng, 1997; 55(5):715–26. CrossRef
De Castro M, Dulay RM. Toxic and teratogenic effects of Lentinus sajor-caju and Pleurotus ostreatus ethanolic extracts in Danio rerio embryo model. Int J Biol Pharm Allied Sci, 2015; 4:2261–9.
Dong X, Song X, Dong C. Nutritional requirements for mycelial growth of milk-white toothed mushroom, Irpex lacteus (Agaricomycetes), in submerged culture. Int J Med Mushrooms, 2017; 19(9):829–38. CrossRef
Duarte LT, Tiba JB, Santiago MF, Garcia TA, Bara MT. Production and characterization of tyrosinase activity in Pycnoporus sanguineus CCT-4518 crude extract. Braz J Microbiol, 2012; 43(1):21–9. CrossRef
Dulay RMR, Alcazar AA, Kalaw SP, Reyes RG, Cabrera EC. Nutritional and physical requirements for mycelial growth and basidiocarp production of Trametes elegans from the Philippines. Asian J Agric Biol, 2021a; 1: 1–9. CrossRef
Dulay RM, Cabrera EC, Kalaw SP, Reyes RG. Optimization of submerged culture conditions for mycelial biomass production of fourteen Lentinus isolates from Luzon Island, Philippines. Biocatal Agricu Biotechnol, 2021b; 38:102226. CrossRef
Dulay RMR, Cabrera EC, Kalaw SP, Reyes RG. Optimal growth conditions for basidiospore germination and morphogenesis of Philippine wild strain of Lentinus tigrinus (Bull.) Fr. Mycosphere, 2012a; 3(6):926–33. CrossRef
Dulay RMR, Cabrera EC, Kalaw SP, Reyes RG, Hou CT. Cultural conditions for basidiospore germination of Lentinus swartzii and Lentinus strigosus and their morphogenesis. Asian J Agric Biol, 2020; 8(4):377–85
Dulay RMR, Flores KS, Tiniola RC, Marquez DHH, Dela Cruz AG, Kalaw SP, Reyes RG. Mycelial biomass production and antioxidant activity of Lentinus tigrinus and Lentinus sajor-caju in indigenous liquid culture. Mycosphere, 2015b; 6(6):659–66. CrossRef
Dulay RMR, Garcia EJB. Optimization and enriched cultivation of Philippine (CLSU) strain of Lentinus strigosus (BIL1324). Biocatal Agric Biotechnol, 2017; (12):323–8. CrossRef
Dulay RM, Kalaw SP, Reyes RG, Alfonso NF, Eguchi F. Teratogenic and toxic effects of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (higher Basidiomycetes), on zebrafish embryo as model. Int J Med Mushrooms, 2012b; 14(5):507–12. CrossRef
Dulay RM, Kalaw SP, Reyes RG, Cabrera EC. Embryo-toxic and teratogenic effects of Philippine strain of Lentinus tigrinus (tiger sawgill basidiomycetes) extract on zebrafish (Danio rerio) embryos. Ann Biol Res, 2014; 5(6):9–14.
Dulay RM, Miranda LA, Malasaga JS, Kalaw SP, Reyes RG, Hou CT. Antioxidant and antibacterial activities of acetonitrile and hexane extracts of Lentinus tigrinus and Pleurotus djamour. Biocatal Agric Biotechnol, 2017; 9:141–4. CrossRef
Dulay RM, Ray K, Hou CT. Optimization of liquid culture conditions of Philippine wild edible mushrooms as potential source of bioactive lipids. Biocatal Agric Biotechnol, 2015a; 4(3):409–15. CrossRef
Elefant E, Hanin C, Cohen D. Pregnant women, prescription, and fetal risk. Handb Clin Neurol, 2020; 173:377–89. CrossRef
Eugenio ME, Carbajo JM, Martín JA, Gonzalez AE, Villar JC. Laccase production by Pycnoporus sanguineus under different culture conditions. J Basic Microbiol, 2009; 49(5):433–40. CrossRef
Falkoski DL, Guimarães VM, de Almeida MN, Alfenas AC, Colodette JL, de Rezende ST. Characterization of cellulolytic extract from Pycnoporus sanguineus PF-2 and its application in biomass saccharification. Appl Biochem Biotechnol, 2012; 166(6):1586–603. CrossRef
Garcia BL, Undan JR, Dulay RM, Kalaw SP, Reyes RG. Molecular identification and optimization of cultural conditions for mycelial biomass production of wild strain of Chlorophyllum molybdites (G. Mey) Massee from the Philippines. J Appl Biol Biotechnol, 2020; 8(6):1–6.
Garcia-Cruz F, Duran-Paramo E, Garin-Aguilar MA, del Toro GV, Chairez I. Parametric characterization of the initial pH effect on the polysaccharides production by Lentinula edodes in submerged culture. Food Bioprod Process, 2020; 119:170–8. CrossRef
Jacob JK, Kalaw SP, Reyes RG. Mycelial growth performance of three species of Pleurotus on coconut water gelatin. Curr Res Environ Appl Mycol, 2015; 5(3):263–8. CrossRef
Jaszek M, Osinska-Jaroszuk M, Sulej J, Matuszewska A, Stefaniuk D, Maciag K, Polak J, Matuszewski L, Grzywnowicz K. Stimulation of the antioxidative and antimicrobial potential of the blood red bracket mushroom Pycnoporus sanguineus (higher Basidiomycetes). Int J Med Mushrooms, 2015; 17(8):701–12. CrossRef
Kalaw SP, Alfonso DO, Dulay RM, De Leon AM, Undan JQ, Undan JR, Reyes RG. Optimization of culture conditions for secondary mycelial growth of wild macrofungi from selected areas in Central Luzon, Philippines. Curr Res Environ Appl Mycol, 2016; 6(4):277–87. CrossRef
Kalpanadevi C, Singh V, Subramanian R. Influence of milling on the nutritional composition of bran from different rice varieties. J Food Sci Technol, 2018; 55(6):2259–69. CrossRef
Landingin HR, Francisco BE, Dulay RM, Kalaw S, Reyes R. Optimization of culture conditions for mycelial growth and basidiocarp production of Cyclocybe cylindracea (Maire). CLSU Int J Sci Technol, 2020; 4(1):1–7. CrossRef
Lee BC, Bae JT, Pyo HB, Choe TB, Kim SW, Hwang HJ, Yun JW. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzyme Microb Technol, 2004; 35(5):369–76. CrossRef
Lima ADL, Costa Fortes R, Garbi Novaes MRC, Percario S. Poisonous mushrooms; a review of the most common intoxications. Nutr Hosp, 2012; 27(2):402–8.
Lin Z. Grass (Juncao). mushroom grower’s handbook 1: oyster mushroom cultivation. MushWorld-Heineart Incorporated, Seoul, South Korea, pp 107–13, 2004.
Marim RA, Oliveira AC, Marquezoni RS, Servantes JP, Cardoso BK, Linde GA, Colauto NB, Valle JS. Use of sugarcane molasses by Pycnoporus sanguineus for the production of laccase for dye decolorization. Genet Mol Res, 2016; 15(4) :1–9. CrossRef
Mendoza WC, Dulay RM, Valentino MJ, Reyes RG. Mycelial biomass and biological activities of Philippine mushroom Pycnoporus sanguineus in time-course submerged culture. J Appl Biol Biotechnol, 2020; 8(05):88–93.
Miao W, He L, Zhang T, Li C. Lentinan impairs the early development of Zebrafish embryos, possibly by disrupting glucose and lipid metabolism. Processes, 2022; 10(1):120. CrossRef
Nagel R. Dart: the embryo test with zebrafish Danio rerio- a general method in eco-toxicology and toxicology. Altex, 2002; 19(1/02):38–48.
Nagendra Prasad MN, Sanjay KR, Shravya Khatokar M, Vismaya MN, Nanjunda Swamy S. Health benefits of rice bran-a review. J Nutr Food Sci, 2011; 1(3):1–7. CrossRef
Narh C, Frimpong C, Mensah A, Wei Q. Rice bran, an alternative nitrogen source for Acetobacter xylinum bacterial cellulose synthesis. Bioresources, 2018; 13(2):4346–63. CrossRef
Park JP, Kim SW, Hwang HJ, Yun JW. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett Appl Microbiol, 2001; 33(1):76–81. CrossRef
Peng J, Lee C, Tsai Y. Effect of rice bran on the production of different king oyster mushroom strains during bottle cultivation. J Agric Res China, 2000; 49(3):60–7.
Piet M, Zaj?c A, Paduch R, Jaszek M, Frant M, Stefaniuk D, Matuszewska A, Grzywnowicz K. Chemopreventive activity of bioactive fungal fractions isolated from milk-supplemented cultures of Cerrena unicolor and Pycnoporus sanguineus on colon cancer cells. 3 Biotech, 2021; 11(1):1–3. CrossRef
Pointing SB, Jones EB, Vrijmoed LL. Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia, 2000; 92(1):139–44. CrossRef
Pointing SB, Vrijmoed LP. Decolorization of azo and triphenylmethane dyes by Pycnoporus sanguineus producing laccase as the sole phenoloxidase. World J Microbiol Biotechnol, 2000; 16:317–8. CrossRef
Poyedinok NL, Mykhailova OB, Shcherba VV, Buchalo AS, Negriyko AM. Light regulation of growth and biosynthetic activity of Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae), in pure culture. Int J Med Mushrooms, 2008; 10(4) :369–78. CrossRef
Quiroz-Castañeda RE, Balcazar-Lopez E, Dantan-Gonzalez E, Martinez A, Folch-Mallol J, Martinez Anaya C. Characterization of cellulolytic activities of Bjerkandera adusta and Pycnoporus sanguineus on solid wheat straw medium. Electron J Biotechnol, 2009; 12(4):5–6. CrossRef
Quiroz-Castaneda RE, Perez-Mejia N, Martinez-Anaya C, Acosta-Urdapilleta L, Folch-Mallol J. Evaluation of different lignocellulosic substrates for the production of cellulases and xylanases by the basidiomycete fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation, 2011; 22(3):565–72. CrossRef
Rosa LH, Machado KM, Jacob CC, Capelari M, Rosa CA, Zani CL. Screening of Brazilian basidiomycetes for antimicrobial activity. Mem Inst Oswaldo Cruz, 2003; 98(7):967–74. CrossRef
Sharma V, Jaitly AK. Optimization of growth of two wild species of Pycnoporus collected from foothill of Uttarakhand. Int J Agric Innov Res, 2017; 6:2319–1473.
Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacogn Phytotherapy, 2013; 1(6):168–82.
Shu CH, Lung MY. Effect of pH on the production and molecular weight distribution of exopolysaccharide by Antrodia camphorata in batch cultures. Process Biochem, 2004; 39(8):931–7. CrossRef
Smania EF, Smania Jr A, Loguercio-Leite C, Gil ML. Optimal parameters for cinnabarin synthesis by Pycnoporus sanguineus. J Chem Technol Biotechnol Int Res Process Environ Clean Technol, 1997; 70(1):57–9. CrossRef
Sogan MM, Maslang JA, Dulay RM. Myco-Chemicals and teratogenic activity of wild mushroom Trichaleurina celebica from Mt. Palali, Quezon, Nueva Vizcaya, Luzon Island, Philippines. CLSU Int J Sci Technol, 2018; 3(2):17–23. CrossRef
Souza EF, Passos DF, Souto F, Calado V, Pereira N. Enhancement of kraft lignin molecular relaxation based on laccases from Pycnoporus sanguineus produced in instrumented bioreactors. Biomass Convers Biorefin, 2021; 28:1–2. CrossRef
Tamboli DP, Lee DS. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram-positive and gram-negative bacteria. J Hazard Mater, 2013; 260:878–84. CrossRef
Teoh YP, Don MM, Ujang S. Media selection for mycelia growth, antifungal activity against wood-degrading fungi, and GC-MS study by Pycnoporus sanguineus. BioResources, 2011; 6(3):2719–31. CrossRef
Tuong TD, Chu DX, Dieu BT. Antioxidant activity of fruiting body extracts from Pycnoporus sanguineus (L.: Fr.) Murrill mushroom. Vietnam J Sci Technol, 2020; 58(2):143–54.
Velioglu Z, Urek Ro. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turk J Biol, 2015; 39(1):160–6. CrossRef
Vieira GR, Liebl M, Tavares LB, Paulert R, Smania Júnior A. Submerged culture conditions for the production of mycelial biomass and antimicrobial metabolites by Polyporus tricholoma Mont. Braz J Microbiol, 2008; 39:561–8. CrossRef
Wang SJ, Zhong JJ. Bioreactor engineering. In: Yang ST (ed.). Bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, The Netherlands, pp 131–61, 2007. CrossRef
Watanabe RA, Sales PD, Campos LC, Garcia TA, Valadares MC, Schimidt F, Santiago MF. Evaluation of the use of Pycnoporus sanguineus fungus for phenolics and genotoxicity decay of a pharmaceutical effluent treatment. Rev Ambiente Agua, 2012; 7:41–50. CrossRef
Yahaya YA, Don MM. Pycnoporus sanguineus as potential biosorbent for heavy metal removal from aqueous solution: a review. J Phys Sci, 2014; 25(1):1.
Zadrazil F. Cultivation of Pleurotus. The biology and cultivation of edible mushrooms by ST Chang and WA Hayes. Academic press Incorporated, Orlando, FL, vol. 1, pp 521–58, 1978. CrossRef
Zetelaki K, Vas K. The role of aeration and agitation in the production of glucose oxidase in submerged culture. Biotechnol Bioeng, 1968; 10(1):45–59. CrossRef