INTRODUCTION
Anaemia, constitutes a major public health concern in developing countries. According to WHO global statistics, 40% of pregnant women, and 42% of children under 5 are anaemic. It alters immune mechanisms and also associated with increased morbidity rates. The economic and social growth of a nation is impacted by anaemia, as it is attributed to delayed cognitive and motor development in children and reduced work capacity in adults (WHO, 2022). Additionally, anaemia during pregnancy is also associated with increased risk of haemorrhage, sepsis, maternal mortality, perinatal mortality, and has poor reproductive outcomes such as preterm birth, low-birth-weight babies and diminished iron reserves for the foetus, etc. It is estimated that approximately all women are iron deficient to some degree and more than half of the pregnant women suffer from anaemia in developing countries. Millions of individuals may suffer from poor health and quality of life if the prevalence of anaemia is not reduced as this would ultimately hinder the development of a nation. The recently released National Family Health Survey (NFHS-5) census reveals that the prevalence of anaemia has increased to 62.6% among children, women of all age groups including pregnant women and men in India [Release of NFHS-5 (2019-21)].
Although the traditional treatment for iron deficiency anaemia involves taking iron supplements like ferrous sulphate or elemental iron, adherence to the medicine is challenging due to variety of adverse side effects like metallic taste, epigastric discomfort, nausea, diarrhea, or constipation, etc. The adverse effects of iron supplements can be reduced by taking medications with food. However, doing so might decrease the absorption of iron. Therefore, there is a need to search for innovative drugs that have better therapeutic value and lesser side effects (Nguyen and Tadi, 2022).
Siddha, a well-known system of Indian medicine owns numerous medicinal preparations for the treatment of anaemia since ages. Siddha medicines are of 64 types viz. 32 internal medicines and 32 external medicines (Rajendra Kumar et al., 2018). All these medicinal formulations were prepared by using plant (herbs), minerals, metals, and animal products. Many types of Siddha formulations like Chooranam, Vadagam, Manappagu, Paanidham, Nei, Mathirai, Elagam, Karpam, Chendhuram, etc of Polyherbal, herbo-mineral/ herbo-metallic origin are available for the treatment of anaemia (Thirunarayanan, 2012). So far, many clinical trials including few randomized clinical trials (RCTs), pre-clinical studies, and other studies have been carried out in these Siddha formulations for the correction of anaemia.
Pre-clinical trials provide the first insight on drug’s suitability for testing on humans by ensuring safety and efficacy in in-vitro, in-vivo or in-silico studies. Therefore, pre-clinical studies of Siddha formulations form the objective of current study in order to compile the data of various studies done so far.
Since, all these Siddha formulations have lesser or minimal side effects, they are successfully used by Siddha Physicians for the treatment of anaemia widely. More so, a medicinal kit including Madhulai Manappagu and Annabedhi Chendhuram was provided by Primary Healthcare Centers to each pregnant women under Tamil Nadu State Government’s Health Program “Amma Magapperu Sanjeevi Thittam” to prevent anaemia during pregnancy since 2016. Therefore, it is the need of the hour to take this traditional Indian system of medicine for its global recognition in anaemia treatment. Hence this study was aimed to conduct from all the available data from 1982 to till date. The objectives of this study includes compilation and summation of all the findings of relevant pre-clinical studies of Siddha formulations in the treatment of anaemia. It also emphasizes the strength of evidence in treating anaemia with Siddha formulations. The outcome of the study may throw light on the efficacy of various Siddha formulations and also will explore the scope of future research prospects in this traditional system of medicine to combat anaemia.
METHODOLOGY
This systematic review was carried out in accordance with the principles of Cochrane Collaboration framework and also by following the guidelines of Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA).
Literature search strategy
Using text word search strategy, the literature search was carried out in electronic databases. Online platforms like PubMed, Cochrane, ScienceDirect, Medline, Scopus, and Google scholar were used in search of articles. The search terms include pandu, paandu, pantu, veluppu, veluppu noi, and sogai. The search was also elaborated by using the search terms annabethi or annabedhi or annabedi, karisalai or karislankanni or karisaalai, ayam, kantham/kaantham/kandham/gandham, maathulai/madhulai to extract a greater number of studies (The search words were selected based on some common Siddha formulations used for anaemia and its correction). To maximize the sensitivity of the search and to avail most of the relevant studies, general keywords like Iron deficiency anaemia and Siddha, Anaemia and Siddha, Anemia and Siddha, Sittha medicine and anaemia were also used.. Furthermore, bibliographies from the extracted studies were also reviewed and researched to identify additional studies.
No limitations were set during the literature search. The literature search was carried out with a time span of about 40 years i.e., from 1982–2022 and they were collected from July to September 2022.
Study selection criteria
The criteria followed for individual studies to be included in this systematic review and meta-analysis are as follows:
The study should be published in English language; published in indexed or peer-reviewed journals; adhered to the Siddha system of Indian medicine; with original data; pre-clinical studies with Siddha interventions; Studies with control and trial groups; Studies with quantitative estimation of Hemoglobin (Hb) between trial and control groups were considered eligible for meta-analysis. The trial results which discuss red blood corpuscles (RBC) studies and other blood investigations along with Hb were also included. If data was not summarized and discussed in the literature, raw data were extracted and statistical analysis was done by the authors to avail the maximum number of studies for meta-analysis.
The studies which did not fulfill the inclusion criteria were excluded. After title and abstract screening, some articles were excluded. Studies with non-available full text articles were also excluded. Furthermore, some articles with poor description of methodology, and statistical methods were excluded after full text article screening.
Data selection and extraction
The selection of studies for this systematic review and data extraction from eligible studies were done manually. Data were extracted from the studies and fetched in excel spreadsheets according to the requirements in different sections. General data including the name of authors, title, literature reference in classical Siddha texts, year of publication and study place were extracted primarily. Other than that, data like number of animals used, type of experimental model used, method of induction of anaemia, route of administration, details of intervention and outcome measures were also collected. For the meta-analysis, the quantitative data of Hb, RBC and other available parameters were entered separately.
Statistical analysis
Collected data were analyzed according to the requirements of the meta-analysis. The Cochrane collaboration tool, Rev-Man 5.2 software was used to carry out the study’s statistical analysis. Pre-clinical Pharmacological studies were only used in meta-analysis. Standard mean deviation, 95% of Confidence interval and p-value were considered as summary statistics of this study. Heterogeneity was calculated using Cochrane’s statistics (I2). If the data is homogenous (I2 ≤ 50%), meta-analysis was done using fixed effect model. In case of heterogeneous data (I2 ≥ 50%), random effect model of meta-analysis was used. Publication bias was addressed using funnel plot analysis. The z-score with a p-value less than 0.05 was considered statistically significant.
RESULTS
Description of included studies
About 79 articles were retrieved from a total of 975 after duplicates removal and title and abstract screening. 3 articles were excluded as their full texts were not available. 35 studies were excluded after full text screening. Finally, this systematic review included a total of 39 papers. Two articles (Padmagreesan, 2008; Shenbagavalli, 2009) were considered twice as they discussed two different formulations for anaemia in pre-clinical aspect. Hence, a total of 41 Siddha formulations with pre-clinical studies were included for systematic review. Among these, pre-clinical pharmacological data were extracted from 33 articles and pre-clinical toxicological data were extracted from 30 articles.
Meta-analysis was carried out in pre-clinical pharmacological data. Out of 33 articles, only 28 studies were found to be eligible for meta-analysis as 5 articles were excluded due to poor description of statistical data. Selection of articles for this review was shown as flowchart in Figure 1 which was designed based on PRISMA 2020.
Systematic review
Characteristics of included studies
Pre-clinical pharmacological studies
A total of 153 albino rats or mice of both sex and standard weight were used as experimental animals in all the studies. The chemical compound Phenyl hydrazine (n = 15) was used to induce anaemia artificially in experimental animals, other (n = 18) studies did not mention about the induction procedure. In all the studies, the oral route was adopted for the administration of medicines. The duration of treatment varies from 28 days to 42 days in pre-clinical pharmacological studies. Detailed characterization of included pre-clinical studies of both polyherbal and herbo-mineral/ herbo-metallic formulations were presented in Table 1.
Pre-clinical toxicological studies
Wistar albino rats or mice of both sex and standard weight according to Organization for Economic Cooperation and Development guidelines were used as experimental animals in all the studies. In all the acute toxicity studies, single oral gavage of trial drug maximum at the dose of 10× were given and observed for 14 days. In most of sub-acute/ repeated oral toxicity studies, oral administration of trial drugs at the doses of 1×, 3×, 5× and 10× were given for the period of 28 days/90 days and observed. Detailed characterization of included pre-clinical toxicological studies of polyherbal and herbo-mineral/ herbo-metallic drugs were presented in Table 2.
Study interventions and systematic review
Pre-clinical pharmacological studies
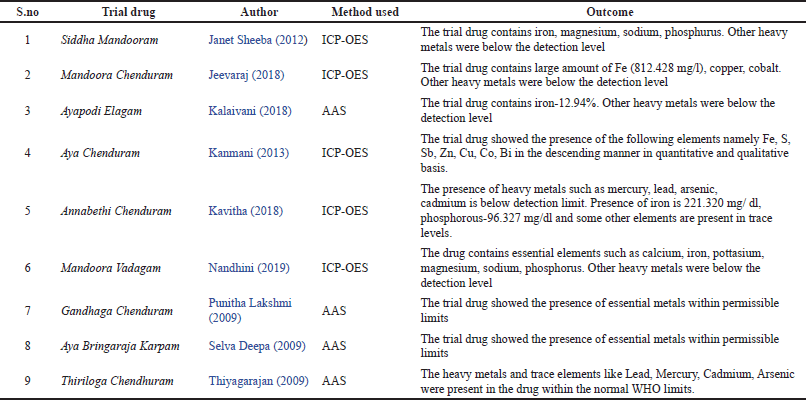
In this review article, 33 Siddha formulations which were used in the treatment of anaemia were included. Out of these 33 formulations, 19 were polyherbal formulations [Chooranam (n = 12); Manappagu (n = 2); Nei (n = 1); Mathirai (n = 1); Elagam (n = 1); Karpam (n = 1); Usidham (n = 1)] and 14 were herbo-mineral/herbo-metal formulations [Vadagam (n = 1); Paanidham (n = 1); Mathirai (n = 2); Elagam (n = 1); Karpam (n = 1); Chenthuram (n = 8)] as depicted in Figure 2. Some formulations were subjected to two or more pre-clinical trials as interventions by different authors to analyze its therapeutic efficacy, for example, in polyherbal formulations, Karisalankanni Chooranam, Madhulai Manappagu were analyzed in 2 different studies and in herbo-mineral formulations, Annabedhi Chenduram, Thiripalai Mathirai were used by 2 authors as interventional drug in their studies. Statistical data of Hb and RBC between trial and control groups as discussed in Table 1.
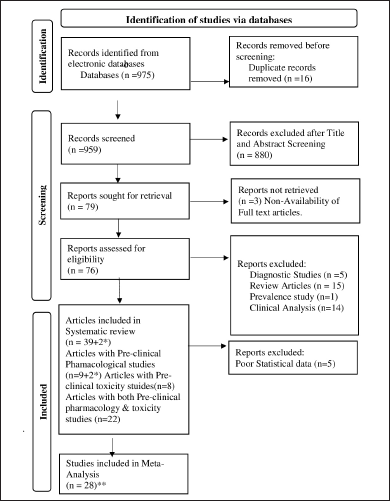 | Figure 1. Prisma flow diagram of included studies, *-No. of studies considered twice. **- Only pre-clinical pharmacological studies considered. [Click here to view] |
Pre-clinical toxicological studies
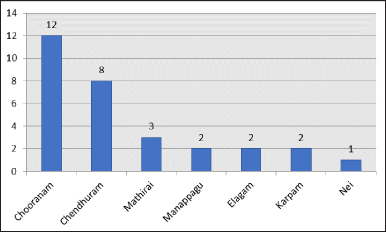
About 30 studies were included and analyzed for toxicity evaluation of Siddha formulations. Acute Toxicity studies were carried out in all 30 Siddha formulations and Sub-Acute or Repeated Oral toxicity studies were carried out in 20 formulations only. Of all the Siddha formulations used in toxicity studies, 16 were polyherbal formulations [Chooranam (n = 11); Manappagu (n = 1); Nei (n = 1); Mathirai (n = 1); Elagam (n = 1); Karpam (n = 1)]; and 14 were herbo-mineral/herbo-metal formulations [Paanidham (n = 1); Vadagam (n = 1); Mathirai (n = 1); Elagam (n = 1); Chenthuram (n = 10)] as depicted in Figure 3.
Heavy metal contents in trial drugs
Out of 33 studies which were included in systematic review, 9 studies have evidenced the presence of minerals and heavy metals in their respective trial drugs. 5 studies used ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) and 4 studies used AAS (Atomic Absorption Spectrometry) to analyze and report the detection limits of heavy metals in the trial drugs. Among these 9 preclinical studies, 3 trial drugs namely Annabedhi Chedhuram (221. 320 mg/dl), Mandoora Chendhuram (812.428 mg/l) and Ayapodi Ilagam (12.94%) has greater Iron content then the other trial drugs (Table 4). The other heavy metals like lead, mercury, Cadmium and arsenic were below the detection limits in all the Siddha drugs studied.
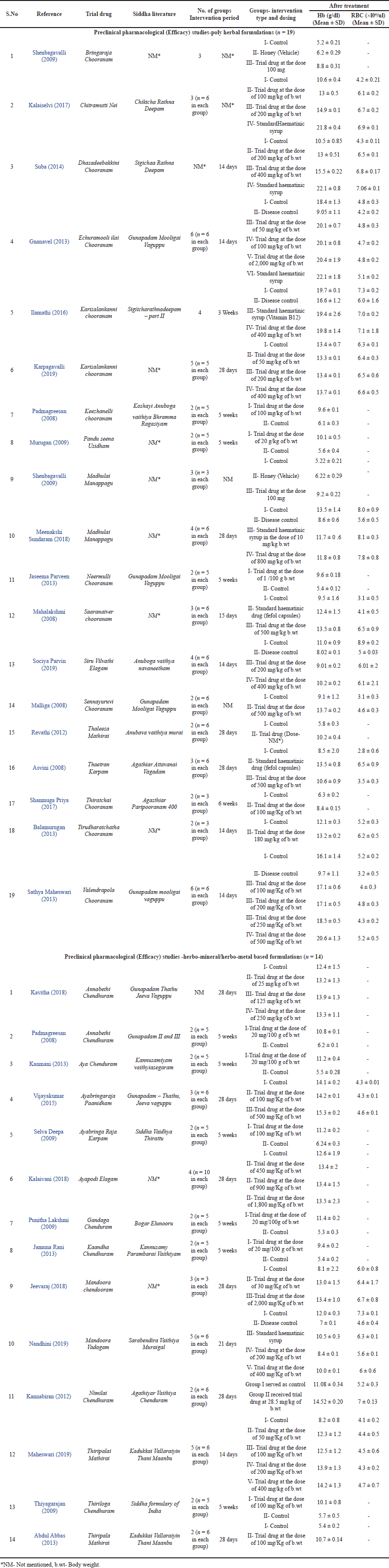 | Table 1. Detailed description and characterization of included pre-clinical pharmacological studies. [Click here to view] |
Risk of bias assessment
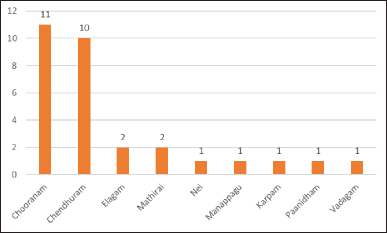
Funnel plot is generated using revman-5.2 to assess publication bias in selected studies. Funnel plot is symmetrical; hence no significant publication bias was observed in this study (Fig. 4).
Meta-analysis
In this meta-analysis, 28 articles evaluating pre-clinical therapeutic effectiveness of Siddha formulations for treating anaemia were included. Quantitative tests for heterogeneity was 95% (I2 = 95%) for the anaemia related outcomes viz. Hb level and Total RBC and thus suggests there was study variability i.e. heterogeneity; which means significant differences across studies. Hence, random effect model was employed for both the outcomes.
Hb levels before and after treatment
Increase in mean Hb of the trial group which received various Siddha formulations before and after treatment was found to be significant. (SMD: 7, 95% CI: 5.43 to 8.57, n = 28 studies; 153 experimental animals each in control and experimental group; Z Value = 8.75; p Value < 0.00001) as shown in Figure 5.
The mean difference in Hb levels of experimental animals which received Siddha polyherbal formulations and Siddha herbo-metallic/herbo-mineral formulations was also observed. Among these two groups, animals which received Herbo-mineral/Herbo-metallic formulations showed significant increase in Hb levels than Polyherbal formulations [Polyherbal formulations- SMD: 7.61, 95% CI: 5.89 to 9.33, n = 15 studies; 85 experimental animals each in control and experimental group; Z Value = 8.65; p Value < 0.00001; Herbo-mineral/Herbo-metallic formulations- SMD: 10.97, 95% CI: 7.27 to 14.68, n = 13 studies; 76 experimental animals each in control and experimental group; Z value = 5.80; p value < 0.00001] and the same was depicted in Figures 6 and 7 respectively. The difference in Hb levels before and after treatment with the trial drugs have been calculated and it was mentioned in Table 3. About 0.4 to 11.4 g/dl improvement in Hb were observed in these pre-clinical studies after treating with the respective trial drugs. The difference in mean Hb between polyherbal and herbo-mineral/ herbo-metallic formulations was shown in Figure 8.
RBC level before and after treatment
Among 28 studies included, only 14 studies have studied the Total RBC count which is another anaemia related outcome of this meta- analysis. These studies have observed the differences in total RBCs and other blood cell indices between trial and control groups. On quantifying the data, there was a notable improvement in mean RBC before and after treatment. (SMD: 2.40, 95% CI: 1.46 to 3.33, n = 14 studies; 163 experimental animals; Z Value = 5.02; p Value < 0.00001) as shown in Figure 9. Besides, there was no appreciable difference was observed between Polyherbal and herbo-mineral/ herbo-metallic formulations study groups due to negligible amount of studies under each groups.
DISCUSSION
On analyzing the data, neither the poly herbal formulations nor any metal or mineral based herbal formulations produced any toxicological behavioral changes, physiological changes, histological changes, bio-chemical changes and mortality up to the therapeutic dose level. In 10× of the therapeutic dose as single oral gavage, the Siddha formulation “Valendrapola Chooranam” showed some moderate toxic symptoms like alertness, increased touch response, writhing and hypnosis. Another drug namely “Thiriloga Chendhuram” at 3× therapeutic dose for a period of 90 days, showed some mild interstitial edema with hemorrhage in heart. However, both the drugs were very much safer at therapeutic dose level as no adverse signs or symptoms were observed. This kind of toxicity at higher doses might be due to non-adherence of standard protocols during trial drug preparations.
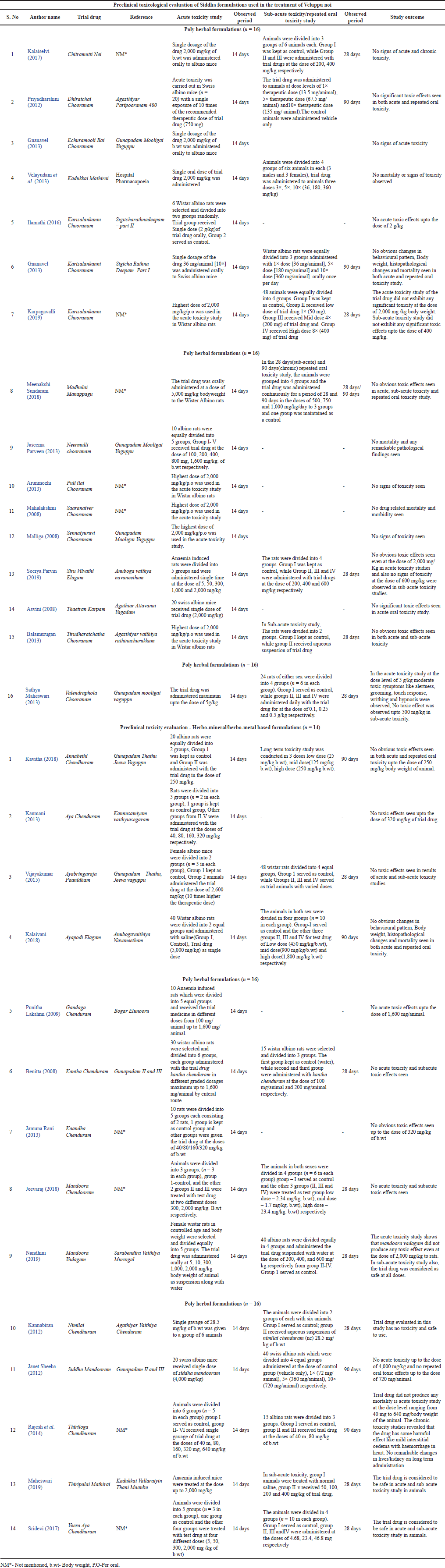 | Table 2. Detailed description and characterization of included pre-clinical toxicological studies. [Click here to view] |
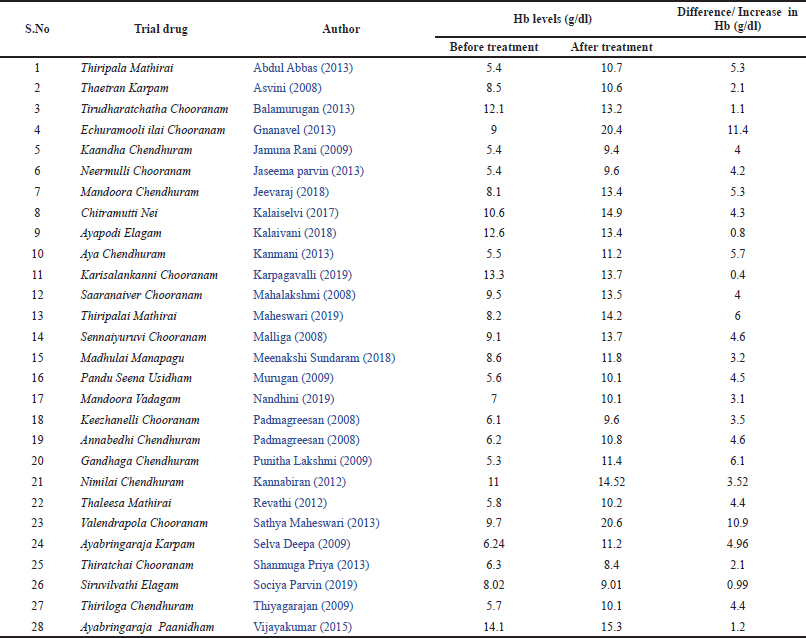 | Table 3. Difference in Hb levels before and after treatment with trial drugs. [Click here to view] |
Metals are toxic in its natural inorganic form. Upon treatment with various polyherbal juices and adopting various techniques like grinding, calcination, etc for herbo-metallic drug preparations, the particle size of the metals would be reduced and reaches nano size which can be easily absorbed under physiological conditions. When a metal remains unprocessed during drug preparation, it leads to accumulation in various major organs and the same might initiate metal induced tissue damage through free radical mechanisms. As Siddhars wrote their experiences in the form of classical literatures, strict adherence to standard protocols during drug preparation, appropriate therapeutic dose, adjuvants used and period of invention were very much essential factors for the usage of herbo-metallic preparations. This might be the reason for the safe nature of the trial drugs at their therapeutic doses and the same implies that the pre-clinical study was done following the appropriate standards and guidelines involved. Though some toxicity were observed at 10× and 3× therapeutic doses of two Siddha formulations, the same may be negligible as that larger doses will never be employed for the treatment of any diseases. Hence it can be established that all the Siddha formulations analyzed in this study was proved to be safe in their respective therapeutic doses.
The presence of heavy metals in a formulation is a highly concerning parameter, regarding safety in human use of the trial drugs. In addition to anaemia related outcomes, heavy metal analysis of many herbo-mineral and herbo-metallic drugs carried out by the included studies were also observed in this analysis. The herbo-mineral and herbo-metallic trial drugs such as Siddha Mandooram, Mandoora Chenduram, Ayapodi Elagam, Aya Chenduram, Annabethi Chenduram, Mandoora Vadagam, Gandhaga Chenduram, Aya Bringaraja Karpam were tested for heavy metals in their respective trials. In all the trials, concentration of heavy metals like Lead, Cadmium, Mercury and Arsenic were found to be below the detection limit. This result ensures the safe usage of the herbo-mineral and herbo-metallic formulations even for a longer treatment period.
 | Table 4. Minerals and heavy metals levels in trial drugs. [Click here to view] |
 | Figure 2. Frequency of type of medicines used in pre-clinical pharmacological analysis. [Click here to view] |
 | Figure 3. Frequency of type of medicines used in pre-clinical toxicological analysis. [Click here to view] |
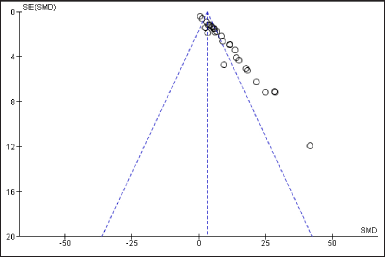 | Figure 4. Funnel plot of included studies. [Click here to view] |
SUMMARY OF FINDINGS
In analyzing the results, in terms of subjective and hematological indicators, most of the Siddha formulations showed statistically significant results and hence proved to be effective in correcting anaemia. Echuramooli Ilai Chooranam in polyherbal formulations, Gandhaga Chendhuram in Herbo-metallic formulations and Thiripalai Mathirai in Herbo-mineral formulations showed highest increase in Hb and RBC. Summary of pre-clinical pharmacological, pre-clinical toxicological studies and clinical studies carried out in Siddha formulations are descripted in Table 5. The Siddha formulations in which all the pre-clinical and clinical trials have been done can be used for therapeutic usage. However, studies with larger sample size and RCT’s should be conducted to ensure its reliability.
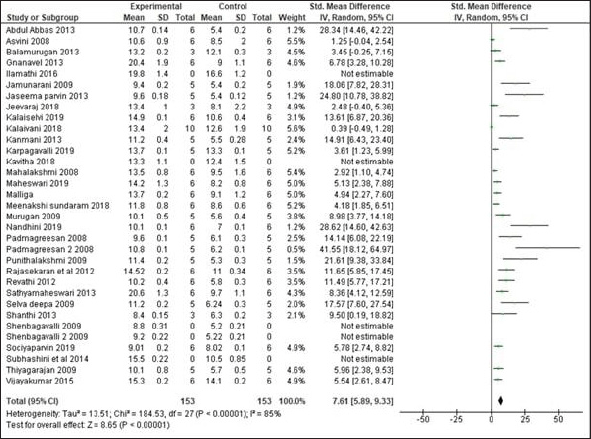 | Figure 5. Forest plot showing Hb difference before and after treatment. [Click here to view] |
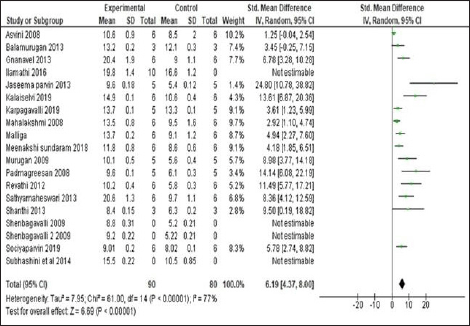 | Figure 6. Forest plot depicting Hb difference in polyherbal formulations. [Click here to view] |
LIMITATIONS
As most of the studies did not mention the levels of other anaemia related outcomes like serum Iron, Ferritin, Transferrin, total iron binding capacity, etc., the efficacy of these trial drugs could not be strongly validated. The heterogeneity of this meta-analysis is very high which could be attributable to varied sample sizes, differences in methodologies, period of intervention and certain limitations in different studies. However, Random effect model was employed in forest plot to address the heterogeneity. Funnel plot showed no publication bias. Further, a greater number of RCTs are needed to provide high level evidences.
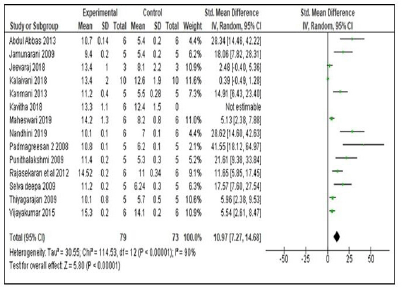 | Figure 7. Forest plot depicting Hb difference in herbo-metallic/herbo-mineral formulations. [Click here to view] |
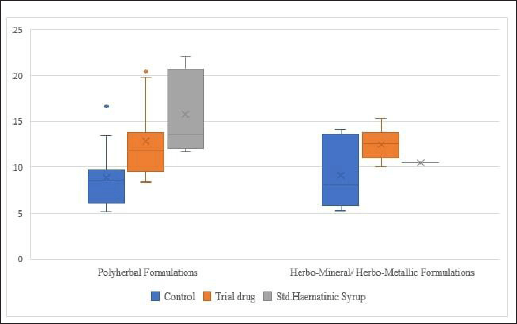 | Figure 8. Box plot showing mean increase in Hb in pre-clinical pharmacological studies. [Click here to view] |
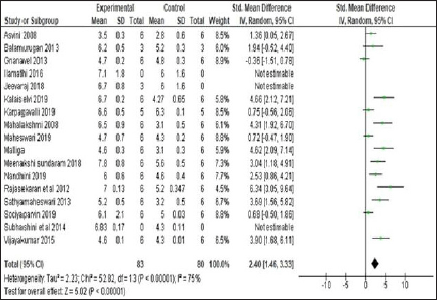 | Figure 9. Forest plot showing total RBC count—before and after treatment. [Click here to view] |
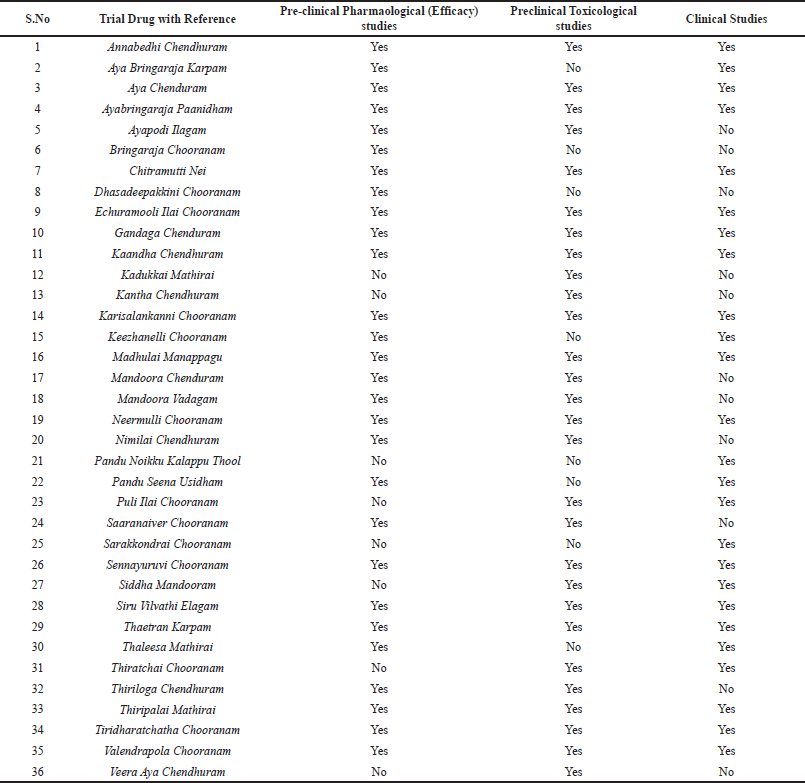 | Table 5. Summary of pre-clinical and clinical studies conducted in Siddha formulations for anaemia. [Click here to view] |
CONCLUSION
Since many ages, the therapeutic effectiveness of the Siddha system of medicine in treating a variety of illnesses has been established. However, there are some impediments limiting the propagation of Siddha medicine globally, including a dearth of supporting evidence and a lack of validation. This study, therefore, sought to establish the safety and efficacy of Siddha formulations in the treatment of anaemia through adequate documentation. The present study showcased that all the Siddha formulations proposed to treat anaemia in Siddha literatures were proved its safety and efficacy in animal models. Besides. many of these Siddha formulations were not in use in daily practice to treat anaemia. Hence, this study suggests that Siddha formulations which proved its efficacy in pre-clinical trials may be taken either to clinical trials with larger samples size or a well- designed RCTs to make it more feasible to treat anaemia. This study will further pave way to prescribe these Siddha formulations in public health initiatives and programs to reduce the incidence and prevalence of anaemia in people of all ages globally.
ACKNOWLEDGMENT
Authors like to acknowledge the research work of all the authors whose papers / dissertation formed a part of this systematic review and meta-analysis.
AUTHOR CONTRIBUTIONS
All the authors have made significant contributions to the concept, design, acquisition of data, analysis and interpretation; and also in drafting and revisions of this article. All the authors are also eligible to be an author based on the guidelines of International Committee of Medical Journal Editors (ICMJE).
FINANCIAL SUPPORT
This article is funded by Central Council for Research in Siddha (Ministry of Ayush, Govt. of India), Chennai.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVALS
This study does not involve any animal or human subjects and hence not applicable.
DATA AVAILABILITY
All data collected, generated and analyzed for the study are included within this manuscript.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdul Abbas. A study on Pandu Noi. Masters thesis, Government Siddha Medical College, Palayamkottai, 2013. Available via http://repository-tnmgrmu.ac.in/7270/ (Accessed 22 July 2022).
Arunmozhi P. Haematinic activity of Puli Ilai Chooranam (Tamarindus indica. L) and hepatoprotective activity of Chara Parpam. Masters thesis, Government Siddha Medical College, Chennai, 2013. Available via http://repository-tnmgrmu.ac.in/7140/ (Accessed 26 July 2022).
Asvini A. A study on Thaetran Karpam (Strychnos Potatorum) for Paandu Noi and a study on Peenisa Choornam for Peenisam. Masters thesis, Government Siddha Medical College, Chennai, 2008. Available via http://repository-tnmgrmu.ac.in/7132/ (Accessed 22 July 2022).
Balamurugan P. A study on Pandu Noi. Masters thesis. National Institute of Siddha, Chennai, 2013. Available via http://repository-tnmgrmu.ac.in/10375/ (Accessed 26 July 2022).
Benitta K. A toxicity study on Kantha Chendhuram. Masters thesis, Govt. Siddha Medical College, Palayamkottai, 2008. Available via http://repository-tnmgrmu.ac.in/1366/ (Accessed 22 July 2022).
Gnanavel IS. Haematinic activity of Echuramooli ilai Chooranam (Aristilochia indica linn.) and spermatogenic activity of Anda Odu Parpam. Masters thesis, Government Siddha Medical College, Chennai, 2013. Available via http://repository-tnmgrmu.ac.in/10360/ (Accessed 22 July 2022).
Ilamathi L. A clinical study of Karisalankanni Chooranam in the management of Vatha Pandu iron deficiency anaemia. Masters thesis, Government Siddha Medical College, Palayamkottai, 2016. Available via http://repository-tnmgrmu.ac.in/3773/ (Accessed 22 July 2022).
Jamuna Rani S. Hypo glycemic activity of Vellalli Poo Chooranam (Nymphaea alba) and haematinic activity of Kaandha Chenduram. Masters thesis, Government Siddha Medical College, Palayamkottai, 2013. Available via http://repository-tnmgrmu.ac.in/7124/ (Accessed 29 July 2022).
Janet Sheeba L. A study on Pithapandu Noi. Masters thesis, National Institute of Siddha, Chennai, 2012. Available via http://repository-tnmgrmu.ac.in/12700/ (Accessed 22 July 2022).
Jaseema Parveen S. Hypo glycemic activity of Neermulli chooranam (Hygrophila auriculata) and haematinic activity of Annabedi Chenduram. Masters thesis, Government Siddha Medical College, Palayamkottai, 2013. Available via http://repository-tnmgrmu.ac.in/7125/ (Accessed 26 July 2022).
Jeevaraj K. Preclinical safety evaluation of Mandura Chenthooram. Masters thesis, National Institute of Siddha, Chennai, 2018. Available via http://repository-tnmgrmu.ac.in/10279/ (Accessed 22 July 2022).
Kalaiselvi GG. An open clinical study on Paandu Noi (Iron Deficiency Anaemia) in children with the evaluation of Siddha drug Chitramutti Nei. Masters thesis, Government Siddha Medical College, Chennai, 2017. Available via http://repository-tnmgrmu.ac.in/9609/ (Accessed 22 July 2022).
Kalaivani A. Preclinical s-afety evaluation of Ayapodi Elagam. Masters thesis, National Institute of Siddha, Chennai, 2018. Available via http://repository-tnmgrmu.ac.in/10280/ (Accessed 22 July 2022).
Kanmani K. Haemostatic and analgesic activity of Madhulai Poo Chooranam and haematinic activity of Aya Chenduram, 2013. Available via https://1library.net/document/lq51mmgy-haemostatic-analgesic-activity-madhulai-chooranam-haematinic-activity-chenduram.html (Accessed 22 July 2022).
Kannabiran R. The study on acute, sub-acute toxicity and hematinic activity of Nimilai Chenduram (Siddha formulation) in Wistar rats. Int J Pharmatech Res, 2012; 4(4):1498–503.
Karpagavalli K. An open clinical study on Paandu Noi (Iron Deficiency Anemia). Masters thesis, Government Siddha Medical College, Chennai, 2019. Available via http://repository-tnmgrmu.ac.in/12146/ (Accessed 22 July 2022).
Kavitha G. Pre-clinical safety evaluation of Annabethi Chendhuram. Masters thesis, National Institute of Siddha, Chennai, 2018. Available via http://repository-tnmgrmu.ac.in/10282/ (Accessed 26 July 2022).
Mahalakshmi VK. A study on Saranaiver (Trianthema Portulacastrum Linn) for Paandu Noi and a study on Sarvanoi Linga Chenduram for Kalladaippu. Masters thesis, Government Siddha Medical College, Chennai, 2008. Available via http://repository-tnmgrmu.ac.in/7136/ (Accessed 29 July 2022).
Maheswari B. A prospective open labelled non randomized phase-II clinical trial to evaluate the therapeutic efficacy of the Siddha medicine “Thiripalai Mathirai” (Internal) for the treatment of “Pitha Paandu” (Iron Deficiency Anaemia). Masters thesis, Government Siddha Medical College, Palayamkottai, 2019. Available via http://repository-tnmgrmu.ac.in/11889/ (Accessed 26 July 2022).
Malliga S. A study on Sennayuruvi (Achyranthes Bidentata Blume) for Paandu Noi and a study on Mandoora Podi for Gunmam. Masters thesis, Government Siddha Medical College, Chennai, 2008. Available via http://repository-tnmgrmu.ac.in/7138/ (Accessed 26 July 2022).
Meenakshi Sundaram M. Pre-clinical and clinical evaluation of a herbal formulation (Madhulai Manappagu) for Pandu Noi (Iron Deficiency Anaemia) in children (6 to 12 years of age). Doctoral thesis, The Tamilnadu Dr. M.G.R. Medical University, Chennai, 2018. Available via http://repository-tnmgrmu.ac.in/19043/ (Accessed 26 July 2022).
Murugan G. Haematinic activity of Paandu Seena Usidham, 2009. Available via http://repository-tnmgrmu.ac.in/7118/1/320201409murugan_G.pdf (Accessed 26 July 2022).
Nandhini D. Pre-clinical study of herbo mineral drug Mandoora Vadagam for its haematinic, hepatoprotective and anti-oxidant activities. Masters thesis, Government Siddha Medical College, Palayamkottai, 2019. Available via http://repository-tnmgrmu.ac.in/12122/ (Accessed 26 July 2022).
Nguyen M, Tadi P. Iron supplementation. StatPearls Publishing, Treasure Island, FL, 2022. Available via https://www.ncbi.nlm.nih.gov/books/NBK557376/ (Accessed 04 July 2022)
Padmagreesan V. Haematinic activity of Keezhanelli Chooranam and Annabedi Chenduram. Masters thesis, Government Siddha Medical College, Palayamkottai, 2008. Available via http://repository-tnmgrmu.ac.in/7108/ (Accessed 26 July 2022).
Priyadharshini K. A study on Pandu Noi (Iron Deficiency Anemia). Masters thesis, National Institute of Siddha, Chennai, 2012. Available via http://repository-tnmgrmu.ac.in/12740/ (Accessed 22 July 2022).
Punitha Lakshmi A. A study on anti-inflammatory, analgesic, antipyretic activity of Sangan Ver Pattai Chooranam (the Bark of Azima tetracantha) and haematinic activity of Gandaga Chenduram. Masters thesis, Government Siddha Medical College, Palayamkottai, 2009. Available via http://repository-tnmgrmu.ac.in/7119/ (Accessed 26 July 2022).
Rajendra Kumar A, Rathinamala R, Gayathri Gunalan, Muthukumar NJ. Morbidity profile of patients attending OPD of siddha regional research institute, Puducherry. J Res Sid Med, 2018; 1(1):33–40.
Rajesh S, Eswaran C, Merish S, Prakash M. Acute and chronic toxicological study on Thiriloga Chendooram - a herbo metallic Siddha formulation in Wistar Rat. World J Pharm Res, 2014; 8(13):1400–06.
Release of NFHS-5 (2019-21). Compendium of factsheets. Ministry of Health and Family Welfare, GOI. Available via https://main.mohfw.gov.in/basicpage-14 (Accessed 05 August 2022).
Revathi P. A study on Mannun Veluppu Noi. Masters thesis, Government Siddha Medical College, Palayamkottai, 2012. Available via http://repository-tnmgrmu.ac.in/12746/ (Accessed 29 July 2022).
Sathya Maheswari T. Pre-clinical and clinical study on Valendrapola Chooranam for haematinic activity in the management of Pandu (Anaemia) and pre-clinical and clinical study on Singathi Chooranam for bronchodilator activity in the management of Eraippu (Bronchial Asthma). Masters thesis, National Institute of Siddha, Chennai, India, 2013. Available via http://repository-tnmgrmu.ac.in/7151/ (Accessed 26 July 2022).
Selva Deepa M. Anti-microbial (Bacterial) activity of Aya Bringa Raja Karpam. Masters thesis, Government Siddha Medical College, Palayamkottai, 2009. Available via http://repository-tnmgrmu.ac.in/7120/ (Accessed 29 July 2022).
Shanmuga Priya M. A clinical study on Pitha Paandu (Iron Deficiency Anaemia) with the evaluation of Siddha drug Sarakondrai Chooranam. Masters thesis, Government Siddha Medical College, Chennai, 2017. Available via http://repository-tnmgrmu.ac.in/9634/ (Accessed 26 July 2022).
Shenbagavalli S. A study on Pandu Noi. Masters thesis, Government Siddha Medical College, Palayamkottai, 2009. Available via http://repository-tnmgrmu.ac.in/7286/ (Accessed 26 July 2022).
Sociya Parvin M. An open clinical study to evaluate the clinical efficacy of Siddha sasthric formulation Siru Vilvathi Elagam for the treatment of “Mannun Veluppu Noi”. Thesis, Government Siddha Medical College, Palayamkottai, 2019. Available via http://repository-tnmgrmu.ac.in/12150/ (Accessed 26 July 2022).
Sridevi J. Acute and 28 days repeated oral toxicity study of the Siddha drug Veera Aya Chendhuram (VAC). World J Pharm Res, 2017; 6(5):1390–401. Available via https://doi.org/10.20959/wjpr20175-8455.
Suba S. Haematinic activity of Dhasadeebaakini Chooranam (A Siddha Herbo Mineral Formulation) in phenyl hydrazine induced anaemic rats. Available via https://1library.net/document/zwv4040q-haematinic-activity-dhasadeebaakini-chooranam-mineral-formulation-hydrazine-induced.html (Accessed 29 July 2022).
Thirunarayanan T. Introduction to Siddha medicine (revised edition). Centre for Traditional Medicine and Research, Chennai, India, vol. 91, no. 101, pp 141–3, 2012.
Thiyagarajan A. Haematinic activity of Thiriloga chendhooram. Doctoral thesis, Government Siddha Medical College, Palayamkottai, 2009. Available via http://repository-tnmgrmu.ac.in/id/eprint/7123 (Accessed 20 September 2022).
Velayudam, Ilavarasan, Arul Amuthan. Acute and 28-day subchronic oral toxicity study of Kadukkai Maathirai, an iron based siddha herbal formulation in wistar albino rats. Int J Pharm Pharm Sci, 2013; 5(4):186–91.
Vijayakumar V. Evaluation of Aya bringaraja Paanidham in the management of Veluppu Noi (Anaemia). Doctoral thesis, The Tamilnadu Dr. M.G.R. Medical University, Chennai, 2015. Available via http://repository-tnmgrmu.ac.in/19047/ (Accessed 26 July 2022).
WHO. Prevalence of anaemia in women of reproductive age (aged 15-49) (%). 2022. Available via https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-women-of-reproductive-age-(-) (Accessed 29 July 2022).