INTRODUCTION
As one of the top cancer-related causes of death among both men and women, lung cancer ranks high among both groups. According to estimates, 1 in 16 individuals will develop lung cancer during their lifetimes; 1 in 15 men and 1 in 17 women (Cancer Facts & Figures, 2022). Despite the fact that non-small cell lung cancer (NSCLC) accounts for the majority of lung malignancies (about 80%), treatment is challenging due to a lack of understanding of the pathological processes involved (Inamura et al., 2010). The mortality rate associated with NSCLC is higher than that of colon, prostate, and breast cancers taken together. Several advances in targeted therapies and immunotherapy have led to a significant increase in the 5-year survival rate for patients with NSCLC. Despite this, the incidence rate remains significantly lower than that of other cancer types, such as prostate and breast cancer (Siege et al., 2018). Genetic and regulatory mutations responsible for suppressing cell death, promoting cell division, and supporting the growth of tumors have been discovered through a deeper understanding of the signaling mechanisms that regulate cell survival. An example of one of these receptors is the epidermal growth factor receptor (EGFR) (Scagliotti et al., 2004). The EGFR tyrosine kinase is expressed in some epithelial, neurogenic, and mesenchymal tissues. Several human cancers, including NSCLC, have been associated with the overexpression of EGFR (Ohsaki et al., 2000). CD44, a transmembrane glycoprotein that serves both as a cancer stem cell marker and as an oncogenic regulator, is implicated in a wide variety of cancer types (Toole and Slomiany, 2008). CD44 overexpression has been associated with multi-drug resistance, progression, and migration of cancer in a number of cancer types, including colorectal, breast, and lung carcinomas (Chen et al., 2018; Park et al., 2016). Several neoplasms have been found to express CD44 in correlation with EGFR levels (Grass et al., 2013; Wobus et al., 2002; Xu et al., 2016).
The development of small-molecule drugs that are capable of targeting specific mutations has been a significant improvement in the treatment of lung cancer in the past several decades. Studies have shown that EGFR-TKIs are effective treatments for lung cancer patients with activating EGFR mutations, including point mutations in exon 21 (L858R) and deletions of exon 19 (19del) of the receptor TK domain (Andrews and Goss, 2019). There are three generations of TKIs currently being used clinically, including first-generation TKIs (such as erlotinib), second-generation TKIs (such as afatinib), and third-generation TKIs [such as Osimertinib (OMB)]. OMB (AZD9291 or Tagrisso) is FDA-approved for the treatment of EGFR-activating mutations and for patients who are unresponsive to first-generation TKIs because of the T790M mutation (Lim et al., 2018). In the kinetic study, OMB was slowly absorbed in an increased dose from 20 to 240 mg and showed a dose-proportional increase in systemic availability over time (Jänne et al., 2015). Considering the half-life of 48.3 hours, the distribution was widespread, and the clearance was short to modest. After 15 days of the administration, a steady state was reached, corresponding to a single-dose PK (Planchard et al., 2016). The high solubility and bioavailability of OMB also contribute to its non-selective ability to accumulate in the tumor core. Additionally, OMB has a large volume of distribution (3,800 l), which indicates that it is widely distributed in tissues and accumulates at non-cancerous sites (Brown et al., 2017). Thus, effective strategies for overcoming nonselective tumor targeting will address a significant clinical challenge and prolong the life expectancy of lung cancer patients.
Since the advent of nanoparticulate drug delivery over the past decades, it has become a primary topic of interest in anticancer treatment. In contrast to conventional chemotherapy, nanoparticles were able to penetrate into the tumor mass and remain at the site, reducing systemic toxicity. Despite this, nanoparticulate therapy for specific cancer cell targeting may not be an ideal approach in all cases (Shi et al., 2016). Although there are numerous types of nanoformulations available, liposomal vesicles possess particular advantages, including low toxicity, biocompatibility, and the capability to encapsulate a broad range of cargo (Choi et al., 2016; Hofheinz et al., 2005; Kim, 2016; Torchilin, 2005). To overcome challenges associated with the conventional delivery of OMB, it has been proposed to conjugate OMB nanoliposomes with a variety of target ligands including antibodies, and their fragments, aptamers, quinic acid, hyaluronic acid (HA), and peptides. The unique characteristics of HA, such as its non-toxicity, biodegradability, and non-immunogenic properties, have attracted considerable attention for its use in enhancing the tumor-tracking effectiveness of liposomes (Ito et al., 2008; Zhang et al., 2016). In addition, HA exhibited a high affinity for CD44 receptors, which are overexpressed in many types of cancer cells (Toole, 2009; Zhou et al., 2000). It has been extensively studied for its potential use as a cancer-targeted therapy. However, recent studies have shown that HA-based nano-liposomes that target CD44 accumulate in the liver rather than in cancerous tissues (Jeannot et al., 2016, 2018). Moreover, the molecular weight and grafting density of HA can significantly influence the interaction between ligand and receptor, as well as liposome stability and cellular uptake (Gasperini et al., 2015; Qhattal and Liu, 2011). Thus, novel and emerging approaches are essential to improving tumor accumulation of HA-based liposomes.
Therefore, we developed PEGylated-HA (PEG-HA) coated OMB nano-liposomes using effective conjugation strategies, for improving in-vitro dissolution, bioavailability, and tumor selectivity of OMB in NSCLC. A scientific hypothesis behind the development of new PEG-HA OMB nanoliposomes is that they could improve tumor-targeting efficiency while maintaining CD44 affinity, eventually accumulating the drug in NSCLC tumors. In this study, OMB was incorporated into PEG-HA to evaluate the effects of varying degrees of substitution (DS) and to investigate how it selectively targets NSCLC tumors through CD44-mediated intracellular accumulation in lung cancer cell models.
MATERIALS AND METHODS
Materials
OMB was procured from Taj Mahal Vision Chemicals Private Limited, Malad West, India. Methoxypolyethyleneglycol amine (mPEG-NH2; Mw 2,000 Da) was obtained from Otto Chemie Pvt Ltd, Mumbai, India. HA (Mw 10–20 kDa), N-hydroxy succinimide (NHS), 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (EDC), 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were acquired from Sigma Aldrich Inc. (USA). 2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP), d-α-tocopherol polyethylene glycol succinate (TPGS), dioleoylphosphatidyl ethanolamine (DOPE), and cholesterol were procured from Merck chemicals, Mumbai, India. Cell culture mediums RPMI 1640, fetal bovine serum (FBS), trypsin, Dulbecco’s modified Eagle’s media (DMEM), phosphate-buffered saline (PBS), glutamine, sodium pyruvate, and antibiotics were obtained from Gibco, BRL (Grand Island, NY). Alexa Fluor 647-anti-mouse/human CD44 antibodies were procured from BioLegend, San Diego, CA. Furthermore, all the compounds and reagents used in various experiments were analytical grades, and all the chemicals were used without any purification. Millipore water (Milli Q water) was used throughout the experiments.
UPLC-based method development for OMB analysis
Using reverse-phase liquid chromatography, we developed a validated method for quantifying OMB with a few modifications to the method described by Van et al. (2020). This method utilizes a conditioned autosampler and waters acquity BEH C18 column (100 mm × 2.1 mm id., 1.7 μm particle size) was employed for OMB chromatographic separation. Acetonitrile: phosphate buffer (pH adjusted to 7.43 by using 10 mM potassium hydroxide) in the ratio of 75:25 (%, v/v) is selected as the mobile phase. An injection volume of 10 µl was injected at a flow rate of 0.8 ml/minute. It was determined that samples were quantitatively evaluated at 271 nm for their retention time (Rt), linearity, range of OMB, and peak area (AUC). According to the results, the retention time of 3.41 minutes was found for OMB, in the total 5 minutes of run time. In the course of collecting, evaluating, and analyzing the data, the Empower 3.0 software was utilized.
Preparation of mPEG-HA
To successfully accomplish this study, it is essential to investigate the dynamic and physicochemical properties of PEG-conjugated HA liposomes and to use varying levels of PEGylation to prepare amphiphilic HA derivatives. Figure 1 illustrates the preparation of HA-modified mPEG in the presence of NHS and EDC, as described in previous studies (Choi et al., 2011). In brief, HA (20 mg) was solubilized in 5 ml PBS solution of pH 7.4, then EDC (51.3 mg, 0.26 mmol) and NHS (30.8 mg, 0.26 mmol) were introduced and agitated at 300 rpm and at room temperature for 2 hours. A 2 ml PBS solution containing 10.72, 21.44, and 42.88 mg of mPEG-NH2 was introduced to the reaction medium drop by drop, while it was being agitated continuously for 24 hours. After dialysis (MWCO 8 kDa) in distilled water for 48 hours and three changes per day, the sample was purified and characterized by 1H NMR spectrometry (Avancore-400 MHz spectrometer, Bruker Corporation, USA) using DMSO-d6 as a solvent, followed by Fourier transform infrared spectroscopy (NicoletTM iS20 FTIR Spectrometer, Thermo Scientific, USA). Using a 1H NMR measurement, and evaluating the “methylene units? proton ratios (–OCH2CH2–: δ = 3.44 ppm) of mPEG with that of methyl groups (–COCH3: δ = 1.82 ppm) of HA, the substitution degree of mPEG was calculated.
Preparation of OMB-LPs, HA- and mPEG-HA coated OMB-LPs
The OMB-LPs were prepared by the freeze-drying method as reported earlier (Chi et al., 2017; Li et al., 2018). In brief, DOPE (20 mg), DOPC (40 mg), TPGS (20 mg), DOTAP (20 mg), cholesterol (10 mg), and the drug OMB (10 mg) were added at the molar ratios of 1:2:1:1:0.5:0.5 respectively, dissolved in tert-butanol of 2 ml, and finally lyophilized (Labconco® FreeZone 4.5 l; at −80°C of lyophilization temp and at 50 mtorr of vacuum for 6 hours) to remove the solvent. For the labeling of liposomes with DiR, lipid mixtures containing 1 ml of 0.5% DiR were used. Following the formation of the lipid cake, it was hydrated for 1 hour using 3 ml of 5% glucose, then sonicated at 4°C for 20 minutes. Free drug from the lipid suspension was removed by using a membrane filter (0.8 mm pore size). Coated liposomes (OMB-LPs) were produced by electrostatic interaction between the negatively charged coating material and the cationic surface of OMB-LPs. A 3 ml solution of HA- or varying DS containing mPEG-HA conjugate (mPEG: HA; 0.5:1, 1:1, and 2:1 for low, medium, and high respectively) (5 mg/ml) was mixed with an equal volume of OMB-LPs suspension (2 mg/ml) under continuous stirring for 30 minutes at 4°C (Table 1). HA-coated OMB-LPs were also prepared using the above-mentioned protocol using similar conditions and parameters, by substituting mPEG-HA conjugate with HA, to investigate the mPEG-HA coating effect on OMB-LPs. After suspension preparation, a membrane filter (MWCO = 30 kDa) was used to centrifuge the resulting solution for 15 minutes at 4°C at 5,000 rpm, in order to remove uncoated liposomes and unconjugated mPEG-HA. The pellets were resuspended in 5% glucose for further analysis.
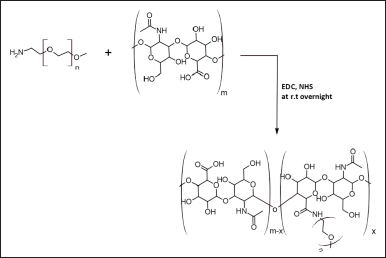 | Figure 1. The synthetic scheme of mPEG-HA conjugate with different DS from low to higher ratios (mPEG:HA; 0.5:1, 1:1, and 2:1). [Click here to view] |
Characterization of liposomes
Zetasizer (Nano-ZS90; Malvern Instruments, Malvern, UK) was used to measure particle size and zeta potential for all formulations using the dynamic Light Scattering method. According to earlier studies (Chi et al., 2017; Li et al., 2018), characterization studies were conducted. Samples were diluted (0.1 mg/ml) with a 1 ml PBS solution of pH 7.4 in order to estimate particle size and zeta potential, with an average of three measurements per sample. The polydispersity index (PDI) which indicates the size distribution, was obtained as a dimensionless number. A transmission electron microscopy (TEM) study was performed on each formulation to examine its morphology. After 2% phosphotungstic acid staining for 20 minutes, TEM was used to monitor air-dried liposome samples. A high-performance liquid chromatography (HPLC) test was used to determine the encapsulation efficiency (%EE) of the five formulations on the basis of the total drug content of the formulations and the amount of drug entrapped in liposomes. To determine %EE OMB-loaded LPs with or without surface coating were lysed, to determine the amount of free OMB. The % EE was calculated using the following formula.
%EE = Wt. of the initial amount of OMB − Wt. of free OMB / Wt. of the initial amount of OMB ×100.
Cell culture
ATCC provided H1975, A549, human non-small cell lung adenocarcinoma cells were cultured in DMEM and PC-9 lung adenocarcinoma cells were harvested in RPMI-1640 with the addition of 2 mM glutamine and growth medium were prepared using PBS of pH 7.4 as described by Rath et al. (2018). Additionally, 10% FBS, 100 mg/ml streptomycin, and 100 U/ml penicillin were added to the medium, and the cells were cultured in an incubator with 5% CO2 and 90% humidity at 37°C. In advance of the trials, the cell viability of both H1975, A549, and PC-9 cells was assumed to be greater than 95%.
Evaluating expression of hyaluronan receptor CD44
The cells were trypsinized before being placed at a density of 1.0 × 105 cells per well on 6-well plates. This study was based on an earlier report by Moura et al. (2021). Cells were incubated for 30 minutes on ice with Alexa Fluor 647-anti-mouse/human CD44 antibody from BioLegend in San Diego, CA. Flow cytometry analysis was performed (Gallios, Tokyo, Japan) after the cells had been washed twice with PBS without magnesium or calcium ions [PBS (−)] containing 1.0% bovine serum albumin and 0.05% sodium azide (NaN3).
Cellular uptake
Using the method described previously by Takenaka et al. (2019), studies were conducted on cellular uptake. Initially, a 6-well plate was seeded with 5 × 105 cells per well. The medium was removed after 24 hours of incubation, and the cells were washed twice with PBS. Following this, the cells were incubated for 2 hours with 2 ml of 15 μM of plain OMB as well as selected liposomal formulations (OMB-LPs, HA-OMB-LPs, and mPEG-HA(mds)-OMB-LPs). Upon completion of the incubation period, the drug solution was removed, and ice-cold PBS was used to wash the cells three times. Immediately following cell lysis, acetonitrile was added to the lysate, which was vortexed vigorously and centrifuged at 5,000 rpm for 10 minutes. An HPLC assay was performed on the supernatant to determine the drug concentration. A BCA protein assay kit was used to determine the protein content of each sample. Before applying each formulation for an additional 2 hours, the cells were pre-treated with free HA (10 mg/ml) for 1 hour in order to determine if CD44 is involved in the absorption of free HA and PEG-HA-coated liposomes.
Cytotoxicity
Selected and HA- and low- to high-degree substituted mPEG-HA-coated OMB-LPs were compared to uncoated OMB LPs using the MTT assay to determine their cytotoxicity against H1975 and PC-9 cells (Zaremba-Czogalla et al., 2020). Essentially, immortalized NSCLC cell lines with specific EGFR mutations (H1975 and PC-9) and wild-type EGFR cell lines (A549) were seeded in 96-well tissue culture-treated plates at a density of 2,500 cells/well and incubated overnight at 37°C under 5% CO2 for adhesion. Different concentrations of plain OMB, OMB-LPs, HA-coated OMB-LPs, and low to high-level substituted mPEG-HA coated OMB-LPs (0.1–25 mM) were formulated in the growth medium and introduced to adherent cells on the next day. An untreated culture medium was used as a control. After 48 hours, the medium was withdrawn from each well, and MTT solution (1 mg/ml in PBS; pH 7.3) was introduced to induce the formation of formazan crystals, which required an additional 3 hours of incubation. To dissolve the formazan crystals generated in each well, 100 µl of DMSO was added after removing the excess MTT solution. Using a multi-modal plate reader (Tecan Spark 10M; Tecan, Mannedorf, Switzerland), absorbance at 570 nm was measured after 30 minutes on a plate shaker. Further assessment of the safety of drug-free NPFs was carried out by incubating HEK-293, a healthy non-cancerous kidney cell line, for 48 hours using the same methodology as used for the 48 hours treatment.
Statistical analysis
Based on three independent experiments (n = 3), the data are presented as mean ± standard deviation (SD). A one-way analysis of variance was used for statistical analysis, and a p-value of less than 0.05 (p < 0.05) was considered statistically significant.
RESULTS AND DISCUSSION
UPLC analysis of OMB
In order to quantify the OMB content, a precise, reliable, and reproducible UPLC technique was devised with a total run duration of 5 minutes. OMB was effectively assessed at 271 nm (Van et al., 2020) by evaluating the retention time (Rt), linearity, range, and peak area employing mobile phase, acetonitrile/phosphate buffer solution (pH 7.43), at 75:25 (%, v/v) (AUC). The area under the curve (Y) is linearly related to the OMB concentration (X), using a regression equation of Y = 0.3907x − 0.2429 (r2 = 0.999). The calibration curve for OMB analysis showed that the concentration range of 0.5–100 ng/ml was effectively assessed. The calibration curve for OMB estimation is shown in Figure 2a, and the UPLC chromatogram showed that OMB was eluted at a retention time of 3.41 minutes, with a strong peak and high resolution (Fig. 2b).
Synthesis and structural characterization of mPEG-HA conjugate
The mPEG-modified HA conjugate with varying DS was successfully synthesized using EDC and NHS, and the synthetic scheme was shown in Figure 1. By comparing the proton ratios of the methylene units in mPEG (–OCH2CH2–) and the methyl groups of HA (–COCH3), the purified mPEG-HA conjugate was structurally characterized using 1H-NMR. In order to study the effect of mPEG DS on HA, three ratios of mPEG: HA ranging from low (0.5:1), medium (1:1), and high (2:1) were synthesized, and the results were shown in Figure 3a–c. The results of proton spectra revealed that methylene units in mPEG (–OCH2CH2–) showed a sharp singlet peak at δ = 3.44 ppm, and methyl groups of HA (–COCH3) showed a highly intensified proton peak at δ = 1.82 ppm demonstrating the formation of C–NH bond connecting the polyethylene glycol moiety at the structural unit of HA (Fig. 3a–c). It was derived from the results that the ratio of the peak intensities corresponding to the methyl group of HA to the methylene group of mPEG significantly increased from low to high stating the elevated levels of DS. According to these results, mPEG substitution on HA increased proportionately with an increase in calculated ratios that proved the successful elevation of the DSs. Further structural confirmation of mPEG-HA conjugate was carried out by FT-IR, and the results were shown in Figure 4. According to the FT-IR spectra, a similar transmittance pattern was observed for the HA and the mPEG-HA conjugate, specifically at 3,513, 3,012, 1,629, 1,174, and 484 cm−1, with the exception of 1,731.29 cm−1, which indicates that mPEG and HA have been conjugated through the formation of a new C–NH bond. These results indicated the formation of mPEG-HA conjugate that proved the synthetic hypothesis. Both Proton NMR and FT-IR postulated the formation of varying degrees of mPEG substitution on HA through the new bond connectivity between the carbonyl moieties of HA and the amino group of polyethylene glycol.
 | Table 1. Various OMB-loaded liposomal formulations with or without coating (LPs). [Click here to view] |
Preparation and characterization of OMB-LPs, HA- and mPEG-HA coated OMB-LPs
Following the conversion of OMB into liposomes using synthetically advanced phospholipids, mPEG-HA-based novel liposomes entrapping OMB were prepared by conjugating OMB with mPEG-HA in distilled water. A freeze-drying method was used for the preparation of the OMB-LPs. Electrostatic interaction between the negatively charged coating materials and cationic surfaces of liposomes enabled the successful formation of the coated liposomes (OMB-LPs). A summary of the characteristics of the liposomes developed is presented in Table 2. It was demonstrated that all preparations, comprising uncoated liposomes (OMB-LPs), and coated preparations (HA-OMB-LPs, mPEG-HA(lds)-OMB-LPs, mPEG-HA(mds)-OMB-LPs, and mPEG-HA (hds)-OMB-LPs,) had shown ideal characteristics of zeta potential, PDI, size and high EE. The particle sizes of all formulations ranged from 116 ± 7.23 to 151 ± 7.93, and OMB-LPs and mPEG-HA(mds)-OMB-LPs exhibited the lowest and highest particle sizes, respectively. It was observed from the results that, coated OMB nanoliposomes showed a good dispersity index of 0.2 compared with uncoated OMB-LPs (0.18). The zeta potential of all formulations was superior (> −40 for coated and >30 for uncoated LPs), with the uncoated OMB-LPs showing a positive value, whereas the coated LPs showed negative values, indicating strong particle-particle repulsive forces. In addition, all liposomal formulations including coated and uncoated LPs resulted in more than 90% of high EE. These findings identified that OMB-LPs had the highest percentage of EE among all formulations, showing 95.37% ± 5.21%. The other coated LPs showed a percentage of EE of around 90% to 93% for all formulations including HA- and mPEG-HA-coated LPs. A TEM study was conducted to observe the liposome morphology (Fig. 5). All formulations were observed to have globular shapes in TEM images, and OMB-LPs, however, exhibited smaller sphere shapes compared to the other formulations. Different DS containing PEGlyated-HA-OMB LPS, showed a fluffy dark layer, with a blurred shade on the liposome surface, which was observed in the image.
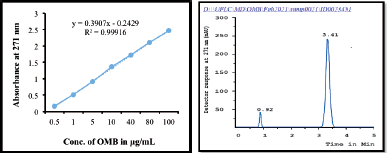 | Figure 2. (a) A calibration curve and (b) UPLC chromatogram of OMB. [Click here to view] |
In-vitro release of OMB from LPs
With the aid of the dialysis bag method, we determined the drug release percentage of OMB from coated and uncoated LPs in two simulated fluids with pH values of 6.5 and 7.4. These results were compared to those obtained with Tagrisso, a commercially available version of OMB (Fig. 6). According to these results, it was found that the release profiles of simulated fluids at pH 7.4 were more promising than the release profiles of OMB at pH 6.5. When the pH value was 7.4 (Fig. 6a), OMB LPs coated with mPEGylated-HA significantly improved the dissolution rate compared to that of the reference dosage form Tagrisso. About 12% of the OMB dissolved from the OMB-LPs during the 60-minute study, while about 55% and 51% of the OMB were released from the standard reference formulation Tagrisso and mPEG-HA(mds)-OMB-LPs, respectively. Both Tagrisso and mPEGylated-HA(mds)-OMB-LPs released OMB with a maximum release of 99.9% after 15 hours of dissolution. It was also observed from the findings that, the highest release profiles of OMB were maintained by both Tagrisso and mPEGylated-HA(mds)-OMB-LPs during 72 hours of the study. The results of the study reveal that OMB-LPs have a maximum release profile of 72% at the end of the study. The mPEGylated-HA(lds)-OMB-LPs also displayed significant release profiles of OMB throughout the dissolution study period compared to the mPEGylated-HA(mds)-OMB-LPs. The study also demonstrated that mPEGylated-HA(hds)-OMB-LPs and HA-coated OMB-LPs failed to exhibit promising releasing profiles throughout the study period. In addition to the main release profile, all formulations, including the reference formulation Tagrisso, displayed ancillary release profiles at pH 6.5 compared to pH 7.4 (Fig. 6b). Therefore the above results demonstrated that mPEGylated-HA(mds)-OMB-LPs have shown enhanced drug release compared to mPEGylated-HA(lds)-OMB-LPs and mPEGylated-HA(hds)-OMB-LPs. Statistical data analysis was conducted using the paired t-test to determine whether there were significant differences between the two sets. According to the data analysis, there is a significant difference for mPEGylated-HA(mds)-OMB-LP. Thus, the mPEGylated-HA coating can serve as a potential carrier, which is capable of releasing and localizing the OMB, resulting in prolonged release and anti-tumor effects.
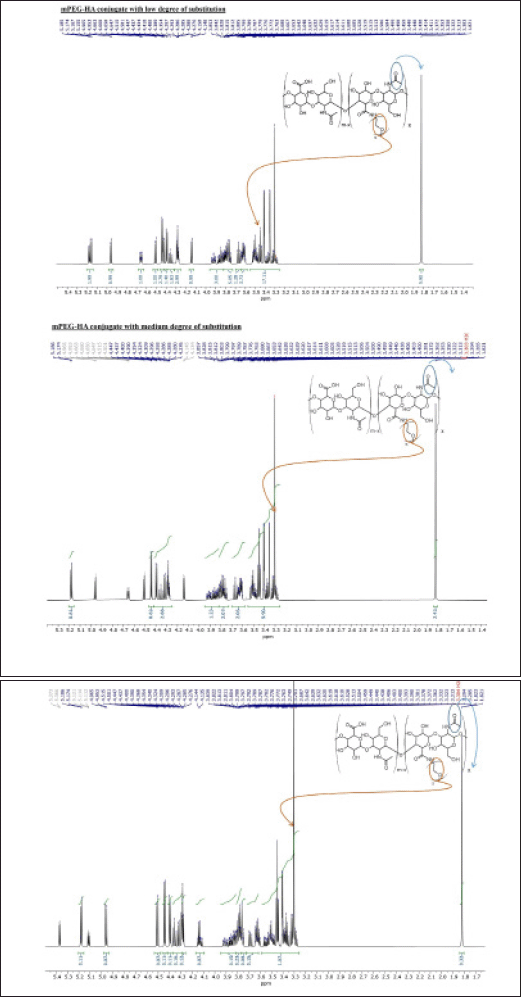 | Figure 3. The synthesis of mPEG-HA conjugates with varying degrees of mPEG substitution on HA; 500 MHz 1H NMR spectra of mPEG-HA with (a) low, (b) medium, and (c) high PEG substitution degrees. [Click here to view] |
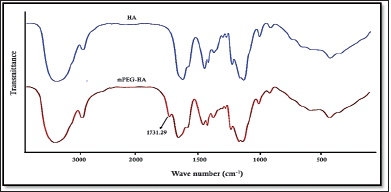 | Figure 4. Comparison of FT-IR spectra of HA and mPEG-HA conjugate. [Click here to view] |
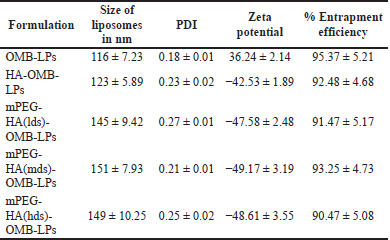 | Table 2. Particle size, PDI, zeta potential, and % EE of coated and uncoated OMB-LPs. [Click here to view] |
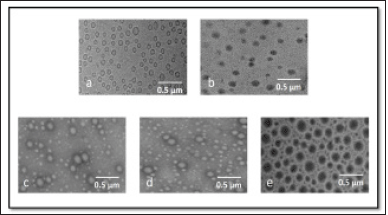 | Figure 5. TEM morphological images of (a) OMB-LPs, (b) HA-OMB-LPs, (c) mPEG-HA(lds)-OMB-LPs, (d) mPEG-HA(mds)-OMB-LPs, and (e) mPEG-HA(hds)-OMB-LPs. [Click here to view] |
Evaluating expression of the hyaluronan receptor CD44
A fluorescently labeled Alexa Fluor 647-anti-mouse/human CD44 antibody was used in initial cell-based assay experiments to determine CD44 expression in mutant EGFR-containing H1975 and PC-9 cells. According to the results, H1975 cells expressed high levels of CD44, while PC-9 cells showed moderate levels of CD44 expression as shown in Figure 8. On the other hand, normal untreated EGFR mutant H1975, and PC-9 cells showed poor expression indicating the inactivated state of CD44 receptors (Fig. 7). It was found that the H1975 cells had high expression of CD44, whereas the PC-9 cells had low expression of CD44. These results successfully differentiated the quantitative expression of CD44 on the cell surface.
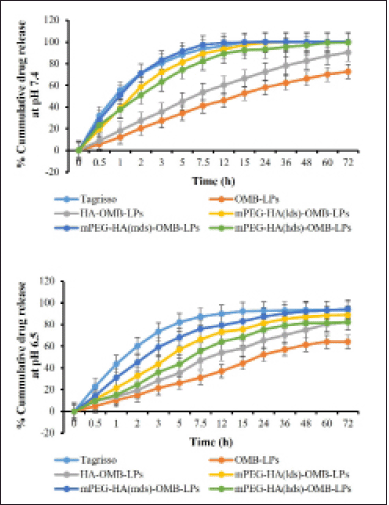 | Figure 6. The in-vitro drug release profile of OMB-LPs, HA-OMB-LPs, and different DS containing mPEG-HA-OMB-LPs nanoliposomes in two simulated fluids having pH of 6.5 and 7.4 respectively. Results were presented as mean ± SD, n = 3. [Click here to view] |
Cellular uptake studies
Results revealed that coated liposomes substantially increased (p < 0.05) the cellular uptake of OMB when compared to uncoated liposomes in both H1975 and PC9 cells overexpressing CD44 (Fig. 8). Among H1975 and PC-9 cells, a mild increase in cellular uptake was achieved in H1975 cells. It was found that HA-OMB-LPs and mPEG-HA(mds)-OMB-LPs increased cellular accumulation of OMB by approximately 1.4 and 1.7-fold, respectively, compared with OMB-LPs in H1975 cells. By blocking CD44 by pretreating with HA, the cellular accumulation of coated liposomes was reduced, resulting in a similar cellular uptake of coated liposomes to uncoated liposomes. It appears that PC-9 cells with low levels of CD44 expression accumulate OMB in the cytoplasm for HA- and mPEG-HA-coated LPs in a different manner from the accumulation profiles observed for H1975 cells with high levels of CD44 expression. The cellular accumulation of OMB was found to be approximately 1.2 and 1.3-fold higher for HA-OMB-LPs and mPEG-HA(mds)-OMB-LPs respectively in PC9 cells than when treated with OMB-LPs. As a result of the pretreatment with HA, blocking CD44 decreased the accumulation of coated liposomes within cells, resulting in similar cellular uptake of coated liposomes and uncoated liposomes. These results are in support of the lower expression levels of CD44 observed in PC-9 cells. On other hand, plain OMB showed poor accumulation of OMB in both cells. Comprehensively these results revealed that HA- ançd mPEG-coated LPs, showed the highest accumulation via CD44 receptor-mediated transport.
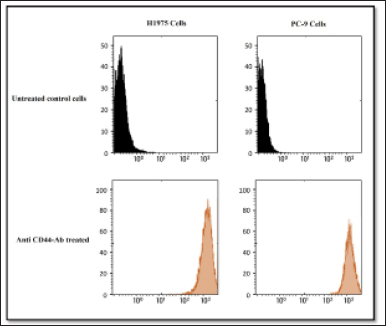 | Figure 7. A comparison of the expression levels of the HA receptor CD44 in H1975 and PC-9 cells. Flow cytometry was used to examine the expression of the HA receptor CD44. An untreated control is shown in the upper panels. Cells treated with Alexa 647-labeled anti-CD44 antibody in H1975 (left) and PC-9 (right) are shown in the lower panels. [Click here to view] |
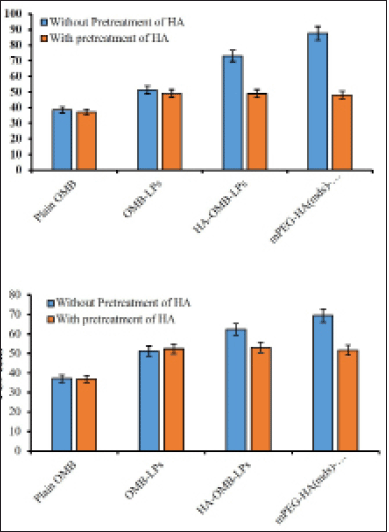 | Figure 8. Quantitative cellular uptake of plain OMB, OMB-LPs, mPEG-HA(mds)-OMB-LPs in (a) H1975 and (b) PC-9 cells. Results are presented as mean + SD (n = 3). Statistical significance of p < 0.05 is considered compared with plain OMB [Click here to view] |
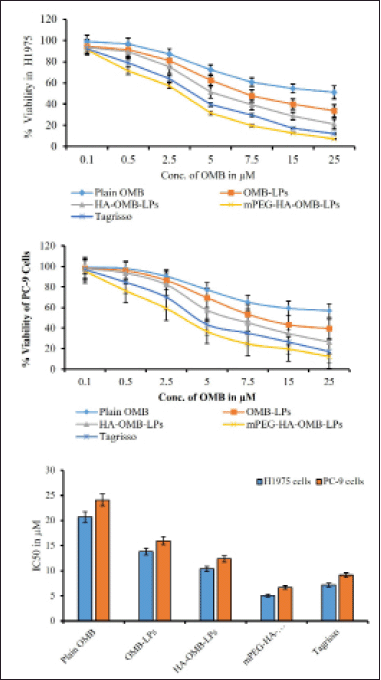 | Figure 9. Cytotoxic effects of plain OMB, OMB-LPs, coated and HA- mPEG-HA coated OMB LPs against H1975 (a) and PC-9 and (b) cells. Comparison of IC50 for plain OMB and OMB-LPs and Tagrisso in different cell lines at 48 hours of treatment (c). This data represents the mean ± SD (n = 6) of three individual experiments. * Statistical significance of p < 0.05 (i.e., 5% significance level) is considered. [Click here to view] |
Cytotoxicity assays
After 48 hours of incubation, the in-vitro cellular cytotoxicity of coated and uncoated OMB LPs was assessed against H1975 and PC-9 (mutant EGFR lines) of selected NSCLCs to establish their therapeutic effectiveness.Figure 8a–c displayed the graphical plots of IC50 values for prepared LPs, plain OMB, and Tagrisso in two cell lines, respectively. The IC50 was determined to be 20.74 ± 1.69 and 24.08 ± 1.95 μM for plain OMB; 13.82 ± 1.54 and 15.98 ± 1.22 μM for OMB-LPs; 10.37 ± 0.95 and 12.34 ± 1.54 μM for HA-OMB-LPs; and 5.02 ± 0.29 and 6.64 ± 0.54 μM for mPEG-HA-OMB-LPs in H1975 cells as shown in Figure 9a and PC-9 cells as shown in Figure 9b respectively. These results revealed that mPEG-HA coated LPs showed great cytotoxic potential which was substantially better than Tagrisso (7.14 ± 0.36 and 9.12 ± 0.68 μM) indicating a significant cytotoxic effect. In addition, mPEG-HA coated LPs showed 4.1 and 3.6 folds increased cytotoxicity against H1975 and PC-9 cells consecutively than plain OMB which was superior to the reference dosage form Tagrisso (2.9 folds for H1975 cells and 2.6 folds for PC-9 cells). On the other hand, mPEG-HA coated LPs showed 2.7- and 2.4-folds higher cytotoxicity against H1975 and PC-9 cells, consecutively than OMB-LPs which were superior to the reference dosage form Tagrisso (1.9 folds for H1975 cells and 1.7 folds for PC-9 cells). Further results demonstrated that mPEG-HA-coated LPs showed twice the cytotoxic activity compared to that of HA-alone coated LPs which indicates, the significance of mPEG conjugation with HA. Figure 9a and b showed that mPEG-HA coated LPs exhibited the lowest IC50 against H1975 cells harboring a double mutant EGFR compared to PC-9 cells harboring a deletion mutation, evidencing the potent anti-proliferative effect of mPEG-HA(mds)-OMB-LPs against both H1975 and PC-9 cells.
CONCLUSION
To summarize our research, we synthesized mPEG-HA molecules with different DS that are highly biocompatible by directly grafting mPEG to HA molecules. These findings revealed that mPEG-HA-coated LPs at a 1:1 ratio formed stable liposomes and promoted sustained release in-vitro. These results demonstrated that mPEG-HA coating enhanced intracellular accumulation of OMB through CD44 receptor-mediated transportation. The cytotoxicity of OMB-loaded liposomes was dose-dependent on EGFR mutant NSCLC cells H1975 and PC-9 line. For the delivery of OMB, mPEG-HA coating could serve as a highly effective carrier, especially in the treatment of cancer.
ACKNOWLEDGMENT
The authors would like to thank the Institute of Pharmacy at Gitam University for allowing them to conduct their studies and providing research facilities. The authors would like to express their appreciation to Mr. Subramanyam Pitchika, a research fellow at the GITAM Institute of Pharmacy at Gitam University, for his assistance, as well as to Dr. Kalakotla Shankar, an assistant professor at JSS University in Mysuru, and Dr. G. Shiva Kumar, a professor at Gitam University in Hyderabad, for their insightful comments and critiques.
AUTHOR CONTRIBUTIONS
Ashadeepti Choppala: Idea, evaluation, editing, Review versions, project management, and monitoring, and guidance; Sanjay kumar Kumar K: Methodology implementation, critical examination, research, data collection, drafting, reviewing, and editing a manuscript
FINANCIAL SUPPORT
The University Grants Commission (UGC) provided funding for this study through UGC-MRP project No. 43-496/2014, while the Institute of Pharmacy at Gitam University in Vishakhapatnam, Andhra Pradesh provided funding through the “Research grant for DRP” (file no. GITAM/DRP/SK-0071/2018).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVALS
There are no animal or human subjects involved in this study.
DATA AVAILABILITY
This article contains all of the data generated and analyzed during the research process.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Andrews Wright NM, Goss GD. Third-generation epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Transl Lung Cancer Res, 2019; 8:247–64. CrossRef
Brown K, Comisar C, Witjes H, Maringwa J, de Greef R, Vishwanathan K, Cantarini M, Cox E. Population pharmacokinetics and exposure-response of osimertinib in patients with non-small cell lung cancer. Br J Clin Pharmacol, 2017; 83(6):1216–26. CrossRef
Cancer Facts & Figures. American Cancer Society, 2022.
Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol, 2018; 11:64. CrossRef
Chi Y, Yin X, Sun K, Feng S, Liu J, Chen D, Guo C, Wu Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Release, 2017; 10(261):113–25. CrossRef
Choi JH, Lee YJ, Kim DI. Image-guided nanomedicine for cancer. Int J Pharm Investig, 2016; 21(3):1–14. CrossRef
Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Choi K, Jeong SY. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials, 2011; 32(7):1880–9. CrossRef
Gasperini AA, Puentes-Martinez XE, Balbino TA, Rigoletto Tde P, Corrêa Gde S, Cassago A, Portugal RV, de La Torre LG, Cavalcanti LP. Association between cationic liposomes and low molecular weight hyaluronic acid. Langmuir, 2015; 31:3308–17. CrossRef
Grass GD, Tolliver LB, Bratoeva M, Toole BP. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J Biol Chem, 2013; 288:26089–104. CrossRef
Hofheinz RD, Gnad Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anti Cancer Drugs, 2005; 16:691–707. CrossRef
Inamura K, Ninomiya H, Ishikawa Y, Matsubara O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features. Arch Pathol Lab Med, 2010; 134:66–72. CrossRef
Ito T, Iida-Tanaka N, Koyama Y. Efficient in vivo gene transfection by stable DNA/PEI complexes coated by hyaluronic acid. J Drug Target, 2008; 16:276–81. CrossRef
Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med, 2015; 372(18):1689–99. CrossRef
Jeannot V, Gauche C, Mazzaferro S, Couvet M, Vanwonterghem L, Henry M, Didier C, Vollaire J, Josserand V, Coll JL, Schatz C, Lecommandoux S, Hurbin A. Anti-tumor efficacy of hyaluronan -based nanoparticles for the co-delivery of drugs in lung cancer. J Control Release, 2018; 275:117–28. CrossRef
Jeannot V, Mazzaferro S, Lavaud J, Vanwonterghem L, Henry M, Arboléas M, Vollaire J, Josserand V, Coll JL, Lecommandoux S, Schatz C, Hurbin A. Targeting CD44 receptor -positive lung tumors using polysaccharide-based nanocarriers: influence of nanoparticle size and administration route. Nanomedicine, 2016; 12:921–32. CrossRef
Kim JS. Liposomal drug delivery system. J Pharm Investig, 2016; 46:387–92. CrossRef
Li C, Chen R, Xu M, Qiao J, Liang Y, Xin DG. Hyaluronic acid modified MPEG-b-PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Deliv, 2018; 25(1):1258–65. CrossRef
Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev, 2018; 65:1–10. CrossRef
Moura J, Gonzaga A, Queiroz S, Martins MD, Pinto LP, Souza LB. Immunohistochemical expression of OCT4 and CD44 in major and minor salivary gland neoplasms. Braz Oral Res, 2021; 16(35):e073. CrossRef
Ohsaki Y, Tanno S, Fujita Y, Toyoshima E, Fujiuchi S, Nishigaki Y, Ishida S, Nagase A, Miyokawa N, Hirata S, Kikuchi K. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep, 2000; 7:603–7. CrossRef
Park NR, Cha JH, Jang JW, Bae SH, Jang B, Kim JH, Hur W, Choi JY, Yoon SK. Synergistic effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem Biophys Res Commun, 2016; 477:568–74. CrossRef
Planchard D, Brown KH, Kim DW, Kim SW, Ohe Y, Felip E, Leese P, Cantarini M, Vishwanathan K, Jänne PA, Ranson M, Dickinson PA. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for the formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol, 2016; 77(4):767–76. CrossRef
Qhattal HS, Liu X. Characterization of CD44-mediated cancer cell uptake and intracellular distribution of hyaluronan-grafted liposomes. Mol Pharm, 2011; 8:1233–46. CrossRef
Rath B, Hochmair M, Plangger A, Hamilton G. Anticancer activity of fascaplysin against lung cancer cell and small cell lung cancer circulating tumor cell lines. Mar Drugs, 2018; 16(10):383. CrossRef
Scagliotti GV, Selvaggi G, Novello S, Hirsch FR. The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res, 2004; 10:4227–32. CrossRef
Shi S, Zhou M, Li X, Hu M, Li C, Li M, Sheng F, Li Z, Wu G, Luo M, Cui H, Li Z, Fu R, Xiang M, Xu J, Zhang Q, Lu L. Synergistic active targeting of dually integrin αvβ3/CD44 -targeted nanoparticles to B16F10 tumors located at different sites of mouse bodies. J Control Release, 2016; 235:1–13. CrossRef
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin, 2018; 68:7–30. CrossRef
Takenaka T, Nakai S, Katayama M, Hirano M, Ueno N, Noguchi K, Takatani-Nakase T, Fujii I, Kobayashi SS, Nakase I. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int J Pharm. 2019; 15(572):118762. CrossRef
Toole BP. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res, 2009; 15:7462–68. CrossRef
Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol, 2008; 18:244–50. CrossRef
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov, 2005; 4:145–60. CrossRef
Van VA, Van GR, Beer DY, Dingemans AM, Stolk L, Ter HR, Vries DF, Croes S. Validation of an analytical method using HPLC-MS/MS to quantify osimertinib in human plasma and supplementary stability results. Biomed Chromatogr, 2020; 34(4):e4771. CrossRef
Wobus M, Rangwala R, Sheyn I, Hennigan R, Coila B, Lower EE, Yassin RS, Sherman LS. CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl Immunohistochem Mol Morphol, 2002; 10:34–39. CrossRef
Xu H, Wu K, Tian Y, Liu Q, Han N, Yuan X, Zhang L, Wu GS, Wu K. CD44 correlates with clinicopathological characteristics and is upregulated by EGFR in breast cancer. Int J Oncol, 2016; 49:1343–50. CrossRef
Zaremba-Czogalla M, Jaromin A, Sidoryk K, Zagórska A, Cybulski M, Gubernator J. Evaluation of the in vitro cytotoxic activity of caffeic acid derivatives and liposomal formulation against pancreatic cancer cell lines. Materials (Basel), 2020; 13(24):5813. CrossRef
Zhang Q, Deng C, Fu Y, Sun X, Gong T, Zhang Z. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol Pharm, 2016; 13:1800–8. CrossRef
Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem, 2000; 275:37733–437741. CrossRef