INTRODUCTION
Cancer is an aberrant proliferation of cells and tissues which produces tumor mass, vascularization, and the subsequent spread to other parts of the body (Kumar and Akdi, 2018) All cancer forms exhibit several characteristics in common, including the capacity to sustain proliferation-associated signals, evade growth suppressors, resist cell death, enable replicative immortality, induce angiogenesis, and activate invasion and metastasis (Hanahan and Weinberg, 2011). Three characteristics of cancer—sustained proliferation, resistance to apoptosis, and activating invasion by cell migration—were taken into account in this study.
The proliferation of cancer cells substantially impacts the development and spread of cancer. This is due to modifications in the expression and/or activity of proteins related to the cell cycle or the constitutive activation of several signal transduction pathways that stimulate cell growth (Feitelson et al., 2015). Growth and metastasis will eventually result from continued cancer cell multiplication. Therefore, it is crucial to find and develop drugs that will block tumor formation and progression early on. On the other hand, resistance to apoptosis is among the important challenges in cancer treatment. It allows cancer cells to survive longer and provides them more time to develop mutations that will speed up the progression, invasiveness, and metastasis of the tumor (Hassan et al., 2014). As a result, finding metabolites that target apoptotic pathways has become an important strategy for creating chemotherapeutic agents. The activation of caspase-3 or -7 is one of the molecular processes displayed by proapoptotic substances (Yadav et al., 2021). Executioner caspase-3 and -7 are downstream effectors of apoptosis. Their activation causes cancer cells to undergo apoptosis, which eventually killed them (Clark and MacKenzie, 2008). Lastly, tumor invasion and metastasis are two pathogenic processes in which cell migration plays a role. Malignant cells invading surrounding tissue and the vascular system constitute the initial stage of tumor metastasis. Cancer cells must migrate to achieve this. The migration of cells, according to Yamaguchi et al. (2005), involves the protrusion of the cell membrane and its adherence to the extracellular matrix (ECM). As a result, several studies focus on looking for substances that can eventually be developed into cancer treatment medications and stop cell migration and growth. For this, the scratch wound assay is frequently utilized to comprehend the molecular mechanisms that regulate cell migration (Walter et al., 2010) and to find pharmaceuticals that can influence cell migration and therefore support therapeutic strategies (Decaestecker et al., 2007).
In recent years, the prevalence of colorectal cancer (CRC) has been rising dramatically across the globe. In 2020, about 1.93 million new cases were diagnosed, and 0.94 million people died from CRC, accounting for 9.4% of all cancer-related deaths and 10% of the world’s 19.29 million new cases of cancer. This makes CRC the third leading cause of cancer-related deaths in both genders worldwide (Xi and Xu, 2021). Furthermore, given the population growth, aging, and human development projections, the number of cases is predicted to increase to 3.2 million in 2040 (Surti et al., 2022). Thus, various studies devoted to the early detection, diagnosis, prevention, and treatment of CRC are being conducted. HCT116 is a commonly used cell line for CRC studies (Zhou et al., 2017).
The majority of conventional cancer treatments have been created to specifically target one of the seven hallmarks (Ercolano et al., 2019). Although there exist cancer treatment choices, their efficacy restrictions and the severe side effects that cancer patients must endure have prompted increased efforts to look for alternative treatments. The development of a novel type of anticancer medication with fewer side effects and enhanced effectiveness would be a breakthrough in cancer therapeutics.
Marine sponges (phylum Porifera) are among the richest sources of chemically diverse and pharmacologically active metabolites (Sipkema et al., 2005). Natural products from sponges have an amazing variety of chemical components, including uncommon nucleosides, bioactive terpenes, sterols, cyclic peptides, alkaloids, fatty acids, peroxides, and derivatives of amino acids which are frequently halogenated (Blunt et al., 2013). The biological actions of sponges, including their antiviral, antioxidant, antiobesity, antihypertensive, antidiabetic, anticancer, and antiproliferative capabilities, are also attributed to these chemicals (Kumar and Adki, 2018). The anticancer potential of marine sponge-derived metabolites relies on a variety of cellular and molecular pathways, including growth inhibition, the induction of apoptosis and autophagy, antiangiogenic effects, and antimigration (Ruiz-Torres et al., 2017).
Philippine marine biodiversity has been evaluated as one of the richest in the world (Carpenter and Springer, 2005). However, the Philippines’ exceptional biodiversity remains largely unexplored (Scheffers et al., 2012). Thus, marine sponges from Iligan Bay, Misamis, Oriental, Philippines, i.e., Ircinia sp., Agelas sp., and Petrosia weinbergi, were collected and studied. Their antiproliferative, proapoptotic, and antimigration activities against colorectal carcinoma cells (HCT116) were evaluated.
MATERIALS ANDMETHODS
Materials
The marine sponges Agelas sp., Ircinia sp., and Petrosia weinbergi were collected off the coast of Iligan Bay, Calangahan, Lugait, Misamis Oriental, Philippines, by hand using scuba at a depth of 6–7 m. Authentication and identification of Agelas sp. were done by Prof. Angelo Responte, a marine biologist from Mindanao State University-Iligan Institute of Technology (MSU-IIT), Iligan City, Philippines, based on its morphological characteristics. For Ircinia sp. and Petrosia weinbergi, deoxyribonucleic acid (DNA) barcoding was employed. The DNA extraction and amplification were done using a commercial genomic DNA extraction kit (QIAGEN’s DNeasy Blood and Tissue Kit) and PCR kit (Dongsheng Biotech), respectively. Amplification using C2 forward and D2 reverse primers was carried out under standard conditions. PCR product purification and DNA sequencing were carried out by Macrogen Inc. (Seoul, South Korea). The BLASTN search software was used to analyze and compare the sequence results with known sequences in the GenBank database to ascertain the samples’ identity. Voucher specimens of Agelas sp., Ircinia sp., and P. weinbergi with voucher specimen numbers KL23, KL26, and KL28, respectively, are kept at Natural Products and Drug Discovery Laboratory, Premier Research Institute of Science and Mathematics (PRISM), MSU-IIT, Iligan City, Philippines.
The HCT116 cancer cells were purchased from the American Type Culture Collection. McCoy’s 5A media and the fetal bovine serum were obtained from Gibco, while the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Celltiter96® Nonradioactive Cell Proliferation Assay) and the Caspase-Glo® 3/7 Assay kits were purchased from Promega Corporation. In the assays, sodium butyrate (Sigma-Aldrich), digitonin (Sigma-Aldrich), and dimethyl sulfoxide (DMSO) (Sigma-Aldrich) were used.
Sample preparation and extraction
The samples were washed thoroughly with distilled water, chopped, and freeze-dried. Each dried sample was powdered and sequentially soaked for 72 hours in H, DCM, and MW (1:1 v/v). After 72 hours, filtration and concentration of each solvent extract under vacuum were done. The obtained crude nonpolar H, mid-polar DCM, and polar MW extracts were weighed and stored at −20°C until further analysis.
Cell culture
The HCT116 cells were cultured in McCoy’s media supplemented with 10% fetal bovine serum (FBS). The cell cultures were incubated and kept at 37°C under a 5% CO2 atmosphere.
MTT assay
The MTT assay was conducted to determine the cytotoxicity of the sponge extracts against the carcinoma cell lines according to the method described by Roy et al. (2020) with modifications. The provided protocol in the Promega kit (Cell Titer 96 ® Nonradioactive Cell Proliferation Assay) was followed. A 90 µl cell suspension containing 2 × 104 cells was seeded in a 96-well microplate except for the blank wells and incubated overnight at 37°C and a 5% CO2 atmosphere.
The next day, 10 µl of 300 µg/ml test samples and positive control (digitonin) were added to their corresponding wells in a 96-well plate to produce a 30 ug/ml assay concentration of test solutions. A vehicle DMSO-treated cell in a medium was used as a negative control, while a vehicle DMSO and cell culture medium without the cells was used as a blank. Incubation at 37°C and 5% CO2 atmosphere was done overnight before the addition of 15 µl MTT reagent and further incubation for 4 hours under 5% CO2 atmosphere at 37°C. A 100 µl of solubilization solution/stop mix was added to all wells which were then incubated for an hour at 37°C and 5% CO2 atmosphere. After incubation, the absorbance was measured and recorded at 570 nm using a spectrophotometer (Perkin Elmer Victor X3 multimode spectrophotometer). The cytotoxicity, expressed as percent cell viability, was then calculated as follows:
Caspase-Glo® 3/7 assay
A commercial kit (Promega G8090 Caspase-Glo® 3/7 Assay, Promega, Madison, WI) was used to measure the proapoptotic property in terms of caspase-3/7 cleavage. The recommended protocol by the manufacturer was followed with minor modifications on the amount of Caspase-Glo 3/7 reagent utilized. In a 96-well flat bottom plate, a 90 µl of cell suspension (1 × 104 cells) was seeded. The plate was then incubated at 37°C and 5% CO2 for 6 hours. The cells were then treated with 10 µl of 300 µg/ml sample extracts to yield a 30 ug/ml final assay concentration and were incubated for a period of 20 hours. After 20 hours, a 10 µl Caspase-Glo 3/7 reagent was added to each well. Incubation for 2 hours at 37°C in 5% CO2 followed. Luminescence was quantified using a luminometer (Perkin Elmer Victor X3 multimode spectrophotometer). A 5 mM sodium butyrate was used as a positive control. On the other hand, DMSO-treated cells in a medium served as a negative control, while a vehicle DMSO and cell culture medium without the cells were used as a blank.
The proapoptotic activity of the samples in terms of caspase-3/7 activity was reported as a mean of the corrected relative light units (RLUs). Higher RLU values are indicative of a stronger caspase-3/7 activity. To further evaluate the proapoptotic activity of the sample, fold change (FC) against the negative control was calculated.
In vitro scratch wound assay
The HCT116 at 5 × 104 density was seeded in a 96-well plate and cultured for 3 days at 37°C under a 5% CO2 atmosphere to attain 90%–100% confluency. A sterile 10 ul tip was used to make a scratch and the culture media were removed carefully. Washing of the cells with 100 ul of PBS to remove detached cells and other cellular debris was then done. The culture media were replaced with 90 μl of media consisting of lower FBS content (2%). The image of the wound area or scratch was taken using an inverted microscope (time 0). Treatment of the cells with 10 μl of the sample extracts (300 ug/ml) and incubation for 18 hours (time 18) at 37°C under a 5% CO2 atmosphere followed. Vehicle DMSO-treated cells in a medium were used as a negative control. The cell migration was observed in the images that were taken using an inverted microscope (AmScope) equipped with a digital camera. ImageJ software was used to assess the wound area at time 0 (A0) and time 18 (A18). Using the formula below, cell migration was calculated as a percentage of wound closure (Almeida et al., 2019):
Statistical analysis
All analysis was done in a three-by-three format (three trials, three replicates per trial). Results are reported as mean values. Significant differences between the negative control (DMSO) and the sample extracts were determined by T-test at a 5% significance level.
RESULTS AND DISCUSSION
Antiproliferative activity
All extracts showed cytotoxicity against the HCT116 cells except for the Ircinia sp. MW extract (Fig. 1). The viability of HCT116, although greater than 50%, was found significantly lower (p < 0.05) than those treated with the vehicle control DMSO. The DCM extract of Ircinia sp. gave the lowest percent cell viability (75.81%) followed by the H extract of Agelas sp. (77.67%).
Cell proliferation is tightly regulated in normal cells, while cancer cells are characterized by their ability to sustain proliferation-related signals as a consequence of stroma or specific gene expression patterns (Deshpande et al., 2005). Marine sponges are known prolific producers of secondary metabolites that have exhibited intriguing chemopreventive and chemotherapeutic properties (Calcabrini et al., 2017). According to Calcabrini et al. (2017), several marine sponge-produced chemicals show antiproliferative activity that targets the regulators of cell-cycle progression.
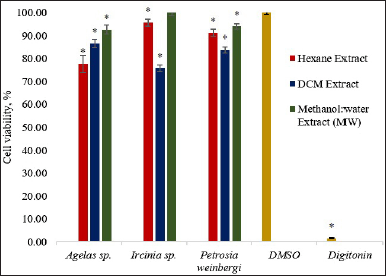 | Figure 1. Percent cell viability of HCT116 using the MTT assay after treatment with 30 ug/ml of the sponge extracts. The asterisk (*) indicates significantly lower percent cell viability than the vehicle control (DMSO) based on the T-test (p < 0.05). Digitonin was used as a positive control. Error bars are SEM (n = 9). [Click here to view] |
Previous studies have reported the cytotoxicity of Ircinia and Agelas sponges against various carcinoma cells. Their cytotoxicity potential against cancer cells is even more evident with the isolation of bioactive secondary metabolites. The genus Ircinia has been a known source of a wide array of steroids, terpenoids, and other metabolites with various pharmacological properties (Heidary et al., 2021). The methanol and diethyl extracts of I. mutans from the Persian Gulf have been found strongly active on KB and HUT cells as determined through XTT assay (Nazemi et al., 2020). Moreover, tedanolide C, an 18-membered macrolide ring, which was obtained from the chloroform partition of the methanolic extract of Ircinia sp., also exhibited potent cytotoxicity against HCT116 cells. Further, treatment of cells with 0.2 ug/ml of tedanolide C led to the arrest of the cell cycle at the S-phase (Chevallier et al., 2006). On the other hand, Agelas sponges have been investigated since the beginning of the 1970s (Zhang et al., 2017). Studies revealed that Agelas sponges produce bioactive compounds belonging to alkaloids (bromopyrrole derivatives), terpenoids, fatty acids, glycosphingolipids, and carotenoids (Bickmeyer et al., 2004). The genus Agelas has been discovered as the most lucrative producer of bioactive alkaloids (Elissawy et al., 2021). Chu et al. (2017) reported the identification of diterpene alkaloids from A. nakamurai collected from the South China Sea. Evaluation of the cytotoxicity of the compounds demonstrated weak to moderate effects against HL60, K560, and HCT116. Two new sceptrin derivatives were also obtained from the combined methanol and DCM extracts of A. kosrae. The sceptrin alkaloids have shown weak cytotoxicity against carcinoma cells including HCT116 (Kwon et al., 2018). Lastly, oroidin, a pyrrole alkaloid from A. oroides, was known to possess moderate cytotoxic activity against many cells, i.e., MCF-7, A2780, and HT29 (Dyson et al., 2014). Ircinia sp. DCM and Agelas sp. H extracts, thus, can be further purified to determine the compounds responsible for their cytotoxicity.
Proapoptotic activity
The proapoptotic activity of the extracts against HCT116 carcinoma cells was monitored by measuring the luminescence produced after the addition of the caspase-3/7 reagent into the cells and sponge extract reaction mixture. The amount of luminescence recorded is proportional to the intensity of caspase activity exhibited by the sample (Payne et al., 2013). To further describe the degree of changes of the measured signals in the treatments relative to the negative control, FC was calculated.
Treatment of the HCT116 carcinoma cells with MW and DCM extract of P. weinbergi and DCM extract of Ircinia sp. led to a significantly higher (p < 0.05) luminescence signal than the vehicle control (Fig. 2). The said extracts enhanced the caspase-3/7 activity greater than onefold relative to DMSO. Among the three extracts, the DCM extract of Ircinia sp. gave the highest RLU and FC value of 563 RLU and 2.69, respectively. The data suggest that the DCM extract of Ircinia sp. may have induced the activation of the caspase-3/7 leading to the apoptosis of HCT 116 cells.
Caspase-3 and -7, which are cysteine-aspartic proteases, are downstream effector caspases in the apoptosis pathway. The caspase-3 regulates DNA fragmentation and morphologic changes of apoptotic cells while caspase-7 was found important in the loss of cellular viability (Lakhani et al., 2006). During apoptosis, caspase-3 and caspase-7 are both activated. Regardless of the death-initiating stimulus, both caspases are considered to coordinate during apoptosis by cleaving a diverse array of protein substrates (Timmer and Salvesen, 2007). The induction of caspase-3/7 activity is proportional to the activation of apoptosis (Butterick et al., 2014).
Only a few literature works have reported on the proapoptotic activity of Ircinia sponges. A compound furanoterpenoid, 10-acetylirciformonin B, was isolated from the ethyl acetate extract of Ircinia sp. This compound exhibited cytotoxicity against HL-60 human leukemia cells by inhibiting cell growth. Interestingly, it also showed proapoptotic properties through the activation of caspase 8, 9, and 3 and induction of PARP cleavage (Su et al., 2012). Chinen et al. (2010) have isolated irciniastatin A (ISA) from I. ramose. ISA, apart from being selectively cytotoxic against certain human cancer cell lines, activates stress-activated JNK in human leukemia Jurkat cells leading to apoptosis. The observed proapoptotic property of the DCM extract of Ircinia sp. on HCT116 carcinoma cells would warrant further investigation to elucidate both the structure of the bioactive compound and the mechanism of action involved in its exhibited activity.
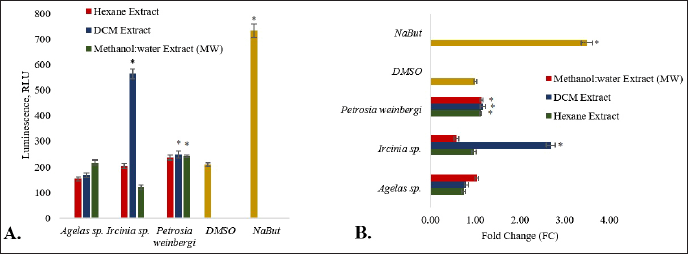 | Figure 2. The proapoptotic activity of the sponge extracts is expressed in terms of A. RLU and B. FC. The asterisk (*) indicates a significantly higher RLU or FC than the vehicle control (DMSO) based on the T-test (p < 0.05). Sodium butyrate (NaBut) was used as a positive control. Error bars are SEM (n = 9). [Click here to view] |
Antimigration property
The MW extracts of Agelas sp. and Ircinia sp., the DCM extract of Ircinia sp., and all extracts of P. weinbergi showed antimigration properties against HCT116 carcinoma cells (Fig. 3). The change in the wound area 18 hours after wounding of the cell layer is smaller in the above-mentioned extracts (Fig. 4). When compared against the vehicle control DMSO, the said extracts gave significantly lower (p < 0.05) percent wound closure. The MW extract of Ircinia sp. and the H extract of P. weinbergi exhibited the highest inhibitory activity against the migration of HCT116 carçcinoma cells after wounding. The results imply that Ircinia sp. and P. weinbergi may have produced compounds that possess antimigration activity.
Cell migration is associated with several physiological and pathological processes. Cell migration facilitates embryonic morphogenesis, aids in tissue repair and regeneration, and promotes the development of diseases including cancer, mental retardation, osteoporosis, atherosclerosis, and arthritis (Ridley et al., 2003). The primary characteristics of tumor biology are cell migration and invasion, which are essential elements of metastasis (van Zijl et al., 2011). As a cyclic process, cell migration is initiated by polarization and extension of protrusions in the direction of migration when a cell responds to a migration-promoting agent. Typically, actin polymerization stimulates the protrusions, which are then stabilized by attachment to the ECM or neighboring cells via transmembrane receptors. These adhesions serve as grip sites for migration as the cells move forward leading to cell movement (Ridley et al., 2003). Wound healing and cancer have been long associated. The mechanisms which regulate wound healing have been found involved in the promotion of the growth and transformation of malignant cells (Arnold et al., 2015). Thus, inhibition of wound healing in cancer cells leads to the suppression of cell migration or invasion (Liew et al., 2017).
The integrins and intracellular signaling proteins found at the regions of focal adhesions stimulate extracellularly and initiate the migration process. The regulation of integrin 1 signaling may prevent cell migration. Basement membranes and extracellular matrices allow tumor cells to move as they spread from the initial site to other subsequent organs (Liew et al., 2017). A study by Yang et al. (2021) reported that astragalin dramatically reduced HCT116 cell migration by preventing matrix metalloproteinases (MMP-2 and MMP-9) from being expressed. MMPs are among the most important regulatory molecules in cancer and metastasis (Egeblad and Werb, 2002), and the expression of these molecules is associated with the emergence and growth of tumors (Song et al., 2016). Cantharidin also suppresses HCT116 cell migration by changing the cytoskeleton structure. Many aspects of cell migration are linked to the dynamic movement of the cytoskeleton network (Zhang et al., 2018).
To the best of our knowledge, however, this is the first report on the antimigration property of Agelas sp., Ircinia sp., and P. weinbergi extracts against HCT116 carcinoma cells. Thus, the results of this study may serve as baseline data for future-related evaluation of their anticancer properties, especially their antimigration activity.
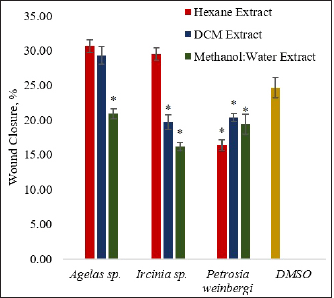 | Figure 3. The antimigration activity of the sponge extracts was expressed in terms of percent wound closure 18 hours after treatment of the wound. The asterisk (*) indicates a significantly lower percent wound closure than the vehicle control (DMSO) based on the T-test (p < 0.05). Sodium butyrate (NaBut) was used as a positive control. The percent wound area of the HCT116 after treatment with NaBut was less than 0 after 18 hours. Error bars are SEM (n = 9). [Click here to view] |
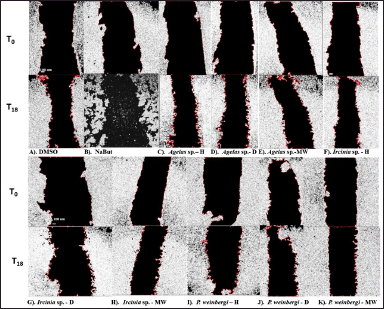 | Figure 4. Images of the wound of the HCT116 cells were taken at 0 hours (T0) after wound and 18 hours (T18) after wound and treatment with (A) DMSO (negative control), (B) NaBut (sodium butyrate as positive control), and (C–K) sponge extracts H, D-DCM, MW). The images and wound area were processed and determined using ImageJ software. [Click here to view] |
CONCLUSION
Ircinia sp. and P. weinbergi were found to show potential antiproliferative, proapoptotic, and antimigration properties against human colorectal carcinoma cells HCT116. Moreover, this is the first report on the antimigration properties of the Agelas sp., Ircinia sp., and P. weinbergi against HCT116. The results of this study warrant the isolation of the bioactive compounds from the studied sponges as well as the elucidation of the mechanism of action responsible for their anticancer property.
LIST OF ABBREVIATIONS
The following abbreviations are used in this manuscript:
HCT: human colorectal carcinoma cell line; FC: fold change; H: hexane; RLU: relative light unit; DCM: Dichloromethane; MW: methanol: water; MTT: (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide).
ACKNOWLEDGMENTS
The authors are grateful to the Philippine Council for Health Research and Development (PCHRD) of the Department of Science and Technology (DOST) of the Philippines for funding this research through the “Tuklas Lunas” Development Center of MSU-IIT. The authors also acknowledge CMU’s Natural Products Research and Development Center (NPRDC) for allowing them to conduct part of this study in their laboratories. Lastly, special thanks are due to Dr. Angelo Responte, Jan Mart T. Daluz, and Tricksie Balatero of PRISM, MSU-IIT for their assistance in the collection of samples, the conduct of the anticancer assays, and DNA barcoding experiments, respectively.
AUTHOR CONTRIBUTIONS
The conceptualization and design of the study were done by M.M. Uy. Acquisition, analysis, and interpretation of the data were performed by A.M.G. Ang. The authentication and identification of samples through DNA barcoding experiments were done by S.R.M. Tabugo and A.M.G. Ang. A.M.G. Ang drafted the article. All authors took part in revising the article; agreed to submit it to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Almeida VM, Bezerra Jr MA, Nascimento JC, Amorim LMF. Anticancer drug screening: standardization of in vitro wound healing assay. J Bras Patol Med Lab, 2019; 55(6):606–19. CrossRef
Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis, 2015; 8:CGM–S11286. CrossRef
Bickmeyer U, Drechsler C, Köck M, Assmann M. Brominated pyrrole alkaloids from marine Agelas sponges reduce depolarization-induced cellular calcium elevation. Toxicon, 2004; 44(1):45–51. CrossRef
Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep, 2013; 30(2):237–323. CrossRef
Butterick TA, Duffy CM, Lee RE, Billington CJ, Kotz CM, Nixon JP. Use of a caspase multiplexing assay to determine apoptosis in a hypothalamic cell model. J Vis Exp, 2014; 86:e51305. CrossRef
Calcabrini C, Catanzaro E, Bishayee A, Turrini E, Fimognari C. Marine sponge natural products with anticancer potential: an updated review. Mar Drugs, 2017; 15(10): 310. CrossRef
Carpenter KE, Springer VG. The center of marine shore fish biodiversity: the Philippine Islands. Environ Biol Fishes, 2005; 72(4):467–80. CrossRef
Chevallier C, Bugni TS, Feng X, Harper MK, Orendt AM, Ireland CM. Tedanolide C: a potent new 18-membered-ring cytotoxic macrolide isolated from the Papua New Guinea marine sponge Ircinia sp. J Org Chem, 2006; 71(6): 2510–3. CrossRef
Chinen T, Nagumo Y, Watanabe T, Imaizumi T, Shibuya M, Kataoka T, Usui T. Irciniastatin A induces JNK activation that is involved in caspase-8-dependent apoptosis via the mitochondrial pathway. Toxicol Lett, 2010; 199(3):341–6. CrossRef
Chu MJ, Tang XL, Qin GF, Sun YT, Li L, de Voogd, NJ, Li GQ. Pyrrole derivatives and diterpene alkaloids from the South China Sea sponge Agelas nakamurai. Chem Biodivers, 2017; 14(7):e1600446. CrossRef
Clark AC, MacKenzie SH. Targeting cell death in tumors by activating caspases. Curr Cancer Drug Targets, 2008; 8(2):98–109. CrossRef
Decaestecker C, Debeir O, Van Ham P, Kiss R. Can anti-migratory drugs be screened in vitro? A review of 2D and 3D assays for the quantitative analysis of cell migration. Med Res Rev, 2007; 27:149–76. CrossRef
Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene, 2005; 24(17):2909–15. CrossRef
Dyson L, Wright AD, Young KA, Sakoff JA, McCluskey A. Synthesis and anticancer activity of focused compound libraries from the natural product lead, Oroidin. Bioorg Med Chem, 2014; 22(5):1690–9. CrossRef
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer, 2002; 2(3):161–74. CrossRef
Elissawy AM, Soleiman Dehkordi E, Mehdinezhad N, Ashour ML, Mohammadi PP. Cytotoxic alkaloids derived from marine sponges: a comprehensive review. Biomolecules, 2021; 11(2):258. CrossRef
Ercolano G, De Cicco P, Ianaro A. New drugs from the sea: pro-apoptotic activity of sponges and algae-derived compounds. Mar Drugs, 2019; 17(1):31. CrossRef
Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Nowsheen S. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol, 2015; 35:S25–54). CrossRef
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell, 2011; 144(5):646–74. CrossRef
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Res Int, 2014; 2014:1–23. CrossRef
Heidary Jamebozorgi F, Yousefzadi M, Firuzi O, Nazemi M, Zare S, Chandran JN, Jassbi AR. Cytotoxic furanosesquiterpenoids and steroids from Ircinia mutans sponges. Pharm Biol, 2021; 59(1):573–81. CrossRef
Kumar MS, Adki KM. Marine natural products for multi-targeted cancer treatment: a future insight. Biomed Pharmacother, 2018; 105:233–45. CrossRef
Kwon OS, Kim D, Kim H, Lee YJ, Lee HS, Sim CJ, Shin J. Bromopyrrole alkaloids from the sponge Agelas kosrae. Mar Drugs, 2018; 16(12):513. CrossRef
Lakhani SA, Masud A, Kuida K, Porter Jr GA, Booth CJ, Mehal WZ, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science, 2006; 311(5762):847–51. CrossRef
Liew SK, Azmi MN, In LL, Awang K, Nagoor NH. Anti-proliferative, apoptotic induction, and anti-migration effects of hemi-synthetic 1′ S-1′-acetoxychavicol acetate analogs on MDA-MB-231 breast cancer cells. Drug Des, Devel Ther, 2017; 11:2763. CrossRef
Nazemi M, Ghaffari H, Moradi Y, Amiran MR, Dargeri SA. Cytotoxic activity of extracts of demosponges Haliclonacaerulea, Axinellasinoxea and Ircinia mutans from Persian Gulf. Indian J Exp Biol, 2020; 58(04):249–53. CrossRef
Payne AM, Zorman J, Horton M, Dubey S, ter Meulen J, Vora KA. Caspase activation as a versatile assay platform for detection of cytotoxic bacterial toxins. J Clin Microbiol, 2013; 51(9):2970–6. CrossRef
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Horwitz AR. Cell migration: integrating signals from front to back. Science, 2003; 302(5651):1704–9. CrossRef
Roy P, Ramanjooloo A, Doorga JRS, Beedessee G, Cresteil T, Van Soest RW, EP D. Cytotoxic potential of sponge extracts from mauritius waters on human cancer cell lines. Hematol Med Oncol, 2020; 5:1–0. CrossRef
Ruiz-Torres V, Encinar JA, Herranz-López M, Pérez-Sánchez A, Galiano V, Barrajón-Catalán E, Micol V. An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small-molecule cancer drugs. Molecules, 2017; 22(7):1037. CrossRef
Scheffers BR, Joppa LN, Pimm SL, Laurance WF. What we know and don’t know about earth’s missing biodiversity. Trends Ecol Evol, 2012; 27(9):501–10. CrossRef
Sipkema D, Franssen MC, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Mar Biotechnol, 2005; 7(3):142. CrossRef
Song J, Peng P, Chang J, Liu MM, Yu JM, Zhou L, Sun X. Selective non-zinc binding MMP-2 inhibitors: novel benzamide Ilomastat analogs with anti-tumor metastasis. Bioorg Medicin Chem Lett, 2016; 26(9):2174–8. CrossRef
Su JH, Chang WB, Chen HM, El-Shazly M, Du YC, Kung TH, Lu MC. 10-acetylirciformonin B, a sponge furanoterpenoid, induces DNA damage and apoptosis in leukemia cells. Molecules, 2012; 17(10):11839–48. CrossRef
Surti M, Patel M, Redhwan A, Al-Keridis LA, Adnan M, Alshammari N, Reddy MN. Ilimaquinone (marine sponge metabolite) induces apoptosis in HCT-116 human colorectal carcinoma cells via mitochondrial-mediated apoptosis pathway. Mar Drugs, 2022; 20(9):582. CrossRef
Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ, 2007; 14(1):66–72. CrossRef
Van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res, 2011; 728(1-2):23–34. CrossRef
Walter MNM, Wright KT, Fuller HR, MacNeil SM, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res, 2010; 316:1271–81. CrossRef
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. (2021). Transl Oncol, 2021; 14(10):101174. CrossRef
Yadav P, Yadav R, Jain S, Vaidya A. Caspase-3: A primary target for natural and synthetic compounds for cancer therapy. Chem Biol Drug Des, 2021; 98(1):144–65. CrossRef
Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Current Opin Cell Biol, 2005; 17(5):559–64. CrossRef
Yang M, Li WY, Xie J, Wang ZL, Wen YL, Zhao CC, Sheng J. Astragalin inhibits the proliferation and migration of human colon cancer HCT116 cells by regulating the NF-κB signaling pathway. Front Pharmacol, 2021; 12:639256. CrossRef
Zhang X, Sui T, Ma Q, Shao H, Hu X, Sheng H, Luo G. Cantharidin suppresses HCT116 colorectal carcinoma cell proliferation and migration by changing the cytoskeleton structure. J Trad Chin Med Sci, 2018; 5(3):302–09. CrossRef
Zhou J, Hu M., Wang F, Song M, Huang Q, Ge B. miR-224 controls human colorectal cancer cell line HCT116 proliferation by targeting Smad4. Int J Med Sci, 2017; 14(10): 937. CrossRef