INTRODUCTION
Type-3 diabetes mellitus (T3D) is a pathological condition that resembles the characteristics of both type-1 and type-2 diabetes mellitus and is characterized by decreased Insulin production and Insulin resistance (IR), confined particularly to the brain, altering brain Insulin signaling, neurotoxins accumulation, and neuronal stress (Ashraf et al., 2014; Caberlotto et al., 2019; Nguyen et al., 2020; Pasquier et al., 2006; Rivera et al., 2005; Sato et al., 2006; Steen et al., 2005). These manifestations affect basic activities such as learning and memory, thus, resulting in neurodegeneration (Nguyen et al., 2020). T3D and Alzheimer’s disease (AD) share common features including hyperinsulinemia, altered Insulin signaling cascade, and/or IR (Baker et al., 2011; Weinstein et al., 2019), aggregation of amyloid proteins such as Aβ and amylin (Cooper et al., 1989; Hardy and Higgins, 1992; Lim et al., 2010; Nguyen et al., 2020) suggesting T3D, a neuro-endocrine disorder, characterized by dysregulated glucose homeostasis (GSK3β, IRS 1, Akt, altered Insulin signaling, Insulin receptor, and IGF 1 receptor), disturbed cell survival (impaired Bcl, Bad), altered lipid metabolism (APOE and JAK-STAT pathway), increased apoptosis (increased activity of caspase 3 and 8, altered p53 pathway) (Mittal et al., 2016). Literature has indicated that the underlying Insulin signaling and/or IR are responsible for the emergence of memory and executive deficits (Ferreira et al., 2018; Ormazabal et al., 2018; Rorbach-Dolata and Piwowar, 2019) suggesting these as the ultimate causes of AD and related pathologies (Moloney et al., 2010; Steen et al., 2005; Talbot et al., 2012).
IR is responsible for tau hyperphosphorylation, and neuroinflammation leading to neurodegeneration (Kroner, 2009). Hyperinsulinemia impairs cognitive function causing cerebral microvascular and macrovascular damage (Den Heijer et al., 2003). Both hyperinsulinemia and IR, are responsible for increasing inflammatory markers such as C-reactive protein and interleukin 6 (Hak et al., 2001). Increased levels of these inflammatory markers result in the development and progression of AD. Sustained increase in the levels of inflammatory markers has been implicated in AD pathogenesis (McGeer and McGeer, 2004). Thus, hyperinsulinemia and IR, act as important factors that keep Insulin at the center stage of both diabetes and AD (Baker et al., 2011). Further, hyperinsulinemia and/or IR may promote the secretion of butyrylcholinesterase (BuChE) (Randell et al., 2005). BuChE and acetylcholinesterase (AChE) may also result in IR in AD (Houstis et al., 2006; Randell et al., 2005; Sharma et al., 2006). Both, altered brain cholinergic activity and IR are known to accelerate the deposition of amyloid β in the AD brain (Das, 2007; Ho et al., 2004; Reyes et al., 2004).
Insulin plays an important role in the regulation of memory and learning. Insulin also regulates the concentrations of various neurotransmitters in the brain and affects long-term potentiation. Insulin receptors are located in the brain, particularly in the hippocampus, entorhinal, perirhinal, parahippocampal cortex, and medial temporal lobe areas (Zhao and Alkon, 2001). Other than being produced by neurons, Insulin may enter the brain by bypassing the blood–brain barrier (BBB) (Soto et al., 2019). There are two different isoforms of the Insulin receptor: IR-A (predominantly present in the brain) and IR-B (predominantly present in peripheral tissues including the muscles, kidneys, and liver) (Mosthaf et al., 1990). IR-A & B, both, are present in astrocytes while IR-A is exclusively present in neurons (Abbott et al., 1999; Garwood et al., 2015). Memory-related functions are regulated by the Insulin in the brain. Brain Insulin signaling pathway exerts a major role in neurons survival along with improving cognitive processes and synaptic plasticity (Beattie et al., 2000; Dou et al., 2005; Huang et al., 2003; Lin et al., 2000; Ma et al., 2003; Mielke et al., 2006; Passafaro et al., 2001;Skeberdis et al., 2001; Wan et al., 1997; Valenciano et al., 2006; Wickelgren, 1998; Zhao et al., 1999). Earlier studies have indicated that Insulin reduces hippocampal inflammation in young rats by decreasing the neuroinflammatory response and enhancing spatial memory in young rats (Adzovic et al., 2015).
Rivastigmine, dual AChE and BuChE inhibitor, is widely used in symptomatic management of AD (Ray et al., 2020). It is known to block hydrolysis of Ach, promotes cholinergic functioning by raising Ach levels and reduces amyloid beta (Aβ) aggregation (Jamshidnejad-Tosaramandani et al., 2021). Also, Rivastigmine has been shown to improve cognitive functions in elder patients without improving peripheral IR. However, brain IR (BIR) is thought to be more serious condition as compared to IR in the periphery. Therefore, BIR and altered cholinergic activity may affect the pathology of AD through various mechanisms (Isik et al., 2012) as impaired Insulin signaling is responsible for the desensitization of the beta-subunit of the Insulin receptor in the brain Insulin signaling pathway rather than in the peripheral signaling pathway. Till now, a clear relationship between IR and AChE inhibition has not been established yet (Isik et al., 2012). So, Rivastigmine may thus be explored for managing BIR as it may promote neuronal survival by activating the PI3K-Akt signaling pathway (Takada-Takatori et al., 2006) and thus may improve Insulin signaling.
As Insulin and Rivastigmine therapy have been shown to counteract the memory deficit, amyloid deposition, and neuronal degeneration in various studies but till date, no study has been conducted to estimate the effect of Insulin and Rivastigmine treatment in T3D. This study was planned to explore the pharmacological effects of Insulin and Rivastigmine in T3D rats.
MATERIAL AND METHODS
Animals and test conditions
The experimental Wistar rats (200–250 g) were procured from Disease Free Small Animal House (DFSAH), Lala Lajpat Rai University of Veterinary and Animal Sciences (LUVAS), Hisar, and housed in the Animal House of Maharshi Dayanand University (MDU), Rohtak under controlled environmental conditions (relative humidity: 60% ± 10%; temperature: 25°C ± 2°C; alternative light and dark cycle of 12 hours). The rats were placed in the standard polypropylene cages in a group of three rats/cage. The rats had free access to water and food. The study design was approved by the Institutional Animal Ethics Committee (IAEC) of Maharshi Dayanand University (Approval No.: 153-165-17/12/2018).
Chemicals and drugs
STZ (Sigma Aldrich), AlCl3 (Loba Chemie), Insulin (Novo Nordisk), and Rivastigmine (Sun Pharma Ltd.) were used. Drugs were dissolved in saline water before use. All the drugs were injected in a volume of 5 ml/kg through the intraperitoneal (i.p.) route except Insulin which was administered via subcutaneous (s.c.) route.
For the induction of T3D, overnight fasted rats were injected with a single dose of STZ (35 mg/kg, i.p., dissolved in 0.1 M citrate buffer of pH 4.5) followed by the confirmation of hyperglycemia on 3rd day after STZ (Andrade et al., 2018). The rats with a blood glucose level greater than 200 mg/dl were further used (Kumar et al., 2011; Qinna and Badwan, 2015). Thereafter, AlCl3 (12.5 mg/kg., i.p.) was administered for 28 days.
Rats in the vehicle control group received saline. Rivastigmine (1 mg/kg, i.p.) and Insulin (1 IU, sc.) were used as the test drugs (Ismail et al., 2013; John et al., 2015; Liu et al., 2020).
Following 28 days of treatment, the rats have undergone behavioral parameters for determining memory-related functions and neurochemical alterations. The rats with increased insulin, glucose, and amyloid levels in the brain and plasma glucose levels were considered T3D rats.
Evaluation of locomotor activity
Open field test (OFT)
Locomotion in the experimental rats was evaluated by placing each rat in the center region of the open field and observed for 5 minutes. Time spent in the peripheral and central squares and total crossings were noted (Rajasankar et al., 2009).
Several other parameters may also be observed to assess the behavior of rodents such as latency (time to initiate the movement), freezing time (period without any movement), grooming and rearing behavior, and frequency of stretch-attend posture (Hira et al., 2020; Temitayo et al., 2020). Location is generally analyzed by evaluating the time spent in the area of the peripheral and central squares and the total crossing of squares crossed by the rodent (Walsh and Cummins, 1976). The peripheral region is considered a protected area (Hira et al., 2020).
Behavioral assessment of cognitive impairment
EPM test
Elevated plus maze (EPM) comprises two open and two closed/covered arms of dimension 50 × 10 cm each Height of the wall of the closed arm was 40 cm and EPM was elevated at 50 cm from the ground. Closed and open arms were linked through the center square. Each rat was gently placed at the end of one of the open arms. Rat explored the maze for 4 minutes. Transfer latency (TL) (time utilized by a rat to enter one of the covered arms) was recorded. If the rat did not enter any of the closed arms themselves up to 90 seconds, then it was guided to enter into one of the closed/covered arms. The second trial was conducted 24 hours after the first trial and TL was recorded (Itoh et al., 1990; Kulkarni, 2012).
Morris Water Maze (MWM) test
MWM of diameter: 210 cm, height: 51 cm, filled with water up to 35 cm having a temperature of 25 C± 2°C was divided into four quadrants (Q4 was considered as the target quadrant here). Platform (height: 28 cm, width: 10 cm) was kept 2 cm beneath the level of water in Quadrant 4 except in the pre-training trial (Day 1), where the platform was placed at the center of the pool, 1 inch above the water level. Training sessions (Days 2–5) were conducted for 4 consecutive days. Platform position remained the same in Quadrant 4 during the everyday trial. The platform was submerged one inch below the water level. Each rat was made to undergo four successive trials each day. During the trials, each rat was gently placed in the pool facing the wall and the maximum time to swim was allowed up to 60 seconds, the rat was shown a platform for 20 seconds. The rat was immediately removed from the pool when it located the platform before 60 seconds. If the rat was not able to locate the platform after 60 seconds, it was guided to locate the platform and placed for additional 20 seconds on the platform. Each test was performed roughly at the same time to reduce the variability in the performance of rats. After 24 hours of the last day of the training session, a probe test was performed on the 6th day to estimate spatial reference memory. In the probe trial, after the removal of the platform from the pool, the rat was allowed to swim for 240 seconds. Time spent in Quadrant 4 indicated memory of rats (Morris, 1984).
Biochemical estimation
Blood and brain sample collection
After the behavioral parameters, rats were sacrificed; blood was collected and centrifuged at 2,500 × g for 15 minutes.
Whole brain of each rat was carefully isolated. The brains were homogenized in an ice bath after adding 10 volumes of 0.1 M phosphate buffer (pH 7.4). The homogenate was then centrifuged for about 15 minutes at 10,000 × g. The resultant supernatant liquid was used for biochemical assessment.
Aβ estimation
In the Congo-Red (CR) fluorescence method, a fresh solution of CR was passed through a filter (0.2 µm) in cold potassium phosphate buffer (5 mm) containing sodium chloride (NaCl) (150 mM) maintained at a pH of 7.4. This CR solution was then added to 100 µg/ml protein solution, finally resulting in a 5 µM concentration and the samples were mixed for 15 seconds. The absorption spectrum was calculated and noted in the range of 400–700 nm in UV–Vis spectrophotometer (Shimadzu-1800). The spectrum of CR solution with protein was contrasted with alone CR solution. An increase in absorption and red shift of the absorption spectrum toward 540 nm indicated the production of amyloid formation (Meehan et al., 2004).
Insulin estimation
Insulin level in the brain was measured using a Beckman Coulter kit based on the chemiluminescent assay method.
Glucose estimation
The glucose level in the brain was measured using the Glucose estimation kit (ERBA) based on the GOD-POD method. The blood glucose levels were estimated using a glucometer (Accucheck).
Brain malondialdehyde (MDA) assay
MDA level was assessed using the UV-Vis spectrophotometer (Shimadzu-1800). Absorbance was noted at 532 nm (Wills, 1966).
Brain nitrite estimation
Brain nitrite estimation was carried out by the method described by Green et al. (1982). The supernatant was mixed with Griess reagent and the mixture was incubated for 10 minutes at 37°C. The absorbance was noted with UV–Vis spectrophotometer (Shimadzu-1,800) at 546 nm.
Experimental groups
Experimental groups (n = 8):
Group-1. Control group: The rat in this group received saline;
Group-2. T3D rats: STZ (35 mg/kg, i.p.) + (After 3 days, AlCl3 12.5 mg/kg., i.p. daily for 28 days).
Group-3. Insulin-treated T3D rats: STZ (35 mg/kg, i.p.) + Insulin (IU s.c. daily for 28 days) 30 min before the AlCl3 administration)
Group-4. Rivastigmine-treated T3D rats: STZ (35 mg/kg, i.p.) + Rivastigmine (1 mg/kg, i.p. daily for 28 days) 30 min before the AlCl3 administration)
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test using the software GraphPad Prism version 9. Data expressed as mean ± SEM. p ? 0.05 were considered as statistically significant.
RESULTS
Insulin and Rivastigmine effect on locomotion in T3D rats
Time spent in the center square (F3,28 = 1.896, p = 0.1533) (Fig. 1A), peripheral square (F3,28 = 1.220, p = 0.3207) (Fig. 1B), and total crossings (F3,28 = 3.043, p = 0.0452) (Fig. 1C) after Insulin and Rivastigmine administration were determined using one-way ANOVA. Tukey’s post hoc analysis suggested no effect of Rivastigmine on the time spent in the center and periphery square and total crossings in comparison to their respective control. However, the Insulin treatment increased the frequency of crossings significantly as compared to its control (p < 0.05).
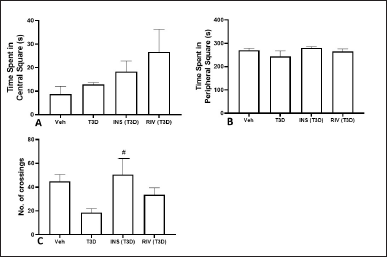 | Figure 1. Effect of various treatments on locomotion in OFT test. Data are expressed as mean ± SEM (n = 8). #p < 0.05 versus type-3 diabetes group. [Click here to view] |
Insulin and Rivastigmine effect on the memory-related alterations in T3D rats in EPM test
The effect of Insulin and Rivastigmine treatment on TL on day 1 (F3,28 = 3.433, p ? 0.0304) (Fig. 2A) and day 2 (F3,28 = 6.448, p = 0.0019) (Fig. 2B) in rats was determined. Tukey’s post hoc analysis suggested significant reduction in TL by the Insulin (p < 0.01) and Rivastigmine (p < 0.05) treatment in comparison to their control.
Insulin and Rivastigmine effect on the memory-related impairment in T3D rats in MWM test
The results suggested that T3D rats spent less time in the target quadrant resulting in impaired cognition. One-way ANOVA indicated the effect of Insulin and Rivastigmine on time in Q4 (F3,28 = 2.869, p = 0.542) (Fig. 3A); frequency of platform crossing (F3,28 = 3.039, p = 0.0454) (Fig. 3B) and frequency of target quadrant crossing (F3,28 = 0.4806, p ? 0.6984) (Fig. 3C). Tukey’s post hoc analysis suggested no effect of Insulin and Rivastigmine on the time spent in Q4, number of center crossings, platform crossings, and crossing across Q4 in comparison to their respective controls. However, Insulin treatment reduced the frequency of platform crossings significantly in comparison to its control (p < 0.05).
Insulin and Rivastigmine effect on blood and brain glucose levels of T3D rats
The results obtained has indicated the effect of Rivastigmine and Insulin administration on glucose level in the blood (F3,28 = 38.12, p < 0.001) (Fig. 4A) and brain (F3,28 = 128, p = 0.001) (Fig. 4B). Tukey’s test has suggested a significant rise in glucose levels in the blood and brain of T3D rats in comparison to the control (p < 0.001). However, the Insulin and Rivastigmine treatment decreased the blood glucose levels significantly in comparison to its control (p < 0.001).
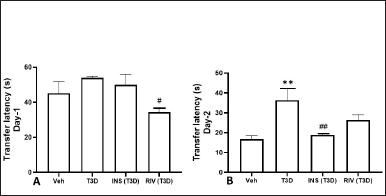 | Figure 2. Effect of insulin and Rivastigmine on the performance of rats in EPM test. Data are expressed as Mean ± SEM (n = 8). #p < 0.05, ##p < 0.01 versus type-3 diabetes group, **p < 0.01 versus vehicle treated group. [Click here to view] |
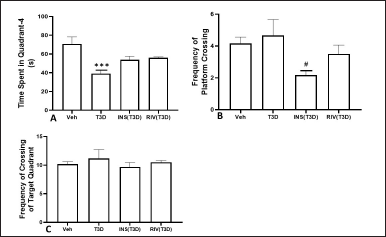 | Figure 3. Effect of insulin and Rivastigmine on the performance of rats in MWM test. Data are expressed as mean ± SEM (n = 8). #p < 0.05 versus type-3 diabetes group, ***p < 0.001 versus vehicle treated group. [Click here to view] |
Insulin and Rivastigmine effect on brain Insulin level of T3D rats
Administration of Rivastigmine has revealed reduced Insulin levels in the brain (F3,28 = 7.858, p = 0.0006) (Fig. 5). Tukey’s test suggested a significant rise in Insulin levels in the brain of T3D rats as compared to its control (p < 0.01). However, Rivastigmine treatment decreased the brain Insulin levels significantly in comparison to its control (p < 0.01).
Insulin and Rivastigmine effect on the brain Aβ level in T3D rats
Insulin and Rivastigmine administration to T3D rats reduced the brain Aβ levels (F3,28 = 431.3, p < 0.001) (Fig. 6). Tukey’s test has suggested a significant rise in Aβ level in the brain in comparison to the control (p < 0.001). However, Rivastigmine and Insulin treatment reduced the brain Aβ levels significantly in comparison to its control (p < 0.001).
Insulin and Rivastigmine effect on the nitrite and MDA level in the brain of T3D rats
One-way ANOVA analysis of the data has indicated the effect of Rivastigmine and Insulin treatment on brain nitrite levels (F3,28 = 0.1880, p = 0.9037) (Fig. 7) and MDA level in the brain (F3,28 = 3.895, p ≤ 0.0192) (Fig. 8). Tukey’s test suggested non-significant effect on MDA and nitrite levels in the brain in comparison to their control.
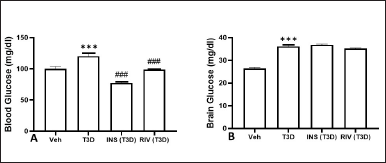 | Figure 4. Effect of insulin and Rivastigmine on the glucose level of rats. Data are expressed as mean ± SEM (n = 8). ###p < 0.001 versus type III diabetes group,***p < 0.001 versus vehicle treated group. [Click here to view] |
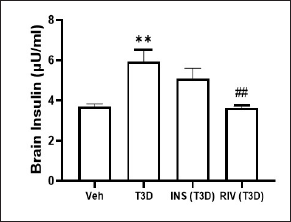 | Figure 5. Effect of insulin and Rivastigmine on the insulin level of rats. Data are expressed as mean ± SEM (n = 8). ##p < 0.01 versus type III diabetes group, **p < 0.01 versus vehicle treated group. [Click here to view] |
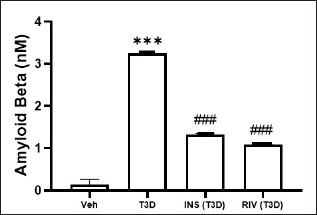 | Figure 6. Effect of insulin and Rivastigmine on the Aβ level of rats. Data are expressed as mean ± SEM (n = 8). ###p < 0.001 versus type III diabetes group, ***p < 0.001 versus vehicle treated group. [Click here to view] |
DISCUSSION
In this study, the combination of STZ (35 mg/kg, i.p., single-dose administration) and AlCl3 [12.5 mg/kg daily (i.p.) × 28 days] was used to induce T3D in experimental rats. It was observed that AlCl3 and STZ administration in rats has caused behavioral changes in EPM and MWM tests. Further, AlCl3 and a single dose of STZ administration in rats have raised the brain glucose, Insulin, and Aβ levels significantly in comparison to the control. Thus, the present experimental study revealed that intraperitoneal administration of STZ (35 mg/kg, once) and AlCl3 (12.5 mg/kg × 28 days) in rats is responsible for the development of hyperglycemia, hyperinsulinemia, and an increase in Aβ level.
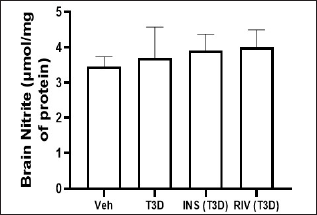 | Figure 7. Effect of insulin and Rivastigmine on brain nitrite level of rats. Data are expressed as mean ± SEM (n = 8). [Click here to view] |
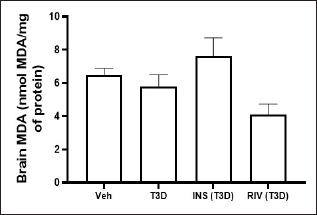 | Figure 8. Effect of insulin and Rivastigmine on brain MDA level of rats. Data are expressed as mean ± SEM (n = 8). [Click here to view] |
The effect of Insulin and Rivastigmine treatment on the various behavioral and neurochemical parameters of T3D rats was estimated which revealed that Rivastigmine has no significant effect on time spent at the center and periphery and the number of square crossings of T3D rats in the OFT. In contrast to this study, Rivastigmine has shown improvement in locomotor and exploratory behavior of aluminum-treated rats in the OFT but did not significantly alter latency to initiate movement suggesting no effect on anxiety in these rats (Abdel-Aal et al., 2011). This effect may be due to the peripheral cholinesterase inhibitory action of Rivastigmine (Wilkinson et al., 2004). However, Insulin administration has increased the number of square crossings indicating exploratory behavior of animals.
In EPM test, Rivastigmine did not affect the TL of day 2 of T3D rats while Insulin decreased the TL of day 2 of T3D rats suggesting the better activity of Insulin. In another similar study, Insulin administration has shown a significant reduction in TL in EPM in Aluminum treated rats (Alam and Bansal, 2020). Peripheral Insulin administration has been shown to improve memory functions in animal models of diabetes (Zhao et al., 2004). In contrast to our study, cognitive decline induced in aluminum-treated rats was reduced by Rivastigmine administration, as it improved the cognitive deficits observed in various behavioral tests. However, this effect was dose-dependent. A low dose of Rivastigmine (0.5 mg/kg) did not exert any significant effect on the rats’ performance in behavioral tests whereas a high dose of Rivastigmine (2.5 mg/kg) produced its maximum effect (Abdel-Aal et al., 2011). This dose-dependent effect was mediated based on the extent of cholinesterase inhibition. Bejar et al. (1999), suggested that Rivastigmine at a dose of 0.5, 0.75, and 2.5 mg/kg produced 21%, 40%, and 62% reduction in cholinesterase activity, respectively (Táyebati et al., 2004). Rivastigmine acts as a dual reversible inhibitor of both AChE and BuChE (Muller, 2007; Táyebati et al., 2004) and has been shown to lower the glycated hemoglobin in diabetic patients (Wills, 2009). It has been reported that Rivastigmine (5 mg/kg) improved the memory and learning deficits caused by AlCl3 (Thippeswamy et al., 2013).
T3D rats have shown a reduction in the time spent in the target quadrant in the MWM test indicating memory impairment. Insulin and Rivastigmine had no effect on the time spent and frequency of target quadrant (Q4) crossing by T3D rats. However, Insulin decreased the frequency of platform crossing by T3D rats. In a previous study, Insulin administration has also shown a significant reduction in escape latency in MWM in Aluminum treated rats (Alam and Bansal, 2020). Further, 6 weeks of intra-nasal Insulin treatment has shown improvement in the spatial memory impairment in MWM in the rats which were administered STZ via intracerebroventricular (icv) route. Thus, confirming the positive effect of Insulin on cognition (Guo et al., 2017).
Administration of Rivastigmine reduced the brain Insulin level of T3D rats suggesting that Rivastigmine may be responsible to regulate brain Insulin signaling. However, no effect of Insulin administration was observed on brain Insulin levels. These findings are in support of previously conducted studies, where there was no difference was observed in the brain Insulin levels between the intraperitoneally administered Insulin (IU/ml) and STZ (3 mg/kg, icv) groups. However, a dose-dependent increase in peripheral Insulin levels was seen in the intraperitoneally administered Insulin group (Lv et al., 2020). In this study, no significant effect was observed in oxidative stress markers such as nitrite and MDA levels whereas previous studies have shown that Rivastigmine (2.5 mg/kg, p.o.) reduced the elevated nitrite level significantly (Khan et al., 2013), diminished the MDA level (Chen et al., 2021; John et al., 2015). The observed beneficial effect may be dose-dependent.
Insulin stimulation promotes the extracellular excretion of Aβ and reduces its intracellular aggregation (Gasparini et al., 2001) whereas altered Insulin signaling affects amyloid precursor protein processing, Aβ clearance, leading to raised neurotoxic effects of Aβ (Delikkaya et al., 2019). Aβ exerts an effect on Insulin signaling, either by reducing its affinity or by competing with and inhibiting the binding of Insulin to the Insulin receptor (Xie et al., 2022). In this study, Insulin and Rivastigmine have reduced the level of amyloid deposits in the brain. These findings are in support of previously conducted studies, where Rivastigmine (0.5 mg/kg) has significantly reduced the plasma Aβ levels of AlCl3-treated rats (Dulla and Bindhu, 2022). Thus, the reduction of amyloid burden improves learning and memory and may halt the process of neurodegeneration. Rivastigmine reduces Aβ brain load by promoting its clearance across the BBB by upregulating Lipoprotein receptor-related protein 1 and P-glycoprotein (Mohamed et al., 2016). Rivastigmine may also alter α-secretase levels and shift amyloid precursor protein processing toward non-amyloidogenic pathway and may modify the disease progression pathway (Ray et al., 2020).
Findings from this study indicate that Rivastigmine might have exerted a beneficial effect by compensating the cholinergic deficits either by increasing the choline acetyltransferase levels or by inhibiting the breakdown of acetylcholine (Táyebati et al., 2004). Rivastigmine has also reduced brain Insulin levels, so, it might have exerted an effect on the Insulin signaling pathway and reduced hyperinsulinemia. Also, Insulin might have exerted a beneficial effect by activating Insulin signaling pathways involving NMDA receptors, PI3K and Akt/PKB activity, and GSK3β inactivation (Neumann et al., 2008).
CONCLUSION
This study has demonstrated that both Insulin and Rivastigmine treatments have reduced brain amyloid levels while Rivastigmine treatment alone has reduced the brain Insulin levels. Further, Insulin treatment in T3D rats has improved memory in the EPM test. In conclusion, the obtained results hold the potential to offer a suitable combination of Insulin and Rivastigmine treatment to control both behavioral and biochemical disturbances in the management of T3D.
AUTHOR CONTRIBUTIONS
Ms. Abhilasha Ahlawat: Collection of data and write the manuscript.
Dr. Vaibhav Walia: Statistical analysis.
Prof. Munish Garg: Study Design and Proof Reading.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental study design was approved by Institutional Animal Ethics Committee of Maharshi Dayanand University (Approval no.: 153-165-17/12/2018).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbott MA, Wells DG, Fallon JR. The Insulin receptor tyrosine kinase substrate p58/53 and the Insulin receptor are components of CNS synapses. J Neurosci, 1999; 19:7300–8. CrossRef
Abdel-Aal RA, Assi AAA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioural changes in rats. Eur J Pharmacol, 2011; 659(2–3):169–76. CrossRef
Adzovic L, Lynn AE, D’Angelo HM, Crockett AM, Kaercher RM, Royer SE, Hopp SC, Wenk GL. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation, 2015; 12:63. CrossRef
Alam MA, Bansal G. Effects of Insulin against aluminium induced Neurotoxicity in Wistar rats. Pharmacol Clin Pharm Res, 2020; 5(2):62–71. CrossRef
Andrade EF, Silva VO, Moura NO, Foureaux RC, Orlando DR, Moura RF, Pereira LJ. Physical exercise improves glycemic and inflammatory profile and attenuates progression of periodontitis in diabetic rats (HFD/STZ). Nutrients, 2018; 10(11):1702. CrossRef
Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, Firoz CK, Naeem A, Alhazza IM, Damanhouri GA, Kamal MA. Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets, 2014; 13(7):1280–93. CrossRef
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol, 2011; 68(1):51–7. CrossRef
Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci, 2000; 3:1291–300. CrossRef
Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, Cedazo-Minguez A, Sita G, Morroni F, Corsi M, Carboni L. Cross-disease analysis of Alzheimer’s disease and type-2 diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep, 2019; 9:3965. CrossRef
Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci, 2021; 28(8):4232–9. CrossRef
Cooper GJ, Day AJ, Willis AC, Roberts AN, Reid KB, Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta Mol Cell Res BBA Mol Cell Res, 1989; 1014(3):247–58. CrossRef
Das UN. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit, 2007; 13:RA214–21.
Delikkaya B, Moriel N, Tong M, Gallucci G, de la Monte SM. Altered expression of Insulin-degrading enzyme and regulator of calcineurin in the rat intracerebral streptozotocin model and human apolipoprotein E-ε4–associated Alzheimer’s disease, Alzheimer’s and dementia: diagnosis. Assess Dis Monit, 2019; 11:392–404. CrossRef
Den HeijerT, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MMB. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia, 2003; 46:1604–10. CrossRef
Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem, 2005; 12:646–55. CrossRef
Dulla BS, Bindhu S. A Study on the effect of valeric acid in Alzheimer’s induced rats by the estimation of Aβ 1-42 biomarker. J Health Allied Sci NU, 2022; 12(02):134–8. CrossRef
Ferreira LSS, Fernandes CS, Vieira MNN, de Felice FG. Insulin resistance in Alzheimer’s disease. Front Neurosci, 2018; 12:830. CrossRef
Garwood CJ, Ratcliffe LE, Morgan SV, Simpson JE, Owens H, Vazquez-Villaseñor I, Heath PR, Romero IA, Ince PG, Wharton SB. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain, 2015; 8:51. CrossRef
Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci, 2001; 21:2561–70. CrossRef
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem, 1982; 126(1):131–8. CrossRef
Guo Z, Chen Y, Mao YF, Zheng T, Jiang Y, Yan Y, Yin X, Zhang B. Long-term treatment with intranasal Insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci Rep, 2017; 7:45971. CrossRef
Hak AE, Pols HA, Stehouwer CD, Meijer J, Kiliaan AJ, Hofman A, Breteler MMB, Witteman JCM. Markers of inflammation and cellular adhesion molecules in relation to Insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab, 2001; 86:4398–405. CrossRef
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science, 1992; 256(5054):184–6. CrossRef
Hira S, Saleem U, Anwar F, Raza Z, Rehman AU, Ahmad B. In silico study and pharmacological evaluation of eplerinone as an anti-Alzheimer’s drug in STZ-induced Alzheimer’s disease model. ACS Omega, 2020; 5(23):13973–83. CrossRef
Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A. Diet-induced Insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J, 2004; 18:902–4. CrossRef
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of Insulin resistance. Nature, 2006; 440:944–8. CrossRef
Hoyer S. Glucose metabolism and Insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol, 2004; 490:115–25. CrossRef
Huang CC, You JL, Lee CC, Hsu KS. Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol Cell Neurosci, 2003; 24:831–41. CrossRef
Isik AT, Bozoglu E, Eker D. aChE and BuChE inhibition by rivastigmin have no effect on peripheral Insulin resistance in elderly patients with Alzheimer disease. J Nutr Health Aging, 2012; 16(2):139–41. CrossRef
Ismail MF, Elmeshad AN, Salem NA. Potential therapeutic effect of nanobased formulation of Rivastigmine on rat model of Alzheimer’s disease. Int J Nanomed, 2013; 8:393–406. CrossRef
Jamshidnejad-Tosaramandani T, Kashanian S, Babaei M, Al-Sabri MH, Schiöth HB. The potential effect of Insulin on AChE and its interactions with Rivastigmine in vitro. Pharmaceuticals (Basel). 2021; 14(11):1136. CrossRef
John J, Nampoothiri M, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR. Sesamol, a lipid lowering agent, ameliorates aluminium chloride induced behavioural and biochemical alterations in rats. Pharmacogn Mag, 2015; 11(42):327–36. CrossRef
Khan KA, Kumar N, Nayak PG, Nampoothiri M, Shenoy RR, Krishnadas N, Rao CM, Mudgal J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J Pharm Pharmacol, 2013; 65(12):1745–52. CrossRef
Kroner Z. The Relationship between Alzheimer’s disease and diabetes: Type 3 diabetes. Altern Med Rev, 2009; 14(4):373–9.
Kulkarni SK. Hand book of experimental pharmacology. 4th edition, Vallabh Prakashan, Delhi, India, pp 146–8, 2012.
Kumar AY, Nandakumar K, Handral M, Talwar S, Dhayabaran D. Hypoglycaemic and anti-diabetic activity of stem bark extracts Erythrina indica in normal and alloxan-induced diabetic rats. Saudi Pharm J, 2011; 19(1):35–42. CrossRef
Lim YA, Rhein V, Baysang G, Meier F, Poljak A, Raftery JM, Götz J. Aβ and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics, 2010; 10(8):1621–33. CrossRef
Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci, 2000; 3:1282–90. CrossRef
Liu KF, Niu CS, Tsai JC, Yang CL, Peng WH, Niu HS. Comparison of area under the curve in various models of diabetic rats receiving chronic medication. Arch Med Sci, 2020; 18(4):1078–87.
Lv H, Tang L, Guo C, Jiang Y, Gao C, Wang Y, Jian C. Intranasal Insulin administration may be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain Insulin resistance. Cogn Neurodyn, 2020; 14(3):323–38. CrossRef
Ma XH, Zhong P, Gu Z, Feng J, Yan Z. Muscarinic potentiation of GABA(A) receptor currents is gated by Insulin signaling in the prefrontal cortex. J Neurosci, 2003; 23:1159–68. CrossRef
McGeer PL, McGeerEG. Inflammation and the degenerative diseases of aging. Ann NY Acad Sci, 2004; 1035:104–16. CrossRef
Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CM. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem, 2004; 279:3413–9. CrossRef
Mielke JG, Taghibiglou C, Wang YT. Endogenous Insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience, 2006; 143:165–73. CrossRef
Mittal K, Mani RJ, Katare DP. Type 3 diabetes: cross talk between differentially regulated proteins of type 2 diabetes mellitus and Alzheimer’s disease. Scientific Rep, 2016; 6(1):1–8. CrossRef
Mohamed LA, Keller JN, Kaddoumi A. Role of P-glycoprotein in mediating Rivastigmine effect on amyloid-β brain load and related pathology in Alzheimer’s disease mouse model. Biochimica et biophysica Acta, 2016; 1862(4):778–87. CrossRef
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, Insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and Insulin signalling. Neurobiol Aging, 2010; 31(2):224–43. CrossRef
Morris R. Development of water maze procedure for studying spatial learning in the rat. J Neurosci Meth, 1984; 11:47–60. CrossRef
Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct Insulin receptors generated by tissue-specific alternative splicing. EMBO J, 1990; 9:2409–13. CrossRef
Muller T. Rivastigmine in the treatment of patients with Alzheimer’s disease. Neuropsychiatr Dis Treat, 2007; 3:211–8. CrossRef
Neumann KF, Rojo L, Navarrete LP, Farías G, ReyesP, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res, 2008; 5(5):438–47. CrossRef
Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Giau VV. Type 3 diabetes and its role implications in Alzheimer’s Disease. Int J Mol Sci, 2020; 21(9):3165. CrossRef
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between Insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol, 2018; 17:122. CrossRef
Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab, 2006; 32(5 Pt 1):403–14. CrossRef
Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci, 2001; 4:917–26. CrossRef
Qinna NA, Badwan AA. Impact of streptozotocin on altering normal glucose homeostasis during Insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther, 2015; 9:2515–25. CrossRef
RajaSankar S, Manivasagam T, Surendran S. Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci Lett, 2009; 454(1):11–5. CrossRef
Randell EW, Mathews MS, Zhang H, Seraj JS, Sun G. Relationship between serum butyrylcholinesterase and the metabolic syndrome. Clin Biochem, 2005 ;38(9):799–805. CrossRef
Ray B, Maloney B, Sambamurti K, Karnati HK, Nelson PT, Greig NH, Lahiri DK. Rivastigmine modifies the α-secretase pathway and potentially early Alzheimer’s disease. Transl Psychiatry, 2020; 10(1):47. CrossRef
Reyes AE, Chacón MA, Dinamarca MC, Cerpa W, Morgan C, Inestrosa NC. Acetylcholinesterase-abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol, 2004; 164:2163–74. CrossRef
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and Insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis, 2005; 8(3):247–68. CrossRef
Rorbach-Dolata A, Piwowar A. Neurometabolic evidence supporting the hypothesis of increased incidence of type 3 diabetes mellitus in the 21st century. BioMed Res Int, 2019; 1435276. CrossRef
Sato T, Shimogaito N, Wu X, Kikuchi S, Yamagishi S, Takeuchi M. Toxic advanced glycation end products (TAGE) theory in Alzheimer’s disease. Am J Alzheimers Dis Other Demen, 2006; 21(3):197–208. CrossRef
Sharma P, Senthilkumar RD, Brahmachari V, Sundaramoorthy E, Mahajan A, Sharma A, Sengupta S. Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis, 2006; 5:1. CrossRef
Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA, 2001; 98:3561–66. CrossRef
Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci, 2019; 116(13):6379–84. CrossRef
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SMJR. Impaired Insulin and Insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis, 2005; 7:63–80. CrossRef
Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of Alzheimer’s disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacol, 2006; 51(3):474–86. CrossRef
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Arnold SE. Demonstrated brain Insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest, 2012; 122(4):1316–38. CrossRef
Táyebati SK, Di Tullio MA, Amenta F. Effect of treatment with the cholinesterase inhibitor Rivastigmine on vesicular acetylcholine transporter and choline acetyltransferase in rat brain. Clin Exp Hypertens, 2004; 26:363–73. CrossRef
Temitayo GI, Olawande B, Emmanuel YO, Timothy AT, Kehinde O, Susan LF, Ezra L, Joseph OO. Inhibitory potentials of Cymbopogon citratus oil against aluminium-induced behavioural deficits and neuropathology in rats. Anat Cell Biol, 2020; 53(3):342–54. CrossRef
Thippeswamy AH, Rafiq M, Viswantha GL, Kavya KJ, Anturlikar SD, Patki PS. Evaluation of bacopa monniera for its synergistic activity with Rivastigmine in reversing aluminum-induced memory loss and learning deficit in rats. Acupunct Meridian Stud, 2013; 6(4):208–13. CrossRef
Valenciano AI, Corrochano S, de Pablo F, de la Villa P, de la Rosa EJ. Proinsulin/insulin is synthesized locally and prevents caspase- and cathepsin-mediated cell death in the embryonic mouse retina. J Neurochem, 2006; 99:524–36. CrossRef
Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature, 1997; 388:686–90. CrossRef
Weinstein G, Davis-Plourde KL, Conner S, Himali JJ, Beiser AS, Lee A, Rawlings AM, Sedaghat S, Ding J, Moshier E, van Duijn CM, Beeri MS, Selvin E, Ikram MA, Launer LJ, Haan MN, Seshadri S. Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer’s disease: pooled analysis from 5 cohorts. PLoS One, 2019; 14:e0212293. CrossRef
Wickelgren I. Tracking insulin to the mind. Science, 1998; 280:517–9. CrossRef
Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging, 2004; 21:453–78. CrossRef
Wills E. Mechanisms of lipid peroxide formation in animal tissues. Biochem J, 1966; 99(3):667. CrossRef
Wills S, inventor. Glycemic control, diabetes treatment, and other treatments with acetyl cholinesterase inhibitors. US; 2009/0081314 A1, 2009.
Xie L, Helmerhorst E, Taddei K, Plewright B, van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci, 2002; 22:RC221. CrossRef
Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem, 1999; 274:34893–902. CrossRef
Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol, 2001; 177:125–34. CrossRef
Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol, 2004; 490(1–3):71–81. CrossRef