INTRODUCTION
Chronic liver disease (CLD) is a clinical syndrome characterized by a rapid decline in liver function progressing over 6 months. It is associated with abnormality in the production of coagulation factors, different proteins, and the inability to detoxify unsafe products. CLD is a gradual process of destruction, inflammation, and regeneration of liver parenchyma finally leads to fibrosis and cirrhosis (Sharma and Nagalli, 2021). About 1.5 billion cases of CLD with different stages were estimated worldwide. It is the 11th driving reason for death and the 15th driving reason for morbidity representing 2.2% of death and 1.5% of disability-adjusted life years in 2016 worldwide (Cheemerla and Balakrishna, 2021). In 2017, CLD caused the deaths of 1.32 million people of which 2/3 were men and 1/3 were women. According to World Health Organization (WHO), around 10 lakh patients with liver disease are newly diagnosed every year. Liver disease may affect every 1 in 5 Indians (Sepanlou et al., 2020).
For several chronic diseases, including liver disease, it is very difficult to develop a suitable tool to identify and predict the outcome of the disease. In CLD, the main tools used to predict the outcome of the disease are the Child-Pugh Score (CPS) and the Model for End-Stage Liver Disease (MELD) Score (Angermayr et al., 2003). The CPS is a system for assessing the prognosis of liver disease using three continuous variables (albumin, prothrombin time, and bilirubin) and two discrete variables (Ascites and hepatic encephalopathy). The patients are categorized into three groups A, B, and C based on the CPS obtained by calculating the score of the 5 variables and their cut-off scores. The values range from 5 to 15 in which scores 5–7 belong to class A, 8–9 belong to Class B, and 10–15 belong to class C indicating good hepatic function, moderate hepatic function, and severely impaired hepatic function respectively (Shetty et al., 1997). MELD is a scoring system that acts as a predictor of survival in patients with CLD and how much the patient needs liver transplantation (Kamath et al., 2001). The parameters used to check MELD scores are creatinine, bilirubin, international normalized ratio (INR), sodium, and dialysis status and the values are ranges from 6 to 40. MELD score >40 is indicative of 71.3% mortality and a score <6 is indicative of 1.9% mortality (Durand and Valla, 2005).
Quality of life (QOL) is defined as the perception of a person’s physical and mental health, along with areas covering their psychological, economic, spiritual, and social well-being (Salehitali et al., 2019). In this study, the World Health Organization quality of life-BREF (WHOQOL-BREF) instrument is used to evaluate the influence of CLD on the QOL as well as to have a comprehensive understanding of the disease outcome in various domains of the patient’s health. The WHOQOL-BREF is a pre-designed and pre-tested 26-item questionnaire, which consists of four major domains, namely, physical health (Phy) (7 items), psychological health (Psy) (6 items), social relationships (SR) (3 items), and environment (E) (8 items). Apart from these domains, two items are assessed separately: question 1 provides the overall perception of an individual’s QOL and question 2 provides the overall perception of an individual’s health (Skevington and Tucker, 1999).
Patient with CLD experiences the ill effects of exhaustion, loss of self-regard, failure to work, tension, other emotional issues, and depression that profoundly diminishes their prosperity and QOL. CLD patients’ QOL can be affected by factors such as age, gender, alcohol consumption, co-morbid conditions, and psychological and environmental factors (Sobhonslidsuk et al., 2006).
The QOL evaluation gives significant data concerning the deficit of particular areas, which require more noteworthy consideration by the workers of the medical care system. CLD is a serious and long-term condition that will affect the QOL of the patients (Cortesi et al., 2020). There is a serious requirement for evaluating the QOL and its domains among such patients for analyzing the factors associated with it.
MATERIALS AND METHOD
Study design
This is a cross-sectional study conducted at the General medicine department for a period of 6 months (October 2021–March 2022) after obtaining permission from the Institutional Ethics Committee (Ref No: NGSMIPS/IEC/19/2021). The study is also registered in Clinical Trial Registry (CTRI No: CTRI/2021/11/038264) under the Government of India.
Study population
The study included patients of either gender, aged above 18 years, undergoing treatment for CLD, diagnosed by history, clinical examination, blood investigation, and ultrasound scanning reports. Patients admitted to intensive care unit, with acute infection or in critical condition, malignancy, and psychiatric problems were excluded from the study. The patients were enrolled after obtaining prior consent.
Sample size
The sample size was calculated based on the literature evidence and the prevalence of CLD cases in the hospital. At a 5% level of significance and margin of error = 2, the required sample size (n) is 165. The sample size was calculated using the software nMaster version 2.0.
Study procedure
The objective of the study was to assess the QOL and associated factors among CLD patients. All the permissions were obtained before the initiation of the study. General wards were visited daily, and information was collected through patient interviews, case sheets, laboratory reports, and other relevant sources using suitable data collection forms.
The WHOQOL-BREF (26 items questionnaire) was used for assessing the QOL in CLD patients. Prior permission was obtained from WHO to use the WHOQOL-BREF questionnaire and its Kannada and Malayalam versions in the study. The questionnaire was given to the patients during their out patients visits and in patients admissions (For illiterate patients, the questionnaire was administered with the help of their caretakers or a person recruited by the investigator) and their answers to each question were marked from 1 to 5. The patients were given 15 to 30 minutes to complete the questionnaire. The 26 questions were divided into 4 domains: Physical, Psychological, Environmental, and SR. The score for each question from each domain was used to calculate the raw score using WHO instructions and it was converted into the transformed score (0–100). Using this, the score of the QOL of patients was calculated (Sharma et al., 2014). From the laboratory values, the CPS and MELD scores were calculated. The CPS uses three continuous variables (albumin, prothrombin time, and bilirubin) and two discrete variables (Ascites and hepatic encephalopathy). According to the CPS, the patients are categorized into three groups A, B, and C based on the values ranging from 5 to 15 in which scores of 5–7 belong to class A, 8–9 belong to Class B, and 10–15 belongs to class C indicating good hepatic function, moderate hepatic function, and severely impaired hepatic function, respectively. The MELD scores were determined using the parameters including, creatinine, bilirubin, INR, sodium, and dialysis status and the values are ranges from 6 to 40. MELD score >40 is indicative of 71.3% mortality and a score < 6 is indicative of 1.9% mortality (Durand and Valla, 2005).
Statistical analysis
The data were collected and electronically documented using Microsoft Excel (2019). Unpaired t-test, one-way ANOVA, and multiple linear regression are used to analyze the results. Qualitative variables (frequency, percentage) and quantitative variables (Mean ± SD) were used; tables and figures were used as appropriate.
RESULTS
Based on the inclusion and exclusion criteria, a total of 165 patients were enrolled in this study.
Socio-demographic characteristics and personal behavior of the patients
The patients diagnosed with CLD had a mean age of 53.06 ± 11.72. The disease proportion was high in males than in females. The majority of the study population had a normal body weight. Most of the study population were alcoholics (Table 1).
The proportion of CLD and decompensated CLD (DCLD) were found to be almost equal (Fig. 1).
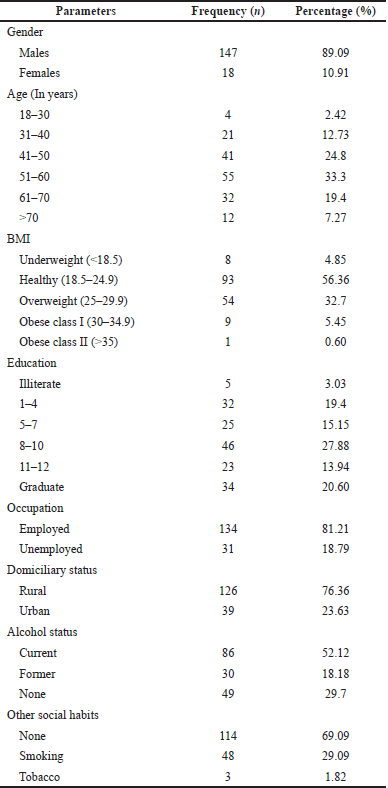 | Table 1. Socio-demographic characteristics and personal behavioural of the patients. [Click here to view] |
Comorbid conditions
The majority of the study population was presented without any comorbid conditions (Fig. 2).
The severity of the disease
The severity of the disease was analyzed by Child-Pugh and MELD score. The severity rate of the disease was found to be moderate in the majority of the study population (class B) followed by severely impaired (class C). The MELD score of the study population was observed to be in the range of 10–19 (6% mortality) followed by 20–29 (19.6% mortality) (Table 2).
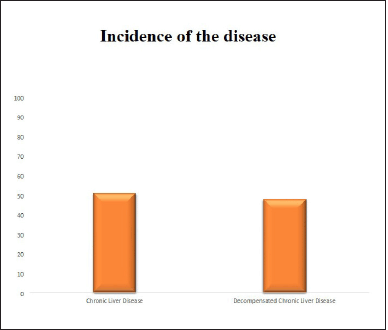 | Figure 1. Incidence of the disease in study population. [Click here to view] |
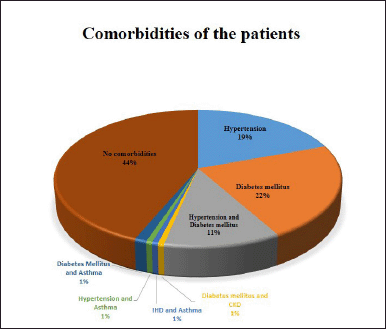 | Figure 2. Different comorbidities in the study population. [Click here to view] |
Quality of life
The overall QOL was 50.96 ± 15.77 SD. It was observed that the QOL was more in the SR domain when compared with the environment, psychological, and physical health domains (Table 3).
In the domains of physical health (p-value = 0.00) and SR (p-value = 0.030), a significant mean difference was observed across the age groups. While considering the education status, there was a significant difference noticed in all four domains (p-value: Phy = 0.000, Psy = 0.000, SR = 0.002, E = 0.000). A statistical significance was observed between urban and rural residents in each domain (p-value: Phy = 0.000, Psy = 0.009, SR = 0.000, E = 0.000). The mean difference between the other social behaviors was significant in the domains of psychological health (p-value = 0.014), social interactions (p-value = 0.003), and environment (p-value = 0.048) (Table 4).
The perception of QOL and health was statistically significant in physical (p-value = 0.000) and psychological health (p-value = 0.000), SR (p-value = 0.000), and environment domains (p-value = 0.000) (Table 5).
Among the various comorbid conditions, a significant difference was obtained exclusively in the physical domain (p-value = 0.009). There was a significant difference between Body mass index (BMI) categories in physical (p-value = 0.045) and psychological health domains (p-value = 0.032) and SR (p-value = 0.045). In terms of the drugs prescribed (p-value: Phy = 0.003, Psy = 0.025, E = 0−0.25) and types of disease (p-value: Phy = 0.003, Psy = 0.016, E = 0.012), there was a significant association between the groups in all domains except SR. A statistically significant relation was observed in all four domains between the classes of Child-Pugh (p-value: Phy = 0.000, Psy = 0.000. SR = 0.000, E = 0.000) and MELD score (p-value: Phy = 0.000, Psy = 0.000, SR = 0.000, E = 0.001) (Table 6).
In physical and psychological health, SR, and environment domains, the highest mean score was for Child-Pugh class A and MELD 6–9 score (Table 7).
BMI (normal weight category) was the only significant explanatory variable in the case of the physical domain (p-value = 0.00181). BMI [normal weight category (p-value = 0.000801)], graduate level of education (p-value = 0.010436), abstinence from alcohol (p-value = 0.022886), and smoking habit (p-value = 0.024504) were the factors associated with the psychological domain. BMI (except class II obese) and tobacco consumption (p-value = 0.002063) were significant in the social relationship domain. There was an association of smoking habits with the environment domain (p-value = 0.02519) (Table 8).
DISCUSSION
In CLD, the health-related QOL can be considered an important parameter to analyze the disease progression and the therapeutic efficacy. In this study, we observed that the disease was dominant in males (89.1%) compared to females (10.9%), with a mean age of 53.06 ± 11.72 SD. This can be due to alcohol consumption among male patients. The result can be compared with the study done by Adhikari (2018) where the prevalence of male patients (61.20%) was predominant over females (38.80%) and Bhattarai et al. (2017), where the prevalence of male patients (70.20%) was predominant over females (29.80%). In this study, 73% of the study population have a history of alcohol use. A study conducted by Kim et al. (2018), also presented a similar result in which 51.4% are currently alcoholics. In the study, we noticed that the majority of the study population is currently alcoholics. The factors associated with their alcohol consumption were workplace stress and tension.
The disease was high in patients with a healthy (BMI; 56.36%) followed by overweight (32.7%). It is evident from the study conducted by Chang et al. (2016), that overweight and obesity were considerably related to the development of liver disease in metabolically healthy men and women.
Among the study population, the majority of the patients belong to class B of the Child-Pugh classification (moderate impairment in hepatic function). The factors contributing to these results were that most of the patients identify their disease condition only when they reach severe stages of the disease due to the lack of disease-related symptoms. These results can be compared to the study conducted by Stine et al. (2020), where 49.5% of the patients belong to the child-Pugh B category with an 80% survival rate for the first year and 60% for the second year and the study conducted by Ray et al. (2010).
On the assessment of the MELD score, it was observed that 41.21% of patients were in the MELD score category 10–19 with a 6.0% of mortality. Similar results were obtained in a study led by Stine et al. (2020), where 45% of patients with CLD were in the class of MELD scores 10–19. Patients with MELD scores above 25 generally have disorientation due to hepatic encephalopathy, but the majority of our study population was properly oriented to time, place, and person.
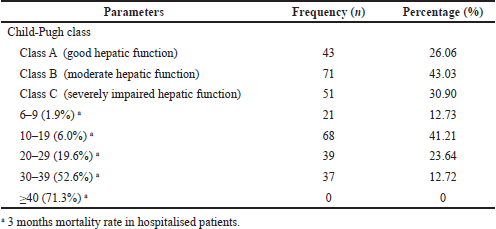 | Table 2. Child-pugh and MELD score of the patients. [Click here to view] |
 | Table 3. Health related QOL of the patients on different domain. [Click here to view] |
While assessing the QOL, the domain scores ranged between 44.08 (physical health) and 58.33 (social relation) out of 100. A similar result was obtained in a study conducted by Adhikari (2018), where the physical health score and environmental scores were 43.78 and 54.67, respectively.
In our study, the mean scores of the domains of physical health, psychological health, SR, and environmental health were 44.08 ± 18.64 SD, 46.70 ± 19.81 SD, 58.33 ± 19.34 SD, and 54.74 ± 16.74 SD, respectively. On the contrary, a study conducted by Pradhan et al. (2020), obtained different mean scores for the domains of physical health (34.4 ± 26.7 SD), psychological health (7.5 ± 17.8 SD), SR (55.2 ± 23.5SD), and environmental health (38.2 ± 17.0 SD). The patient-related and disease-specific characteristics were diverse in both studies which could be the reason for the variation.
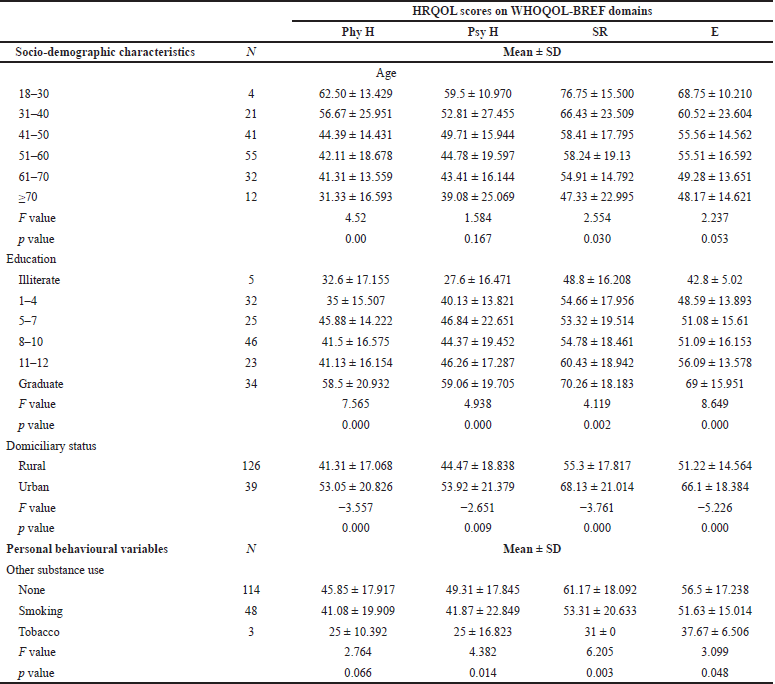 | Table 4. Mean scores of socio-demographic characteristics and personal behaviour. [Click here to view] |
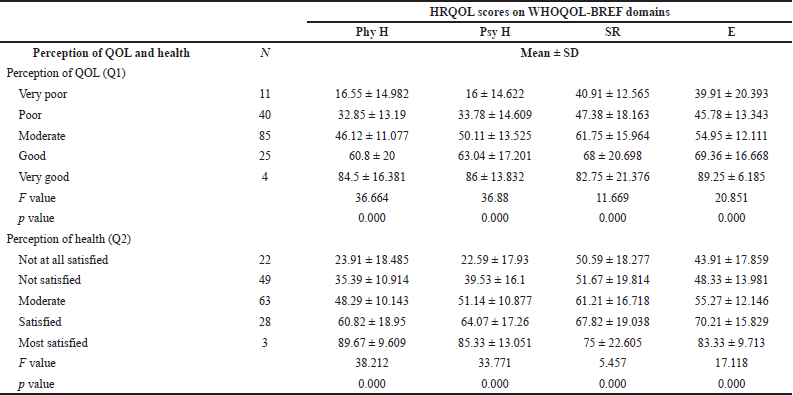 | Table 5. Mean scores of perceptions of QOL and health. [Click here to view] |
Age was significantly associated with the domains of physical health (p-value = 0.000) and social relationship (0.030), similar findings were observed in the study led by Adhikari (2018) and Marchesini et al. (2001) also.
The educational status was having a significant difference in all four domains (p-value = 0.000 in Physical health and Psychological health, 0.002 in SR, and 0.000 in Environmental health). A significant association was found for domiciliary status in all domains also (p-value = 0.000 for Physical health, Social relationship, and Environmental, 0.009 for Psychological health). There was a significant difference between substance use in psychological (p = 0.014), SR (p = 0.003), and environmental (p = 0.048). These findings were similar to that of the study directed by Salama et al. (2016).
The finding of the study depicted a significant association between comorbidities in the physical domain (p-value = 0.009) which is similar to the study by Hussain et al. (2001) and contradicts Afendy et al. (2009).
In physical (p = 0.045), psychological (p = 0.032), and social relationship (0.045) domains the BMI was found to have a significant association. The number of drugs prescribed had a significant association with physical (p = 0.003), psychological (p = 0.025), and environmental (p = 0.025) domains.
Child-Pugh (p = 0.000 in all domains) and MELD score had a significant association in all four domains (p = 0.000 in Physical health, Psychological health, and social relationship and 0.001 in Environmental health).
In all four domains, perception of health and QOL had a significant association (p = 0.000 in all domains). The patient’s perspective toward the treatment and their life will have an impact on their physical, psychological, and environmental health and their social relationship.
The highest score for health-related QOL in the Child-Pugh class was found in category A (61.16 ± 17.84SD) whereas the lowest was in category C (39.63 ± 18.18SD). These results can be correlated with the findings of Stine et al. (2020).
In class A, the social relationship (68.05 ± 17.30) domain has the highest health-related QOL and the physical health domain (55.05 ± 18.41 SD) has the least. But in class C the domains of Social relationship (47.12 ± 19.32 SD) and Physical health (31.37 ± 15.64 SD) were lower when compared to other classes.
In the MELD class, the highest health-related QOL score was found in the MELD scores ranging from 6 to 9 and the least was in the scores of 30 to 39. The result was similar to the study conducted by Stine et al. (2020) wherein the MELD score: was 6–9, the highest health-related QOL score was for the Social domain (73.57 ± 12.94 SD), and the lowest for the physical domain (58.67 ± 19.14SD). Whereas in MELD score: 30–39, the lowest and the highest for physical (29.19 ± 16.48 SD) and environmental domain (46.76 ± 15.54 SD) respectively.
This study also analyzed the mean and SD of MELD and Child-Pugh in different domains. In the physical domain, the highest value was found for Child-Pugh class A and MELD scores 6–9 (60 ± 21 SD). Similarly in psychological, social relations, and environmental also, Child-Pugh class A and MELD score 6–9 is having a high QOL score (64 ± 19 SD, 74 ± 16 SD, and 67 ± 17 SD, respectively). The patients who fall under the category of Child-Pugh class A and MELD score 6–9 are having least disease activity. So they usually have a higher QOL when compared to other Child-Pugh and MELD classes. Those who belong to this category have a QOL similar to the healthy population. These observations can be supported by the research conducted by Stine et al. (2020).
STRENGTH AND LIMITATIONS
In our study, we could determine the overall QOL and associated factors, the severity of the disease, and its impact on health-related QOL. This enables health care providers to identify areas to be focused more for improving QOL.
The study has the limitation of being a single-center study with a small number of patients for a short period of time.
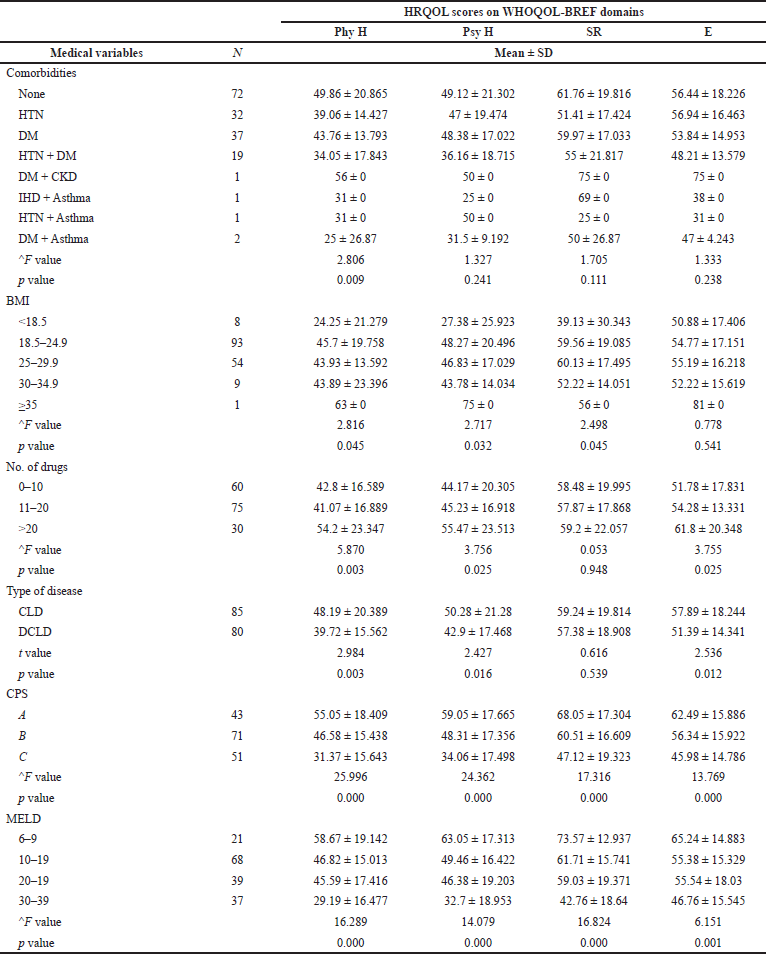 | Table 6. Mean scores of medical variables. [Click here to view] |
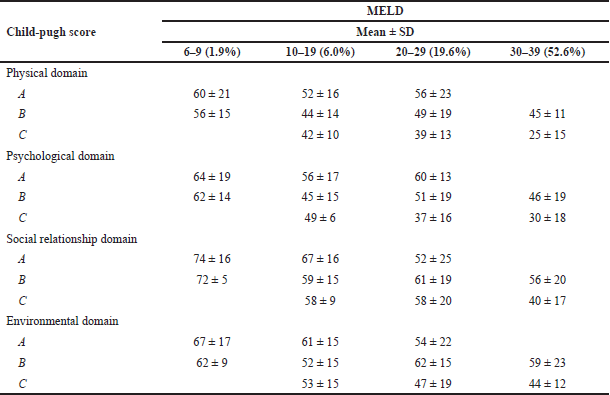 | Table 7. Cross-tabulation of child-pugh and MELD. [Click here to view] |
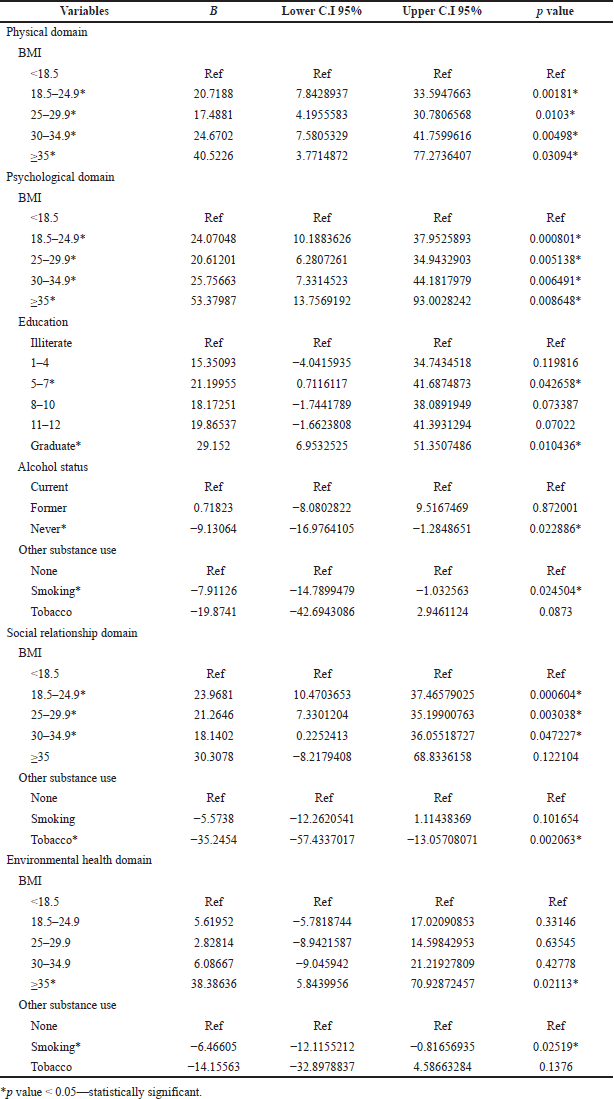 | Table 8. Association of factors in different domain using univariate linear regression analysis. [Click here to view] |
CONCLUSION
CLD has a substantial impact on QOL. The overall QOL of patients with CLD is lower than normal. The highest score was noticed in SR and the lowest was in the physical health domain. A healthy BMI is a crucial component in the physical domain, whereas education, alcohol status, and smoking contributed to the psychological domain. Tobacco chewing and BMI were linked to the SR domain. The environmental domain was linked to smoking and BMI. The Child-Pugh class A and MELD score 6–9 have a higher QOL.
The results of the study demonstrated the importance of health-related QOL in patients with CLD. Clinical pharmacy services collaborating with medical care will be helpful to CLD patients to improve their clinical outcomes and QOL.
ACKNOWLEDGMENTS
Researchers extend sincere gratitude to the Department of General Medicine, Justice K S Hegde Charitable Hospital, and respective faculties and participants of the study for completing this research work.
AUTHOR CONTRIBUTIONS
All authors agreed to submit the article to the current journal, gave financial approval for the version to be published, made significant contributions to the design data collection form, data collection, analysis, and interpretation, participated in its writing, or critically revised it for important intellectual content, and agreed to be responsible for all aspects of the work. According to the requirements/guidelines of the International Committee of Medical Journal Editors (ICMJE), all of the authors are qualified to be authors.
FUNDING
This research did not receive any financial support from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The researchers declare that they have no conflicts of interest.
ETHICAL APPROVAL
The ethical approval was obtained from Institution Ethics Committee on 12th November 2021 (Ref No: NGSMIPS/IEC/19/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adhikari S. Health related quality of life of patient with chronic liver disease in a tertiary hospital, Kathmandu. IJHSR, 2018; 8(10):178–88.
Afendy A, Kallman JP, Stepanova M, Younoszai Z, Aquino RD, Bianchi G, Marchesini G, Younossi ZM. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009; 30(5):469–76.
Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A, Schimid M, Ferlitsch A, Lipinski E, Brunner H, Lammer J, Ferenci P, Gangl A, Peck-Radosavljevic M. Child–Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut, 2003; 52(6):879–85.
Bhattarai S, Gyawali M, Dewan KR, Shrestha G. Demographic and clinical profile in patients with liver cirrhosis in a tertiary care hospital in central Nepal. JNMA J Nepal Med Assoc, 2017; 56(1):401–6.
Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso R, Ahn J, Kim CW, Rampal S, Cainzos-Achirica M, Zhao D, Chung EC, Shin H, Guallar E, Ryu S. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroeneterol, 2016; 111(8):1133–40.
Cheemerla S, Balakrishna M. Global epidemiology of chronic liver disease. Clin Liver Dis, 2021; 17(5):365–70.
Cortesi PA, Conti S, Scalone L, Jaffe A, Ciaccio A, Okolicsanyi S, Rota M, Fabris L, Colledan M, Fagiuoli S, Belli LS, Cesana G, Strazzabosco M, Mantovani LG. Health related quality of life in chronic liver diseases. Liver Int, 2020; 40(11):2630–42.
Durand F, Valla D. Assessment of the prognosis of cirrhosis: child-pugh versusMELD. J Hepatol, 2005; 42(1):S100–7.
Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed-pee N, Lok AS. Comorbidillness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol, 2001; 96(9):2737–44.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology, 2001; 33(2):464–70.
Kim HJ, Chu H, Lee S. Factors influencing on health-related quality of life in South Korean with chronic liver disease. Health Qual Life Outcomes, 2018; 16(1):1–8.
Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R, Italian Study Group for Quality of Life in Cirrhosis. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology, 2001; 120(1):170–8.
Pradhan RR, Bhandari BK, Pathak R, Poudyal S, Anees S, Sharma S, Khadga P. The assessment of health-related quality of life in patients with chronic liver disease: a single-center study. Cureus, 2020; 12(9):1–10.
Ray I, Dutta D, Basu P, De BK. Quality of life assessment of patients with chronic liver disease in eastern India using a Bengali translation chronic liver disease questionnaire. Indian J Gastroenterol, 2010; 29(5):187–95.
Salama ZA, Darweesh SK, Shehab HM, Abd-Elhameed MA. Etiology and prevalence of fatigue in chronic liver disease: clinical view. Egypt J Intern Med, 2016; 28(2):78–85.
Salehitali S, Nooriyan K, Hafizi M, Dehkordi AK. Quality of life and its effectivefactors in tuberculosis patients receiving directly observed treatment short-course (DOTS). J Clin Tuberc Other Mycobact Dis, 2019; 15:100.
Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, Poustchi H. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol, 2020; 5:245–66.
Sharma A, Nagalli S. Chronic liver disease [book on the internet]. StatPearls Publishing, Treasure Island, FL, 2021. Available via https://www.ncbi.nlm.nih.gov/books/NBK554597/.
Sharma R, Yadav R, Sharma M, Saini V, Koushal V. Quality of life of multi drug resistant tuberculosis patient: a study of North India. Acta Medica Iranica, 2014; 52(6):448–53.
Shetty K, Rybicki L, Carey WD. The child–pugh classification as a prognostic indicator for survival in primary sclerosing cholangitis. Hepatology, 1997; 25(5):1049–53.
Skevington SM, Tucker C. Designing response scales for cross-cultural use in health care: data from the development of the UK WHOQOL. Br J Med Psychol, 1999; 72(1):51–61.
Sobhonslidsuk A, Silpakit C, Kongsakon R, Satitpornkul P, Sripetch C, Khanthavit A. Factors influencing health-related quality of life in chronic liver disease. World J Gastroenterol, 2006; 12(48):7786.
Stine JG, Stukenborg GJ, Wang J, Adkins A, Niccum B, Zimmet A, Argo CK. Liver transplant candidates have impaired quality of life across health domainsas assessed by computerized testing. Ann Hepatol, 2020; 19(1):62–8.