INTRODUCTION
Ultraviolet (UV) radiation causes the process of melanogenesis to start producing melanin. Its bio-radical character and susceptibility to UV radiation make melanin a natural photoprotector of the skin against damage caused by UV radiation. UV radiation causes damage to the skin where the absorbed UV will form reactive oxygen species (ROS), a free radical found in the skin (Berman and Cockerell, 2013). Melanin is the only natural UV radiation protection on the skin by eliminating the effects of free radicals, but the result is a brownish tint to the skin. If the formation of melanin is not evenly distributed on the skin, it will cause black spots or patches on the skin (Herrling et al., 2007; Mujahid et al., 2017). Depigmenting agents generally have a mechanism of action by inhibiting melanin formation by inhibiting tyrosinase (TYR) including inhibition of maturation and increased degradation, down-regulation of melanocortin 1 receptor activity, inhibition of microphthalmia-associated transcription factor (MITF), disturbances in the process of maturation and transfer of melanosomes, and a decrease in melanocytes. The inhibition of MITF activity by depigmenting agents will inhibit the work of TYR, TYR-related protein-1, and TYR-related protein-2 so that the rate of melanogenesis process can also be inhibited (Ando, 2017; Hsiao and Fisher, 2014).
Depigmenting agent used can be derived from synthetic materials or natural materials. Synthetic depigmenting agents that are often used are hydroquinone and its derivatives. Hydroquinone when used continuously with levels below 2% can cause contact leukoderma and exogenous ochronosis (Burger et al., 2016; Coiffard and Couteau, 2016). Hydroquinone has an inhibitory effect on melanogenesis, where the enzyme TYR oxidizes hydroquinone and produces benzoquinone which is toxic to melanocyte cells, which can cause contact leukoderma (Palumbo et al., 1991). Depigmenting agents from natural ingredients are usually in the form of herbal extracts or phytochemical compounds, which are tested as depigmenting agents that are safer and more effective against cells (Masaki, 2017). Several studies have shown that plant extracts are able to inhibit melanogenesis in vitro and furthermore show potential after the isolation of active compounds (Burger et al., 2016). Catechins are phytochemical compounds that are included in the secondary metabolites of flavonoids. Catechins are the main components of gambir plants. Isolation of gambir (Uncaria gambir (Hunter) Roxb.) can produce catechins with a purity of 96.1% (Rahmawati et al., 2012).
Research on catechins as inhibition of melanogenesis was carried out on several compounds contained in tea (Camelia sinensis), where the depigmentation effect that occurred in the administration of catechins was caused by direct inhibition of TYR activity on B16 cells. The results showed that epigallocatechin-3-gallate (EGCG), EGC, catechin (C), and gallic acid had significant inhibitory activity of melanin synthesis (Sato and Toriyama, 2009). Catechins protect keratinocytes against UVB radiation and ROS. Catechins prevent damage to keratinocytes mainly through UVB and ROS induction, which is very different from the mechanism of action of EGCG (Wu et al., 2006). Catechins are less cytotoxic than EGCG. Catechins found in red wine (Vitis vinifera) in vitro have activity as depigmenting agents. TYR inhibitory activity was observed in the fraction containing oligomeric proanthocyanidins which contained catechins and epicatechins. This compound can inhibit melanin synthesis on B16 cells (Fujimaki et al., 2018).
The melanogenesis inhibitory effect of a depigmenting agent can be done by looking at the activity of the TYR enzyme. Decreased enzyme activity can affect the formation of melanin. Expression of the TYR gene can be associated with protein formation in cultured B16F10 cells. The decrease in the amount of melanin is due to a decrease in the expression of the TYR gene in the mRNA transcription process (Hartman and Czyz, 2015). There was no difference in the TYR mRNA level; the difference in TYR expression arose from the post-translational modification of the enzyme leading to its activation or inhibition in B16F10 cells (Rodriguez-lopez et al., 1992). Mouse melanoma cells are a melanoma cell model that is often used in testing the effect of a depigmenting agent on the melanogenesis process in vitro (Beaumont et al., 2014). B16 cells are the stem cells of mouse melanoma. The use of B16F0 cells was more effective than B16 cells in an in vitro experiment (Nakamura et al., 2002). In vitro assays on the activity of quantitative depigmenting agents in cell culture include inhibition of TYR activity, decreased levels of melanin, and analysis of microscopic images of melanin. One of the three test methods can be carried out to analyze the effect of depigmenting agents (Kim et al., 2019). In addition to these three parameters, tests on cytotoxicity, solubility, absorption, and penetration into the skin as well as the stability of the test substance can also be considered in the test (Solano et al., 2006).
This study aims to determine the effect of depigmenting agent catechin isolated from gambir with different purity (90% and 98%). Tests were carried out in vitro including cell viability, TYR activity, and melanin content using B16F0 cells. The results of this study are expected to provide information about the potential of gambir catechins as depigmenting agents. In this study, 3-isobutyl-1-methylxanthine (IBMX) was used to stimulate the process of melanogenesis in B16F0 cells (Cha and Kim, 2013). This study provides an additional list of compounds that can be formulated in cosmetic preparations as lightening agents.
MATERIALS AND METHODS
Materials
Catechin 90% (C90, PE-001) and catechin 98% (C98, PE-003) isolated from U. gambir were obtained from Andalas Sitawa Fitolab, West Sumatra, Indonesia. Other materials are as follows: kojic acid (KA, Sigma Aldrich), mouse melanoma B16F0 cells from the European Collection of Cell Cultures (B16F0, ECACC 92101204, United Kingdom), Dulbecco’s modified eagle’s medium (DMEM, Sigma Aldrich), fetal bovine serum (FBS, Sigma Aldrich), penicillin-streptomycin, amphotericin B (Sigma Aldrich), phosphate buffer saline (PBS, Bioneer), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Merck), trypsin-ethylene diamine tetra acetic acid (Corning), dimethylsulfoxide (Sigma-Aldrich), IBMX (Sigma Aldrich), sodium dodecyl sulfate (SDS, Sigma Aldrich), trichloroacetic acid (Sigma Aldrich), 3,4-dihydroxy-L-phenylalanin (L-DOPA, Sigma-Aldrich), Tris–HCl and Triton X-100 (Merck), TYR from mushroom (T3824, Sigma Aldrich), melanin synthetic (M8631, Sigma Aldrich), HCl, NaOH, and NH4OH.
Equipment
Equipment is as follows: incubator (Thermo), laminar air flow cabinet (Nuaire), ELISA reader (Benchmark Bio-Rad), centrifuge (Thermo), microscope (Olympus FE 125), hemocytometer (Neubauer), vortex, and water bath.
Cell culture
B16F0 cells were cultured in DMEM media using 10% FBS, 2% penicillin-streptomycin, and 0.5% amphotericin B. The culture was maintained at 37°C and in an atmosphere containing 5% CO2. The cell culture density was 5 × 104 cells/ml. Every 2 days the media is replaced with a new one and cells are counted and replanted to get the desired density.
Cell viability test
The cell viability was determined by the MTT assay. The 96-well plate was filled with B16F0 cells with a density of 5 × 103 cells/well. It was incubated for 24 hours at 37°C and in an atmosphere containing 5% CO2. After 24 hours of changing the medium, it was incubated for 24 hours. C90, C98, and KA were each dissolved with distilled water and made a series of concentrations of 25–500 µg/ml. The test solution was put into the well about 100 µl, and we incubated the cells in an incubator at 37°C and in an atmosphere containing 5% CO2 for 72 hours. We discarded cell media, washed with PBS, and added 100 µl MTT reagent to each well, including media control (without cells). It was incubated for 2–4 hours in an incubator at 37°C, and in an atmosphere containing 5% CO2, if formazan has clearly formed, add a stopper (10% SDS in 0.01 N HCl). After incubation overnight, cell density was determined by reading the absorbance at a wavelength of 595 nm. The percentage of cell viability is calculated using the following equation (Kamiloglu et al., 2020):
Create a concentration graph with cell viability, determine the linear regression equation, and calculate the IC50 value.
Assay of TYR activity
Inhibitory activity of mushroom TYR
A cell-free assay system was used to test for the direct effects of catechin on mushroom TYR activity. C90, C98, and KA were each dissolved with distilled water and made a series of concentrations of 5–80 µg/ml. An aliquot of 70 µl of each test solution was added into the well. Then 20 µl mushroom TYR 200 U/ml was added, and we let it stand for 10 minutes, left in a dark place. Then 10 µl of 10 mM L-DOPA solution was added, and we let it stand for 10 minutes, left in a dark place. Absorption was measured at a wavelength of 475 nm as dopachrome. The percentage of inhibition was calculated using a blank. Graph the concentration with TYR inhibition, determine the linear regression equation, and calculate the IC50 value.
Assay of cellular TYR activity
We measured cellular TYR activity assay using B16F0 cells. Each well added B16-F0 cells with a density of 5 × 103 cells/well, incubated for 24 hours. Media was replaced and incubated again for 24 hours. An aliquot of 100 µl of each test solution with various concentrations (5–80 µg/ml) was added to the wells; 50 µl IBMX 0.1 mM was added and incubated for 24, 48, and 72 hours. Cells were washed with PBS and lysed using Tris–HCl 0.1% Triton X-100 (pH 7.5). Lysates were collected at each sample concentration and then centrifuged at 1,500 rpm for 10 minutes at 4°C. Each precipitate formed was dissolved with the medium and then put into the well. 10 µl L-DOPA 10 mM was added to each well, allowed to stand for 60 minutes, and left in a dark place. Measure the absorption at a wavelength of 475 nm as dopaquinone. The absorbance obtained was calculated as the percentage of the control, that is, cells that were only treated with IBMX.
Measurement of melanin content
Measurement of extracellular melanin content and microscopy
Each well added B16F0 cells with a density of 5 × 103 cells/well, and incubated for 24 hours; the medium was changed and incubated again for 24 hours. An aliquot of 100 µl of each test solution with various concentrations (5–80 µg/ml) was added to the wells; 50 µl IBMX 0.1 mM was added and incubated for 24, 48, and 72 hours. Absorption was measured at a wavelength of 400 nm. The test results were calculated as a percentage of the control, that is, cells that were only treated with IBMX. Before measuring the melanin content, cells were observed under a light microscope and photographed using a digital camera.
Measurement of extracellular melanin concentrations
Each well added B16-F0 cells with a density of 5 × 103 cells/well, incubated for 24 hours; the medium was changed and incubated again for 24 hours. An aliquot of 100 µl of each test solution with various concentrations (5–80 µg/ml) was added to the wells; 50 µl IBMX 0.1 mM was added and incubated for 24, 48, and 72 hours. Cells were washed with PBS, then lysed using 20 mM Tris-0.1% Triton X-100 (pH 7.5), and incubated for 5 minutes. Cell lysates were collected, transferred to tubes, and centrifuged at 4°C for 10 minutes at 1,200 rpm. The supernatant was transferred to the well, adding Bradford reagent; the absorbance was measured at a wavelength of 450 nm. Calculate the protein content of melanin in cells (%). The precipitate was dissolved with NaOH 1 N and incubated at 60°C for 60 minutes; the solution was centrifuged at 4°C for 10 minutes at 12,000 rpm; the supernatant was transferred to a well; the absorbance was measured at a wavelength of 400 nm. Standard curve of synthetic melanin (0–400 µg/ml) was prepared.
Statistical analysis
All measurements were presented as group mean ± SD, which was replicated three times. The data were processed statistically using two-way analysis of variance. Tukey’s post hoc test was applied to clarify the change in group mean; statistical significance was defined as p < 0.05.
RESULTS AND DISCUSSION
Research on the effects of catechins and their benefits for the skin has been carried out, including antioxidants, anti-microbials, anti-inflammatory, anti-allergic, anti-diabetic, anti-obesity, anti-cancer, and others including nutraceuticals and biocosmetics on the skin (Bae et al., 2020; Saad et al., 2020). There is no research on catechins derived from gambir as inhibitors of melanogenesis or depigmenting agents. Uncaria gambir Roxb. or often referred to as gambir is included in the Rubiaceae family and is a plant that contains high levels of catechins. Research on the isolation and purification of catechin isolates derived from the first quality gambir extract obtained from West Sumatra obtained the purity of catechin isolates which obtained 99.80% ± 0.132%. The content of catechins is the main determinant of the quality of gambir (Kurniatri et al., 2019).
This study used KA for comparison to see the effect of the depigmenting agent. KA has the same mechanism of action as hydroquinone, which is to competitively inhibit the TYR enzyme and is used at a concentration of 1%–4%. Patients who do not respond to hydroquinone can be treated with KA. The carcinogenic effect of hydroquinone can be replaced by KA (Sarkar et al., 2012). KA and hydroquinone have very strong inhibitory activity against B16BL6 cells which have IC50 < 100 µM compared to arbutin and vitamin C which have weak inhibitory activity of 100 and 400–500 µM, respectively. In vitro assays on B16FBL6 cells, hydroquinone, KA, and arbutin have a very strong inhibitory effect on melanogenesis compared to vitamin C (Park et al., 2003).
The study was conducted using B16F0 cells to see the activity of catechins derived from gambir against melanogenesis inhibition. Research on the effect of depigmenting agents on extracts and phytochemical compounds in biomolecular terms uses cell-line mouse melanoma for in vitro testing, which can see the activity of several groups of enzymes involved in the process of melanin pigment synthesis. Cell culture is an in vitro model for melanoma in humans (Overwijk and Restifo, 2001). Cell-line mouse melanoma has several characteristics based on its metastatic ability and mortality, namely, B16F0, B16F1, B16F10, and B16BL6 (Nakamura et al., 2002).
Cell viability was defined as the number of live cells in the sample. In particular, the viability test is used to evaluate the effect of the developed material on the cells. A standardized evaluation to determine whether a material contains a biologically hazardous (toxic) substance is called a cytotoxicity test. The conditions that must be met for a cytotoxicity test system include the fact that the test system must be able to produce a dose-response curve that is in line with the effects that appear in vivo. One of the commonly used methods to determine the number of cells is the MTT method (Kamiloglu et al., 2020). The biochemical mechanism of MTT assay involves the nicotinamide adenine dinucleotide phosphate-dependent cellular oxidoreductase enzyme which converts yellow MTT to purple formazan by the action of mitochondrial reductase. The intensity of the purple color is directly proportional to the number of cells, thus indicating cell viability (Han et al., 2010). One of the cell viability tests is to determine the IC50 parameter, which is to show the potential toxicity of a test compound. The IC50 value is also used as a basis for determining the maximum concentration of the test compound to be tested (Kumar et al., 2018). The activity of C90, C98, and KA on the viability of B16F0 cells is shown in Figure 1. Based on the results of cell viability, the IC50 value of catechins was obtained as shown in Table 1, so the maximum concentration for the next test was around 170 µg/ml.
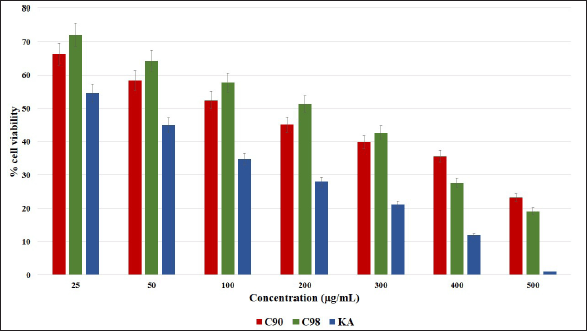 | Figure 1. Cell viability assay using the MTT assay, B16F0 cells were treated with C90, C98, and KA at different concentrations (25, 50, 100, 200, 300, 400, and 500 μg/ml) in 72 hours. Error bars represent the standard deviation (n = 4). [Click here to view] |
The IC50 values of C90 and C98 in this study were different from those obtained for catechins from green tea, where at a concentration of 20 µM cell viability ranged from 60% to 80% against B16 melanoma cells for 5 days (Sato and Toriyama, 2009). Extracts of black tea, green tea, and white tea were tested for cell viability with a concentration of 50 µg/ml on Melan-A cells for 48 hours and cell viability was 62%–76% (Kim et al., 2015). To determine the anti-melanogenesis effect of green tea compound EGCG, UVA irradiated B16 cells at a concentration of 100 µg/ml showed cell viability of 67.09% ± 3.27% (Liang et al., 2014). This shows that C90 and C98 are less toxic to cells than catechins derived from tea.
The most common approach to look at the effects of depigmenting agents on the skin in vitro is to involve inhibition of the enzyme TYR, which is a copper-containing enzyme that catalyzes the process of melanogenesis. The use of TYR enzyme has been widely used for research on natural ingredients as a TYR inhibitor in vitro, where the results obtained have an effect that is close to a TYR inhibitor on human skin (Panzella and Napolitano, 2019). Although in vitro test results are not always reproduced on human skin, this method is widely used in melanogenesis research, especially as a first step to identify potential depigmenting agent activity. Testing of TYR inhibitors was done using mushroom TYR without using cultured cells. Activity is assessed based on the formation of dopachrome which can be measured spectrophotometrically at a wavelength of 475 nm where the test is to see the IC50 value (Zolghadri et al., 2019).
 | Table 1. The linear regression equation and IC50 value of cell viability. [Click here to view] |
Research conducted using the mushroom TYR enzyme showed that increasing concentrations of C90, C98, and KA would increase the percentage of enzyme inhibition; this is shown in Figure 2. Based on the results of the concentration and percentage of enzyme inhibition, an equation was obtained to determine the IC50 value where the IC50 value of C98 with KA was almost the same (Table 2). The results obtained fall within the range of IC50 values of catechins reported by Panzella and Napolitano (2019), where the IC50 values of catechins range from 20 to 150 µM (~5.8–43.5 µg/ml). C98 derived from gambir in this study showed a better value than catechins derived from tea, which had an IC50 value of 57.12 µM (~16.57 µg/ml), but not for C90 (Tang et al., 2018). The inhibitory effect on mushroom TYR depends on the dose used significantly, where the KA concentration of 3.91–250 µg/ml shows an effect as TYR inhibition (Lajis et al., 2012).
The reaction of the TYR enzyme to dopaquinone is a rate-limiting step in the melanogenesis of melanin formation. These reactions are rate-limiting steps in melanogenesis of melanin formation; therefore, depigmenting agent activity was measured by directly assessing TYR activity. Many other factors were found that regulate melanogenesis such as the activity of additional enzymes (dopachrome tautomerase, peroxidase, and so on) and certain metal ions, especially copper and iron. An additional level of genetic control is involved in melanin synthesis; therefore, TYR activity is an important, but not the only, factor for determining the level of melanin production (Hu, 2008). Measurement of TYR activity in melanocytes in cell culture was measured as dopaquinone at a wavelength of 475 nm. The depigmenting agent activity resulted in the formation of less dopaquinone compared to the control (Kim et al., 2019).
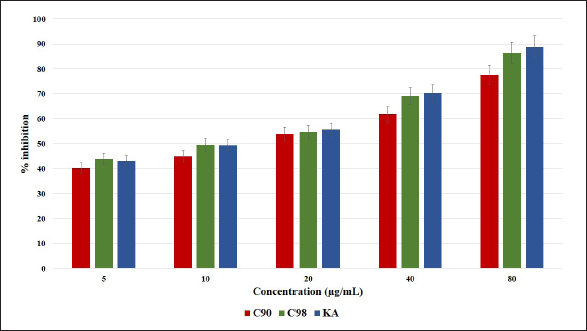 | Figure 2. Percentage of inhibition of C90, C98, and KA on mushroom tyrosinase enzymes with different concentrations (5, 10, 20, 40, and 80 μg/ml). Error bars represent the standard deviation (n = 3). [Click here to view] |
 | Table 2. The linear regression equation and IC50 value of inhibition TYR. [Click here to view] |
The research that was conducted found that C90, C98, and KA could reduce the dopaquinone content in B16F0 cells, which are presented in Table 3. The results of testing the effects of C90, C98, and KA on TYR activity showed that the higher the concentration and duration of the test were, the lower the dopaquinone content was significant (p = 0.000); this is shown in Figure 3. At the 24 hours duration of the test, all concentrations had a significant difference in reducing the dopaquinone content (p = 0.000). At the test duration of 48 hours, there was no significant difference between C90, C98, and KA in reducing the dopaquinone content with concentrations of 20 µg/ml (p = 0.510) and 40 µg/ml (p = 0.417). There was no significant difference between C98 and KA (p > 0.05) at a concentration of 80 µg/ml. In the 72 hours test duration, all concentrations had a significant difference in reducing dopaquinone content (p = 0.000); except for C98 with KA there was no significant difference (p > 0.05).
The research showed that, at a concentration of 10 µg/ml for 48 hours, C90 and C98 decreased dopaquinone in B16F0 cells, where the results obtained were almost the same as EGCG contained in tea at a concentration of 12.5 µg/ml for 48 hours by 45%–58%; the test was carried out on Melan-A cells (Kim et al., 2015). EGCG can significantly inhibit the concentration-dependent increase in TYR (Sato and Toriyama, 2009).
Melanin, the main pigment in melanocytes, is synthesized in response to various cellular and environmental factors. There are two parameters used to measure the melanin content, namely, the extracellular melanin content and the intracellular melanin content. The extracellular melanin content was determined by comparing the density of the treated melanin with the density in the untreated cells. The intracellular melanin content is determined by counting the amount of melanin in the cells. These two parameters can be used to determine the melanin content or one of the two parameters (Chung et al., 2019; Hu, 2008).
Measuring melanin content extracellularly and intracellularly, the results obtained are presented in Table 4 and Figure 4. Increasing the concentration and duration of the test at C90, C98, and KA showed a significant difference in reducing the extracellular melanin content in B16F0 cells (p = 0.000). At the 24 hours test duration, there was a significant difference between C90, C98, and KA in reducing melanin content (p = 0.000); except at a concentration of 80 µg/ml, there was no significant difference between C90, C98, and KA in reducing melanin content (p = 0.066). At the 48 hours test duration, there were significant differences in all samples in reducing the melanin content (p = 0.000 and p = 0.001). At the test duration of 72 hours, there was a significant difference in all samples in reducing the melanin content (p = 0.000 and p = 0.032).
C90 with increasing concentration and duration of the test showed a significant difference in reducing the melanin content (p = 0.000); except at a concentration of 40 µg/ml, the duration of the test did not show a significant difference (p = 0.051). The same thing also happened to C98, where with increasing concentration and duration of the test showed a significant difference in reducing the melanin content (p = 0.000 and p = 0.016); except at a concentration of 40 µg/ml, the duration of the test did not show a significant difference (p = 0.114). This result is different from KA, which shows that with increasing concentration and duration of the test there is a significant difference in decreasing melanin content (p = 0.000).
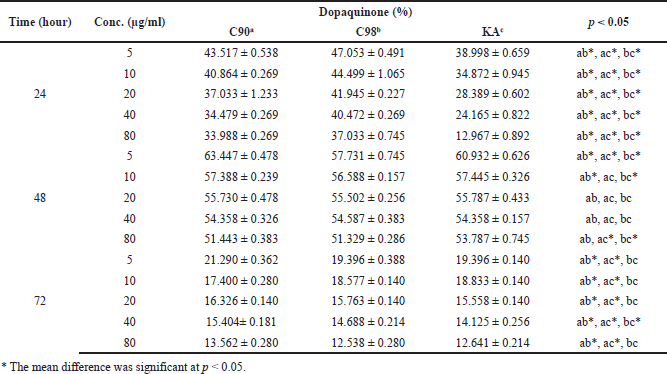 | Table 3. The dopaquinone content on B16F0 cells. [Click here to view] |
 | Figure 3. The reaction of the TYR enzyme to dopaquinone is a rate-limiting step in the melanogenesis of melanin formation. [Click here to view] |
The decrease in extracellular melanin content was also evidenced by morphological observations (Fig. 5) using concentrations of C90, C98, and KA of 80 µg/ml. The picture shows that giving IBMX will increase the formation of melanin cells in B16F0 cells. Administration of C90, C98, and KA will reduce the amount of melanin in the visual observations for the duration of the test.
C90 and C98 at a test duration of 72 hours showed better results in reducing the melanin content than the water extract of Cordyceps militaris, where at a concentration of 5 mg/ml for 72 hours the melanin content was 59.8% in B16F0 cells (Cha and Kim, 2013). This result is different when compared with EGCG from tea extract, where at a concentration of 12.5 µg/ml for 72 hours the melanin content in cells ranges from 20% to 52% in Melan-A cells (Kim et al., 2015), compared to C90 and C98 where at a concentration of 10 µg/ml for 72 hours the melanin content was 76.7% and 62.1% in B16F0 cells, respectively.
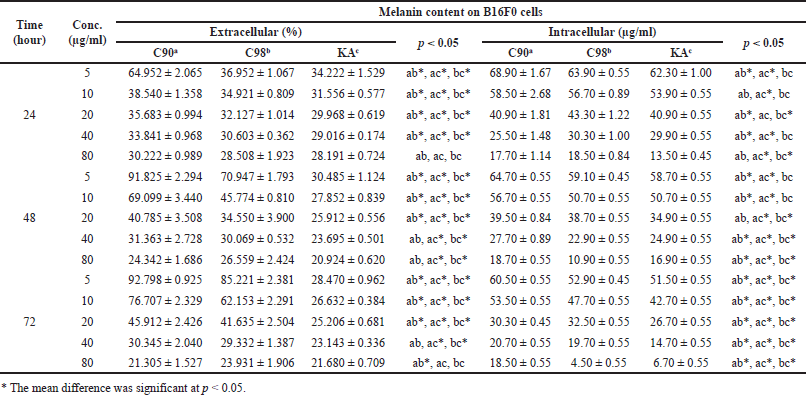 | Table 4. The extracellular and intracellular melanin content on B16F0 cells. [Click here to view] |
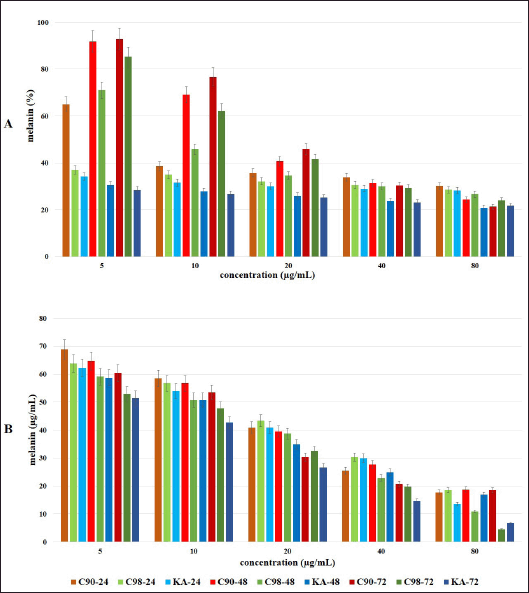 | Figure 4. Effect of C90, C98, and KA on melanin content extracellular (A) and intracellular (B) in B16F0 cells at different concentrations (5, 10, 20, 40, and 80 μg/ml) with the test duration of 24, 48 and 72 hours. Error bars represent the standard deviation (n = 3). [Click here to view] |
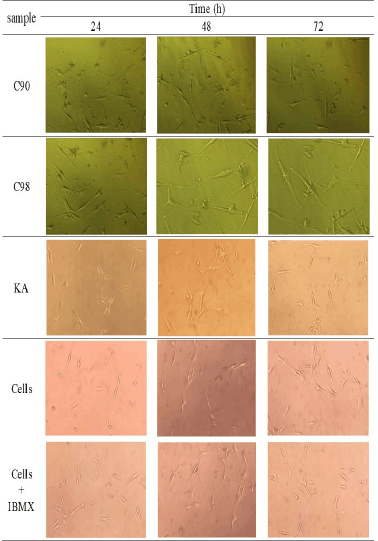 | Figure 5. Morphological form of decreased extracellular melanin content from C90, C98, and KA at a concentration of 80 μg/ml. [Click here to view] |
Measuring melanin content intracellularly using a straight line equation of synthetic melanin concentration, in this study the line equation y = 0.001x + 0.0143 with R2 of 0.9967 was obtained.From the obtained equation, absorbance values were entered to calculate the intracellular melanin content (Table 4). The results showed that increasing concentrations and duration of testing at C90, C98, and KA significantly decreased intracellular melanin levels in B16F0 cells (p = 0.000). There was no significant difference in the test duration at C90 concentration of 80 µg/ml (p = 0.152); this was different from C98 and KA where the concentration and duration of the test significantly affected intracellular melanin levels (p = 0.000).
The research is still in the in vitro testing stage using B16F0 cells. Further testing of other cell cultures related to the process of melanogenesis can be carried out to strengthen the data regarding in vitro studies of gambir catechins, for example using B16F16, B16BL6, Melan-A cells, and also with other in vitro methods on melanogenesis as a depigmenting agent effect.
CONCLUSION
C90 and C98 affect the viability of B16F0 cells without being toxic to cells. C98 has a mushroom TYR (IC50) inhibition value which is almost the same as KA. C90 and C98 decreased TYR activity and melanin content in B16F0 cells significantly (p = 0.000) depending on concentration and duration of the assay. The melanogenesis inhibitory activity of C98 was not significantly different from that of C90 and KA. Based on in vitro testing, C90 and C98 can be used as depigmenting agents.
AUTHORS’ CONTRIBUTIONS
All the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors would like to thank Lembaga Pengelola Dana Pendidikan (LPDP) of the Ministry of Finance of the Republic of Indonesia for the funds provided for this research, contract no. PRJ-6073/LPDP.3/2016.
CONFLICTS OF INTEREST
The authors have no conflicts of interest regarding this investigation.
ETHICAL APPROVAL
This research does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed is included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ando H. Melanogenesis. In: Sakamoto K, Lochhead H, Maibach H, Yamashita Y (Eds.). Cosmetic science and technology: theoretical principles and applications, Elsevier, Amsterdam, Netherlands, pp 729–36, 2017.
Bae J, Kim N, Shin Y, Kim SY, Kim YJ. Activity of catechins and their applications. Biomed Dermatol, 2020; 4(8):1–10.
Beaumont KA, Mohana-Kumaran N, Haass NK. Modeling melanoma in vitro and in vivo. Healthcare, 2014; 2:27–46.
Berman B, Cockerell CJ. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Dermatol, 2013; 68(1):S10–9.
Burger P, Landreau A, Azoulay S, Michel T, Fernandez X. Skin whitening cosmetics: feedback and challenges in the development of natural skin lighteners. Cosmetics, 2016; 3(36):1–24.
Cha J, Kim S. Anti-melanogenesis in B16F0 melanoma cells by extract of fermented Cordyceps militaris containing high cordycepin. J Life Sci, 2013; 23(12):1516–24.
Chung S, Lim GJ, Lee JY. Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci Rep, 2019; 9(780):1–9.
Coiffard L, Couteau C. Overview of skin whitening agents: drugs and cosmetic products. Cosmetics, 2016; 3(27):1–16.
Fujimaki T, Mori S, Horikawa M, Fukui Y. Isolation of proanthocyanidins from red wine, and their inhibitory effects on melanin synthesis in vitro. Food Chem, 2018; 248:61–9.
Han M, Li J, Tan Q, Sun Y, Wang Y. Limitations of the use of MTT assay for screening in drug discovery. J Chin Pharm Sci, 2010; 9:195–200.
Hartman ML, Czyz M. MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci, 2015; 72:1249–60.
Herrling T, Jung K, Fuchs J. The important role of melanin as protector against free radicals in skin. SOFW-J, 2007; 133(9):26–33.
Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys, 2014; 563:28–34.
Hu D. Methodology for evaluation of melanin content and production of pigment cells in vitro. Photochem Photobiol, 2008; 84:645–9.
Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Front, 2020; 1:332–49.
Kim YC, Choi SY, Park EY. Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes. J Vet Sci, 2015; 16(2):135–43.
Kim Y, Kim M, Kweon D, Lim S, Lee S. Quantification of hypopigmentation activity in vitro. J Vis Exp, 2019; 145:1–6.
Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harbor Protoc, 2018; 6:469–72.
Kurniatri AA, Sulistyaningrum N, Rustanti L. Purifikasi katekin dari ekstrak gambir (Uncaria gambir Roxb.). Media Litbangkes, 2019; 29(2):153–60.
Lajis AFB, Hamid M, Ariff AB. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J Biomed Biotechnol, 2012; 952452:1–9.
Liang YR, Kang S, Deng L, Xiang L, Zheng XQ. Inhibitory effects of (-)-epigallocatechin-3-gallate on melanogenesis in ultraviolet A-induced B16 murine melanoma cell. Trop J Pharm Res, 2014; 13(11):1825–31.
Masaki H. Bioactive ingredients: benefits of cosmetics stimulated through biological aspects. In: Sakamoto K, Lochhead H, Maibach H, Yamashita Y (Eds.). Cosmetic science and technology: theoretical principles and applications, Elsevier, Amsterdam, Netherlands, pp 255–65, 2017.
Mujahid N, Liang Y, Murakami R, Roider EM, Gray NS, Fisher DE, Wang J. A UV-independent topical small-molecule approach for melanin production in human skin. CellReports, 2017; 19(11):2177–84.
Nakamura K, Yoshikawa N, Yamaguchi Y. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci, 2002; 70:791–98.
Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol, 2001; 39:20–1.
Palumbo A, D’Ischia M, Misuraca G, Prota G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta, 1991; 1073(1):85–90.
Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics, 2019; 6(57):1–33.
Park Y, Lee J, Park D, Park J. Effects of kojic acid, arbutin and vitamin C on cell viability and melanin synthesis in B16BL6 cells. J Soc Cosmet Sci Korea, 2003; 29(1):151–67.
Rahmawati N, Bakhtiar A, Putra DP. Isolasi katekin dari gambir (Uncaria gambir (Hunter). Roxb) untuk sediaan farmasi dan kosmetik. J Penelitian Farm Indones, 2012; 1(1):6–10.
Rodriguez-lopez JN, Tudelap J, Varons R, Garcia-carmonap F, Garcia-canovaspll F. Analysis of a kinetic model for melanin biosiynthesis pathway. J Biol Chem, 1992; 267(6):3801–10.
Saad MFM, Goh H, Rajikan R, Yusof TRT, Baharum SN, Bunawan H. Uncaria gambir (W. Hunter) Roxb: from phytochemical composition to pharmacological importance. Trop J Pharm Res, 2020; 19(8):1767–73.
Sarkar R, Chugh S, Garg VK. Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol, 2012; 78(4):417–28.
Sato K, Toriyama M. Depigmenting effect of catechins. Molecules, 2009; 14:4425–32.
Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res, 2006; 19:550–71.
Tang H, Cui F, Li H, Huang Q, Li Y. Understanding the inhibitory mechanism of tea polyphenols against tyrosinase using fluorescence spectroscopy, cyclic voltammetry, oximetry, and molecular simulations. R Soc Chem, 2018; 8:8310–8.
Wu W, Chiang H, Fang J, Chen S, Huang CC, Hung CF. (+)-Catechin prevents ultraviolet B-induced human keratinocyte death via inhibition of JNK phosphorylation. Life Sci, 2006; 79:801–7.
Zolghadri S, Bahrami A, Tareq M, Khan H, Saboury AA. A comçprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem, 2019; 34(1):279–309.