INTRODUCTION
Datura (Datura metel L.) is a typical Asian plant commonly found throughout South Asia’s tropics to Southeast Asia (Indonesia). This plant has an annual cycle, characterized by thorny fruit, 0.40–1 m high. The leaf size is ±15 cm, and the colors of the flower are either purple or white (Gaire and Subedi, 2013). The use of Datura as traditional medicine has been documented for centuries, and empirically, it is used as an asthma medication by utilizing its dried leaves. Datura is also used as an anti-bacterial, antiseptic, narcotic, and sedative (Carpa et al., 2017; Wu et al., 2020). Datura metel, which has a big population but has not been widely developed as an active ingredient in this therapy since the study of chemicals and fragmentation, has not been thoroughly applied. In the previous study, all components of the Datura plant were analyzed, but just phytochemical screening was done and no particular analysis of the active compounds was done. Antony et al. (2019) identified all parts of the Datura plant: the roots, stalks, leaves, flowers, fruits, and seeds, containing terpene and alkaloid compounds. The most alkaloid content is found in its roots and seeds. The Withanolide group, which is a steroid, is also a terpene group. The known alkaloid groups are atropine, hyoscyamine, scopolamine, and hyoscine. Besides, fatty substances and calcium oxalate were also found in the fruit (Antony et al., 2019; Carpa et al., 2017; Guo et al., 2018). Datura is classified as a toxic plant, with alkaloid compounds as the trigger factor. Several types of alkaloid compounds were found in hyoscyamine, atropine, and scopolamine (Carpa et al., 2017; Iranbakhsh et al., 2006). Identification of various secondary metabolites is needed to determine the specific effectiveness of Datura (D. metel L.). Identification can be done using ultra-performance liquid chromatography-mass spectroscopy (UPLC-MS) by looking at the specific fragmentation of Datura (D. metel L.). Several previous studies have tested the content of D. metel using high-performance liquid chromatography and gas chromatography-mass spectra (Hossain et al., 2013a, 2013b). This analysis was carried out to identify the phenolic content of Datura (D. metel L.).
Vadlapudi and Kaladhar (2012) researched Datura leaf extract. Methanol and n-hexane extracts at a concentration of 2 mg/ml formed inhibition zones of 6 and 8 mm, respectively, against Staphylococcus aureus. Yang et al. (2014) successfully isolated nine new isolates of the Withanolide group. Isolate 5,7-dimethyl 6-hydroxyl 3-amine sitosterol is isolated from Datura leaves, showing inhibition of P. aeruginosa, B. subtilis, S. typhi, K. pneumonia, S. aureus, and P. mirabilis (Okwu and Igara, 2009). One of them is the Datura folisides, a compound that shows a promising anti-inflammatory effect. Anti-inflammatory activity test used rat macrophages (murine) RAW 264.7 stimulated by Lipopolysaccharide. The study on the cytotoxic potential of Datura leaves has also been carried out by Bellila et al. (2011). The Datura leaf isolates of the Withanolide group, Withametelin, and 12α-hydroxydaturametelin B, showed cytotoxic effects on human lung carcinoma cells (A549) and human colorectal adenocarcinoma cells (DLD-1). Both isolates showed cytotoxicity to A549 and DLD-1 cells with IC50 values of 7 and 2.0 μM, respectively. Therefore, further research is needed to identify compounds and their cytotoxic activities using the brine shrimp lethality test (BSLT). The compound’s toxicity potential can be determined based on the number of deaths of the tested animals.
Furthermore, the identification and isolation of secondary metabolites were carried out. The maceration stage was conducted by fractionation using the liquid-liquid partition. The isolation and purification used preparative thin-layer chromatography (TLC). The purification results obtained pure isolates, which were then identified using UPLC-MS. In this study, an analysis of the cytotoxicity and fragmentation pattern of D. metel L. leaves using is conducted. The UPLC-MS technique combines two methods in one analysis system. The isolate purity identification can use the UPLC chromatography system to detect compounds using mass spectroscopy by looking at the compound’s fragmentation pattern and molecular weight (Jian, 2017; Yang et al., 2020). This research is expected to be continued with research on the formulation of active pharmaceutical ingredients using Datura metel essential oil.
MATERIALS AND METHODS
Extraction
In this study, extraction was performed by maceration using methanol 96% solvent (Dirks et al., 2016). The extraction stage was conducted by soaking 150 g of Datura (D. metel L.) leaf powder in 3,000 ml n-hexane. Maceration was carried out for 3 days and stirred every 12 hours. The maceration of the n-hexane extract was filtered. The filtrate obtained was concentrated using a rotary evaporator (IKA Germany) until it became a thick extract, and the remaining residue was evaporated in an oven at 45°C for 48 hours. The residue that was evaporated was then macerated again using 3,000 ml ethyl acetate. Maceration was carried out for 3 days and stirred every 12 hours. The result of the maceration of ethyl acetate extract was filtered. The obtained filtrate was concentrated using an evaporator until the extract became thick, and the remaining residue was evaporated in an oven at 45°C for 48 hours. The evaporated residue is macerated. The maceration of the methanol extract was filtered, and the filtrate obtained was concentrated using an evaporator. The concentrated thick extract was weighed afterward (Arivalagan et al., 2018; Dirks et al., 2016).
Phytochemical screening
Alkaloid identification
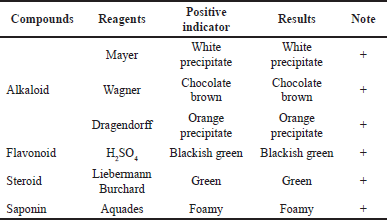
A total of 2 g of the extract was put into a test tube, dripped with HCl 2N, and then divided into several test tubes. Each tube was added with each reagent. If the extract forms a white or yellow precipitate after adding Mayer’s positive reagent, the extract contains alkaloids. The extract contains alkaloids if an orange precipitate is formed after positive Dragendorff’s reagent (De Silva et al., 2017).
Flavonoid identification
Several extracts were put into a test tube; then 10 ml of hot water was added, boiled for 5 minutes, and then filtered. From the filtrate obtained 5 ml was taken and added with magnesium powder and 1 ml of concentrated HCl was taken and added with amyl alcohol; shake the mixture till separation. If an orange, red, or yellow precipitate is formed, it is positive for containing flavonoids (De Silva et al., 2017).
Saponin identification
Several extracts were put into a test tube; then 10 ml of hot water was added and cooled; then they were shaken vigorously for 10 seconds. Being positive for saponins is indicated by forming foam as high as 1–10 cm for not less than 10 minutes, and with the addition of 1 drop of 1% HCl, the foam will be stable (Pandey and Tripathi, 2014).
Steroid and terpenoid identification
Several extracts were macerated with 10 ml ether for 2 hours and then filtered. From the filtrate obtained 5 ml was taken and then evaporated in a cup. To the residue obtained, 2 drops of anhydrous acetic acid and 1 drop of concentrated H2SO4 were added. When we see what is produced, red is positive for steroids, while purple is positive for terpenoids (Pandey and Tripathi, 2014).
Cytotoxic test
To determine the activity of Datura leaves, a cytotoxic test was carried out using the BSLT method. BSLT is one of the initial screening methods for testing cytotoxic compounds (Ramachandran et al., 2011). The first thing to do was to incubate the Artemia salina L. eggs in the A. salina L. egg-hatching vessel for 24 hours of hatching and within 48 hours. Preparation of the standard solution in each extract is done by dissolving 100 mg of the extract in 100 ml of seawater to obtain a 1,000 ppm standard solution. The standard solution was diluted to a series concentration of 10, 20, 40, 80, and 160 ppm. Tests were carried out by inserting 10 larvae of A. salina L. in a test tube containing the extract. After 24 hours, the number of dead larvae was counted and a probit analysis was performed to determine cytotoxic activity (Ramachandran et al., 2011).
Separation of active compound D. metel L. leaves
The sample of Datura (D. metel L.) leaf extract was diluted with methanol for the dotting process. The mobile phase was prepared with a ratio of chloroform:n-hexane (1:1), methanol:ethyl acetate (3:2), and n-hexane:ethyl acetate (2:3). The sample was dotted on the TLC plate and put into the chamber. The purpose of this process is to find the best eluent composition that will be used to separate the compounds contained in the Datura leaf extract in the next TLC process (Gandjar and Rohman, 2012).
Separation by preparative TLC was performed using silica gel F254 with 20 × 20 cm. The plate was activated by heating it in an oven at 100°C for ± 30 minutes to remove any moisture on the plate. The concentrated extract was dissolved in the solvent; then 7 spots were dotted along the lower boundary line using a capillary tube. Then it was eluted using an eluent, which produced the best separation on analytical TLC. Elution was stopped after the developer solution reached the boundary line. The eluted plate was dried, and the stains were observed using a UV lamp with a wavelength of 254 and 366 nm (Ferey et al., 2017; Wang et al., 2012).
Isolate identification
A total of 10 mg of isolates were weighed using analytical scales. Then they were dissolved by adding 10 ml of methanol to obtain a concentration of 1,000 ppm, filtered, and then used as a standard solution. A solution with a concentration of 10 ppm was made by pipetting 50 µl, each of which was put into a 5 ml volumetric flask, and then methanol was added to the marked line. The standard solution was filtered and put into the vial liquid chromatography. Then it was injected into the UPLC-MS QTOF (Waters) system with a mobile phase flow rate of 200 µl/minute; then we viewed the fragmentation spectrum data on the mass spectroscopy data.
The stationary phase for the liquid chromatography mass spectrometer (LCMS) test was a C18 column, and the elution method was isocratic with an acetonitrile:water (15:85 v/v) solvent ratio. An injection volume of 5 microliters and a flow rate of 0.2 ml/minutes were used for the test.
RESULTS
Datura (D. metel L.) leaves were screened for phytochemicals. The results obtained are positive and contain alkaloids, flavonoids, steroids, and saponins.
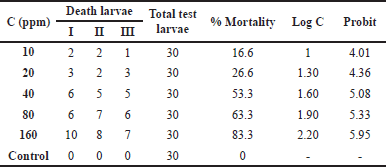
The main solution from the thick methanol extract of Datura leaves (Datura stramonium) was diluted to 5 concentrations, namely, 10, 20, 40, 80, and 160 ppm, to be used in concentration orientation tests. Negative controls were seawater and shrimp larvae without the extracts. Datura (D. metel L.) leaves were tested for cytotoxicity. From the test, we obtained linear results. After that, a concentration orientation test was carried out to obtain the percentage of larval mortality in the range of 10%–90%, the test concentrations were 160, 80, 40, 20, and 10 ppm. This experiment was performed in three repetitions (triple) to obtain more accurate data. Larvae that were 48 hours old were put into vials containing 10 different concentrations each. After 24 hours of extract addition, the larvae mortality was observed. Table 3 shows the results of the cytotoxic test of Datura leaf extract.
Table 4 shows the testing of isolates from Datura (D. metel L.) using LCMS. The result obtained is the presence of a Baimantuoluoline E component in Datura leaves.
Larval mortality is monitored 24 hours after the extract was administered. The above number is put into the equation y = bx + a, where y is the 50% probit value of the death percentage, X is the concentration log, and the antilog X is the lethal concentration (LC50).
Figure 2 shows the outcomes of the TLC-based isolate purification procedure. Scraped off are the primary compound bands that have been projected to be present. To acquire the crystals of the isolate powder, the scraped powder was extracted with methanol, filtered, and evaporated.
 | Table 1. LCMS optimum condition. [Click here to view] |
 | Table 2. Phytochemical screening of Datura metel leaf extract. [Click here to view] |
 | Table 3. Cytotoxicity study of Datura metel leaf extract. [Click here to view] |
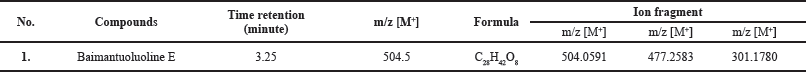 | Table 4. LCMS results of Datura metel isolate. [Click here to view] |
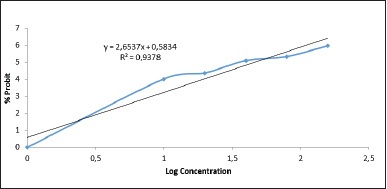 | Figure 1. Linear regression between log concentration of D. metel leaf extract and the % probit of shrimp mortality. [Click here to view] |
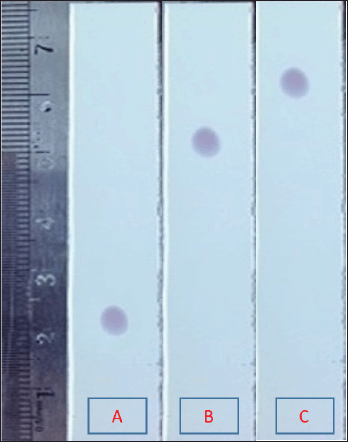 | Figure 2. TLC results of D. metel isolate in different variations of mobile phase methanol:n-hexane. A: 4:1 v/v; B: 1:1 v/v; C: 1:4 v/v. [Click here to view] |
Figure 3 shows one of the semi-polar elutions of the mobile phase of methanol:n-hexane (1:1 v/v) as a result of the isolate purity evaluation process.
Figure 4 shows the chromatograms of the isolates. The sharper the chromatogram peak gets the higher the indication that the compound only consists of one compound.
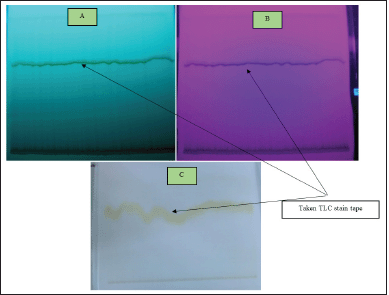 | Figure 3. TLC results of D. metel with mobile phase chloroform:n-hexane (1:1 v/v). A: UV 254; B: UV 366; C: visible wavelength. [Click here to view] |
Figure 5 shows the results of the detection of isolates using a mass spectrometer. The mass spectrometer detector will identify the compound eluted from the LCMS column by ionizing it first and then measuring the mass ratio (m/z) and molecular fragments into small pieces. Furthermore, specific isolates were identified using a mass spectrometer to analyze these isolates’ molecular ions and fragmentation patterns. The results of the detection of isolates using a mass spectrometer are shown in Figure 5.
Figure 6 shows the mass spectrum of Datura (D. metel L.) leaves. The molecular weight of the isolated substance was 504.0591 m/z, and it fragmented into smaller molecules with masses of 477.2583 m/z [M+ - CH3-OH] and 301.1780 m/z [M+ -C9H11O4]. More than 600 Withanolide-related actions have been reported.
DISCUSSION
Research has been carried out on Datura leaves. This research was preceded by an extraction process using 96% methanol. The extract obtained was then used for phytochemical screening tests. Furthermore, the extract’s toxicity test was performed using the BSLT method to perceive its potential toxicity. The isolation process used preparative TLC using the appropriate eluent. The isolates obtained were then identified by the UPLC-MS technique.
Phytochemical screening
About 200 g of Datura leaf samples were extracted by the maceration method using 3,000 ml of methanol as a solvent (Carpa et al., 2017). Maceration was carried out for 6 × 24 hours. The macerated filtrate is evaporated using a rotary evaporator until a thick extract is obtained. The extract was obtained with a weight of 28.19 g and a yield of 14.09% from this stage. The physical properties of the extracts obtained are blackish-green, paste-like, and sticky.
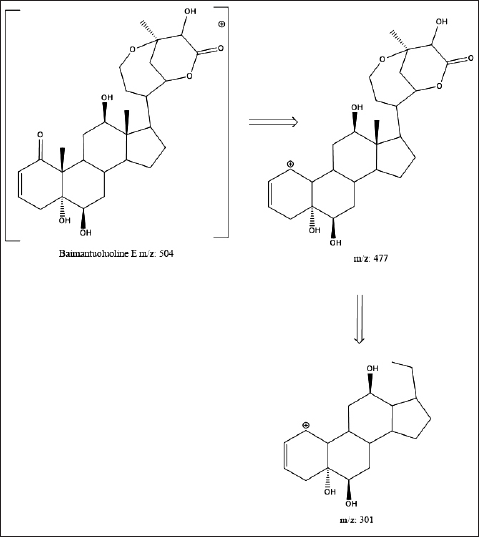 | Figure 4. Fragmentation pattern of D. metel isolate (Xia et al., 2019; Yang et al., 2020). [Click here to view] |
The phytochemical compounds of the thick methanol extract produced from the extraction process were tested, which aims to determine the secondary metabolite compounds present in the sample. The phytochemical test in this study included identifying alkaloids, flavonoids, steroids, saponins, and tannins. Phytochemical test results can be seen in Table 2.
Based on the analysis conducted, samples of Datura leaves tested positive for containing flavonoids. It is indicated by the change in color to blackish green when added with the H2SO4 reagent. There is a change in color because flavonoids are phenolic compounds. Therefore, the color will change when an alkaline solution or ammonia is added (Hossain et al., 2013b, 2013b).
Datura leaf samples tested positive for alkaloid compounds. The deposits’ formation indicates this in the three test tubes after the drops of Mayer, Wagner, and Dragendorff reagents. The reaction with Mayer’s reagent formed a white precipitate, with Wagner’s reagent, it formed a brown precipitate, and with Dragendorff’s reagent, it formed a red-orange precipitate (De Silva et al., 2017). Based on the analysis carried out, the Datura leaves tested positive for containing steroid compounds. The color change can be seen after the Liebermann Burchard reagent addition, namely, green (Pandey and Tripathi, 2014; Tiwari et al., 2012). Based on the analysis, the Datura leaves tested positive for containing saponin compounds indicated by foam formation after the shaking process. The formation of foam indicates glycosides, which can form foam in the water (De Silva et al., 2017; Tiwari et al., 2012).
The same results were obtained by Dhawan and Gupta (2016), who carried out a phytochemical screening of Datura leaves. The results showed that the methanol extract of Datura leaves contains flavonoids, alkaloids, steroids, and saponins. The study also reported that the ethyl acetate and n-hexane extracts positively contained flavonoids, alkaloids, steroids, and saponins. Alabri et al. (2014) found that the methanol extract of Datura leaves contains alkaloids, flavonoids, and saponins but is negative in steroids. Many factors can influence these different results. Various factors cause secondary metabolite variability of the same species, including physiological variations, environmental conditions, geographical variations, and genetic and evolutionary factors (Figueiredo et al., 2008). Given that the samples obtained also show different geographic locawtions and environments, there are several studies that state that the influence of the environment, climate, and land affects the production of secondary metabolites in plants and in producing food reserves (Deduke et al., 2012; Sunic et al., 2021).
Secondary metabolites’ content plays a role in providing antioxidant effects but through different biological mechanisms (Hossain and Nagooru, 2011; Suresh and Nagarajan, 2009). Most of the secondary metabolite components that have been isolated from Datura plants that show biological activity are extracts dissolved with polar solvents (Alabri et al., 2014; Gonzalez-Guevara et al., 2004). Several research results on the flavonoid group show high potential biological activity as antioxidant, anti-inflammatory, anti-microbial, anti-cancer, and anti-allergic reactions (Anyasor et al., 2010; Chao et al., 2002; Igbinosa et al., 2009; Thitilertdecha et al., 2008). Saponins are secondary metabolites that play a role in the plant defense system. Therefore, saponins show anti-microbial activity (Ayoola et al., 2008; Banso and Adeyemo, 2006). Phenolic compounds such as tannins and their derivatives are considered compounds that act as antioxidants or free radical scavengers (Akharaiyi, 2021; Sekar et al., 2012; Vadlapudi and Kaladhar, 2012).
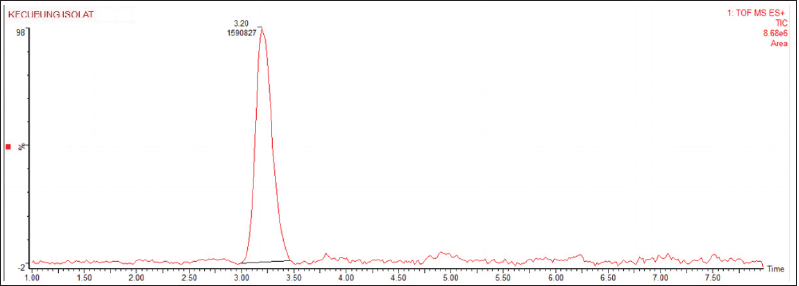 | Figure 5. Peak chromatogram of D. metel isolate. [Click here to view] |
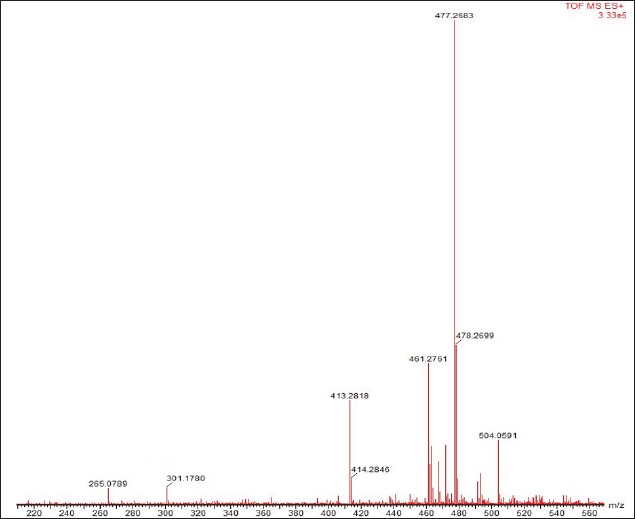 | Figure 6. Mass spectrum of D. metel isolate. [Click here to view] |
BSLT cytotoxicity test
Cytotoxicity test was used to determine seawater and other larval mortality factors (Ramachandran et al., 2011). Thus, it can be ascertained that the addition of the extract only causes the death of larvae. The mortality results of A. salina Leach larvae in each vial were compared, including negative control vials. The total larvae used in each concentration with 3 repetitions of the experiment were 30 individuals. Therefore, the total number of larvae used in the entire experiment was 180. The percentage of mortality is obtained by multiplying the average mortality rate for larvae by 100%. The use of A. salina larvae in this BSLT test is because shrimp larvae have an affinity with mammals, such as having a DNA-dependent RNA polymerase as that of mammals (Bagheri et al., 2010).
The eggs of A. salina Leach used were shrimp larvae that were 48 hours old. At the age of 24 hours, the new larvae will enter the first instar phase, where the larvae cannot eat because their mouth and digestive tract have not been fully formed yet. Meanwhile, at the age of 36–48 hours after hatching, the larvae will metamorphose into a second instar, where the larvae will already have a perfect mouth and digestive system. Thus, the larval environment extract enters the larvae’s bodies and causes death in the larvae (Chanda and Baravalia, 2011). Based on standard criteria, larvae are said to be dead if they do not move for 10 seconds of observation. Observation of larval mortality is carried out after 24 hours after giving the extract (Bagheri et al., 2010). The value above is entered into the straight-line equation y = bx + a, where the y value is the 50% probit value of the death percentage, X is the concentration log, and the antilog X is LC50 (Fig. 1).
The results of the toxicity test of the Datura leaf extract are shown in Figure 1. It can be concluded that the higher the concentration of an extract is, the higher the mortality rate of the larvae is. Calculate the LC50 using the straight-line equation Y = 2.6537X + 0.5833; then input the number 5 in the Y value to obtain LC50 46.1636 µg/ml. An extract indicates a strong cytotoxic presence and is further analyzed if the LC50 value is <100 ppm. Calculation using this method produces LC50, which is included in the toxic category. It indicates that Datura leaf extract has a very cytotoxic ability and should be further analyzed (Chanda and Baravalia, 2011; Ramachandran et al., 2011).
The biological response of plants does not come from one component but a mixture of various bioactive components from plants (Baravalia et al., 2012). The BSLT test results showed a correlation with the results of the phytochemical screening test. Phytochemical screening showed the presence of flavonoids, alkaloids, steroids, and saponins. Flavonoids and alkaloids are thought to form a complementary effect to kill shrimp larvae (Alabri et al., 2014; Subhadra et al., 2011). They work by acting as stomach poisoning. Therefore, when these compounds enter the larvae’s bodies, their digestive organs will be disturbed. This compound will inhibit the taste receptors in the larvae’s mouths. This results in the fact that the larvae fail to get a taste stimulus; thus they are unable to recognize their food, and as a result, the larvae die out of starvation (De Padua et al., 1999; Djali et al., Khairunnisa et al., 2018). Meanwhile, other bioactive compounds that cause shrimp larvae’s death are steroids. Steroids and saponins in plants are toxic to insects, bacteria, and fungi and can be used as drugs to prohibit tumor cell growth in plants and animals (Nugrahaningsih et al., 2019; Rohmawati and Sutoyo, 2018).
BSLT is very sensitive because the Artemia larvae have fragile skin, making it easy for the solution to diffuse. Furthermore, the rapid growth of Artemia larvae resembles cancer cells’ growth, making it easier for researchers to detect changes in biological responses (Mirzaei and Mirzaei, 2013; Thangapandi and Pushpanathan, 2014). Several previous studies have concluded that there is a direct relationship between toxic activity in BSLT and antiproliferative effects (Handayani et al., 2018; Sandrawati et al., 2019; Suzery and Cahyono, 2014). BSLT has a synergistic correlation with cytotoxic activity in some solid human tumors and pesticide activity. It has led to the discovery of a new class of natural pesticides and active antitumor agents (Chanda and Baravalia, 2011). Therefore, it might be suggested that the BSLT is an inexpensive, easy-to-master, and suitable preliminary test for predicting cytotoxic activity (McLaughlin et al., 1998).
Separation and identification of compounds
The fractionation of the compound components in the chloroform extract of Datura leaves began with determining the eluent (mobile phase) through TLC. The stationary phase used was the G60 F254 silica plate. Observations of the stains on the TLC plate were carried out using 254 and 366 nm UV lamps. The use of UV 254 lamps will cause fluorescent silica gel. It distinguishes it from silica gel, which binds to the compound (stain) and will appear blurry and differently clear (Fried, 2017). The search for the mobile phase (eluent) begins with a single eluent and then a combination of eluents to get the best separation shown through the separation stage between spots. TLC’s mobile phase optimization is carried out until a suitable solvent is obtained based on a trial and error system (Coskun, 2016). The result is a combination of 2 solvents, namely, chloroform:n-hexane with the ratio of 1: 1 v/v. The combination of these solvents produces spots that are relatively far apart. Thus, it is used as an eluent in preparative TLC. From the calculation results, the Rf stain value is 0.61. The Rf value is still vulnerable to the recommended Rf value of 0.2–0.8 (Gandjar and Rohman, 2012).
The isolation process used TLC with a stationary phase of silica gel G60 F254 using a mobile phase of chlorophyll:n-hexane (1:1 v/v). The elution result consists of one dominant band and is separated from the other bands that are far apart to facilitate the scraping process. The results were observed under 254 and 366 nm UV lamps. The visible band at UV 254 nm is due to the interaction between UV rays and the plate’s indicator, namely, silica gel F254 (Spangenberg et al., 2011). The plate will glow in UV light 254, while the stained area will cover the plate’s light. Thus, the stain can be seen (Fried, 2017), whereas at a wavelength of UV 366, it will show the band’s fluorosis and indicate the presence of a conjugated double bond (Ferey et al., 2017; Wang et al., 2012).
The results of the TLC scraped off were then refined using the same method. The isolates produced yellowish stains after being sprayed with cerium sulfate (Fig. 1). Cerium sulfate functions as a chelating agent. Thus, stains can appear in visible light (Harbone, 2001; Pandey and Tripathi, 2014; Wall, 2005). The main compound bands which have been predicted to be located are scraped off. The scraped powder was extracted with methanol, filtered, and then evaporated to obtain the isolate powder’s crystals. Figure 2 shows the results of the isolate purification process by performing the TLC.
To confirm the purity of the isolates, an evaluation was carried out using TLC (Coskun, 2016). The isolates obtained were eluted with three different types of eluents: polar, semi-polar, and non-polar. Figure 3 shows one of the semi-polar elutions of the mobile phase of methanol:n-hexane (1: 1 v/v) as a result of the isolate purity evaluation process.
The TLC technique was used to confirm the purity of the isolates, using eluent with 3 different variations, namely, polar, semi-polar, and non-polar with variations in the polar mobile phase of methanol:n-hexane (4: 1 v/v), semi-polar methanol:n-hexane (1: 1 v/v), and non-polar methanol:n-hexane (1: 4 v/v). The elution evaluation results of the isolates’ purity showed that only one stain appeared in the three eluent concentrations. It indicates that the isolates obtained contain only one compound based on the TLC method (Coskun, 2016). Isolates are said to be pure should they are eluted using three variations of polar, semi-polar, and non-polar mobile phases, showing the consistency of only having one spot (Bajpai et al., 2016). The stains formed also showed that the polar eluent Rf was below 0.28, semi-polar Rf was in the middle of 0.61, and non-polar Rf was above 0.85. It occurs due to the eluent’s influence, where the polar eluent will cause the isolate not to be eluted upward because hydrogen bonds are formed between the isolate and silanol on the TLC plate. When the eluent’s polarity is reduced, the dimer interaction between the isolate and the TLC plate is reduced, causing the isolate to be more susceptible to Rf (Marston, 2011; Rossing and Chiaverina, 2000; Waksmundzka-Hajnos et al., 2008).
Wasnik et al. (2009) identified Withanolide using the TLC which documented the Rf. The approximate Withanolide was 0.61 with methanol:n-hexane (50:50%) as eluent. The spray reagent used to identify phytosterol groups in plants uses cerium sulfate. The presence of a steroid group from the alcohol group is indicated by a yellow to brown stain in visible light and a blue fluorescence on UV light 366 nm (Harbone, 2001; Sarker and Nahar, 2012). Based on the isolate chromatogram results of the spray, the cerium sulfate reagent shows a yellow-brown color. It indicates the presence of phytosterol compounds. The color that occurs is due to a substituted hydroxyl group on the steroid ring with a positively charged Ce (Harbone, 2001; Rossing and Chiaverina, 2000).
Isolate identification using LCMS
The initial step before carrying out the LCMS analysis is to optimize the instrument and its mobile phase. The purpose of optimizing the LCMS tool is to see the most suitable conditions for analyzing paracetamol in herbal samples. Based on the LCMS conditions’ optimization results (Table 1), the optimal conditions for analyzing paracetamol are best obtained by using an isocratic elution system. According to Yu et al. (2016), in the isocratic elution system, the mobile phase used is regulated in a constant concentration that is pumped into the column. This study’s type of column is the type of octadecyl silica column (ODS or C18) with the silica gel component in it. This C18 column has a reversed-phase column and can produce the best separation with high levels of purity and accuracy (Jian, 2017).
In this analysis, an isolate chromatogram was obtained, which showed a sharp peak at the retention time (tR) of 3.20 minutes. According to Termopoli et al. (2019), the use of LCMS in the qualitative analysis was carried out by looking at the chromatogram peaks. The sharper chromatogram peak indicates that the compound only consists of one compound. The chromatograms of the isolates are shown in Figure 4.
Retention time (tR) is the time required for the analyte, which starts during the injection process until the column’s separation process. The separation response will be sent in the form of a signal read by the detector. The small peaks formed around it indicate that impurities are present (Niessen and Correa, 2017). These impurities usually come from solvents or during the sample preparation process (Termopoli et al., 2019). However, the presence of impurities did not have a significant effect on the peaks of the isolates. It can be seen from the chromatogram peaks that are relatively far apart.
Table 4 shows the detected molecular weight and fragmentation patterns. The isolate is assumed to be a Baimantuoluoline D (m/z 504) compound, a steroid derivative from the Withanolide group (Xia et al., 2019). The Withanolide family has been detected in more than 600 different plants (Misico et al., 2011). Withanolide is formed from the Ergostane skeleton with side-chain modifications in the δ-lactone ring substituted on the C22 and C26 carbon chains (Guo et al., 2018). Withanolide derivatives have been known to have anti-inflammatory, antitumor, cytotoxic, and immunomodulatory activities (Wu et al., 2020; Xu et al., 2018). The isolate’s molecular weight is known to be [M +] 504.0591 m/z, with a base peak of 477.2583 m/z. 477.2583 m/z [M + -CH3-OH] and 301.1780 m/z [M + -C9H11O4] daughter fragments were produced (Fig. 4). At the 477.2583 m/z fragments, there was a breakdown of hydroxy at Ring A of C1 carbon and methyl at C19. The termination of the hydroxy bonds is due to non-bonding bonds on the C1 carbon; thus it is easier to be released. Meanwhile, the effect of Lewis acid on the proton of the neighboring hydroxy group (C1) is to form hydrogen bonds, which result in the instability of C18 carbon. The formation of the 301.1780 m/z fragment is more due to the instability of the δ-lactone ring in the Ergostane framework due to epoxy in carbon substitution C22 C26 (Niessen and Correa, 2017; Yang et al., 2020).
The mass spectrum is an accurate, valid, and decisive identification because it can directly identify the structure of an unknown compound in a complex mixture even with a minimal concentration (Evard et al., 2016). Based on the mass spectra data obtained, the isolates analyzed showed 504, 427, and 301 m/z. This pattern is in line with that obtained by Yang et al. (2020), who confirmed that one of the secondary metabolites in Datura leaves is Baimantuoluoline D with a molecular weight of 504.5 m/z with ion fragments forming 504,477.301 m/z (Niessen and Correa, 2017; Yang et al., 2020). This fragmentation pattern confirms that the isolate is a Baimantuoluoline D.
The fragmentation pattern that is formed is also not abundant because the ionizer system used is electron spray ionization. The electron ionization (EI) system uses the Bombardment technique with a potential energy of 70 eV, which causes many fragments to be formed (Wei et al., 2019). In the ESI system using electrospraying technology, the isolate’s molecular ion is obtained by evaporation, where the charged liquid particles are reduced in size and the electric charge becomes closer together. The reduction in grain size due to evaporation continues to occur, to the point where the coulombic repulsive force overcomes and opposes the granules’ cohesive force, resulting in the desolvation or breakdown of the solvent (Banerjee and Mazumdar, 2012; Schröder, 2012). The sample in the granules will be released/desorbed out in the form of [M + H] + or [M-H]−. The formed fragments are not as many as in the EI system because the potential difference given is only 3–5 eV (Chen et al., 2011).
Research conducted by Yang et al. (2020) managed to find 85 Withanolide isolates. One of them is the Datura mentaline compound found in all parts of plants. Datura foliside compounds are found in all parts of the plant except in the seeds. Literature studies show the leaves’ antifungal potential, especially related to the Withanolide content in Datura leaves (Chukunda et al., 2019; Dabur et al., 2004a, 2004b). Antifungal activity in Datura is found in leaves and fruit, while other parts are of very low activity. Roots are generally less active than leaves, fruit, and stems (Javaid and Saddique, 2012). Geographical differences significantly affect the existence of secondary metabolites in plant organs (Al-Snafi, 2017).
Apart from Datura, Withanolide is also found in the roots of Withania somnifera, which is a well-known ginseng from India (Misra et al., 2008; Trivedi et al., 2017). Several studies also show the Withanolide groups such as β-hydroxy-2,3-dihydro-withanolide F, Withanolide A, Withaferin A, Withanolide D, Ixocarpalactone A, Withanolide S, and Thiowithanolide (Chatterjee et al., 2010; Trivedi et al., 2017). Besides, it shows that isolates from Withania somnifera have various pharmacological activities including antioxidant, anti-cancer, immunomodulatory, hepatoprotective, neuroprotective, anti-inflammatory, anti-microbial, hypoglycemic effects (Budhiraja et al., 2000; Chen et al., 2011; Gorelick et al., 2015; Singh et al., 2010).
CONCLUSION
The phytochemical screening results showed that the Datura leaves contained alkaloids, flavonoids, steroids, and saponins. The cytotoxic activity of Datura leaf extract obtained LC50 46.1636 µg/ml. The isolate obtained from Datura leaves is Baimantuoluoline D, a steroid base framework classified as a steroid derivative compound from the Withanolide group. This research helps to find a way to isolate the Withanolide group compounds, one of which is Baimantuoluoline D. Furthermore, activity tests are needed on Baimantuoluoline D isolates to develop medicinal compounds and to perceive their usefulness.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no financial support from the external subject. This study is supported by our own finance.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akharaiyi F. Antibacterial, phytochemical and antioxidant activities of Datura metel. Int J PharmTech Res, 2021; 3(1):478–83.
Alabri THA, Al Musalami AHS, Hossain MA, Weli AM, Al-Riyami Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J King Saud Univ Sci, 2014; 26(3):237–43.
Al-Snafi A. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium-a review. IOSR J Pharm, 2017; 7(2):43–58.
Antony VS, Silky VI, Raji P, SaiPriya C, Jenifer Selvarani A. Bioactivity studies of Datura metel, Aegle marmelos, Annona reficulata and Saraca indica and their green synthesized silver nanoparticle. J Pure Appl Microbiol, 2019; 13(1):329–38.
Anyasor GN, Ogunwenmo O, Oyelana OA, Akpofunure BE. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae). Afr J Biotechnol, 2010; 9(31):4880–4.
Arivalagan M, Roy TK, Yasmeen AM, Pavithra KC, Jwala PN, Shivasankara KS, Kanade SR. Extraction of phenolic compounds with antioxidant potential from coconut (Cocos nucifera L.) testa and identification of phenolic acids and flavonoids using UPLC coupled with TQD-MS/MS. LWT, 2018; 92:116–26.
Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, Atangbayila TO. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res, 2008; 7(3):1019–24.
Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol, 2010; 48(3):242–6.
Bajpai V, Majumder R, Park J. Isolation and purification of plant secondary metabolites using column-chromatographic technique. Bangladesh J Pharmacol, 2016; 11(4):844–8.
Banerjee S, Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int J Anal Chem, 2012; 2012:1–41.
Banso A, Adeyemo S. Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monifera and Datura stramonium. Biokemistri, 2006; 18(1):39–44.
Baravalia Y, Vaghasiya Y, Chanda S. Brine shrimp cytotoxicity, anti-inflammatory and analgesic properties of Woodfordia fruticosa Kurz flowers. Iran J Pharm Res, 2012; 11(3):851–61.
Bellila A, Tremblay C, Pichette A, Marzouk B, Mshvildadze V, Lavoie S, Legault J. Cytotoxic activity of withanolides isolated from Tunisian Datura metel L.’ Phytochemistry, 2011; 72(16):2031–6.
Budhiraja R, Krishan P, Sudhir S. Biological activity of withanolides. J Sci Ind Res, 2000; 59(11):904–11.
Carpa R, Dumitru DV, Burtescu RF, Maior MC, Dobrot? C, Olah NK. Bio-chemical analysis of Datura stramonium extrac. Studia Univ Babes-Bolyai Biologia, 2017; 62(2):5–19.
Chanda S, Baravalia Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrima aerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat Prod Res, 2011; 25(20):1955–64.
Chao P, Hsiu S, Hou Y. Flavonoids in herbs: biological fates and potential interactions with xenobiotics’. J Food Drug Anal, 2002; 10(4):219–27.
Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, Tuli R. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry, 2010; 71:1085–94.
Chen LX, He H, Qiu F. Natural withanolides: an overview’. Nat Prod Rep, 2011; 28(4):705–40.
Chukunda F, Baraka R, Umoren G. In-vitro evaluation of Datura metel leaves for potential antifungal activity against Lasiodiplodia theobromae Pat’. Microbiol Res Int, 2019; 7(2):10–6.
Coskun O. Separation techniques: chromatography’. North Clin Istanb, 2016; 3(2):156–60.
Dabur R, Ali M, Singh H, Gupta J, Sharma GL. A novel antifungal pyrrole derivative from Datura metel leaves’. Die Pharmazie Int J Pharm Sci, 2004a; 59(7):568–70.
Dabur R, Singh H, Chhillar AK, Ali M, Sharma GL. Antifungal potential of Indian medicinal plants. Fitoterapia, 2004b; 75(3–4):389–91.
Deduke C, Timsina B, Piercey-Normore MD. Effect of environmental change on secondary metabolite production in lichen-forming fungi. In: Young SS, Silvern SE (Eds.). International perspectives on global environmental change. IntechOpen, London, UK, pp 197–230, 2012.
De Silva KKH, Huang HH, Joshi RK, Yoshimura M. Chemical reduction of graphene oxide using green reductants’. Carbon, 2017; 119:190–9.
De Padua LS, Bunyapraphatsara N, Lemmens R. Medicinal and poisonous plant. Plant resources of South-East Asia. Backhuys Publishers, Leiden, The Netherlands, vol. 12, no. 1, 1999.
Dhawan D, Gupta J. Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int J Biol Chem, 2016; 11:17–22.
Dirks NF, Martens F, Vanderschueren D, Billen J, Pauwels S, Ackermans MT, Endert E, den Heijer M, Blankenstein MA, Heijboer AC. Determination of human reference values for serum total 1, 25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC–MS/MS method’. J Steroid Biochem Mol Biol, 2016; 164:127–33.
Djali M, Setiasih IS, Rindiantika TS. AJAB. Asian J Agri Biol, 2018; 6(1):103–14.
Evard H, Kruve A, Leito I. Tutorial on estimating the limit of detection using LC-MS analysis, part I: theoretical review. Anal Chim Acta, 2016; 942:23–39.
Ferey J, Da Silva D, Lafite P, Daniellou R, Maunit B. TLC-UV hyphenated with MALDI-TOFMS for the screening of invertase substrates in plant extracts. Talanta, 2017; 170:419–24.
Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJ. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J, 2008; 23(4):213–26.
Fried B. Thin-Layer chromatography in the study of entomology. In: Richard S (ed.). Practical thin-layer chromatography, CRC Press, London, UK, pp 71–104, 2017.
Gaire B, Subedi L. A review on the pharmacological and toxicological aspects of Datura stramonium L. J Integr Med, 2013; 11(2):73–9.
Gandjar I, Rohman A. Analisis obat secara spektrofotometri dan kromatografi. Pustaka Pelajar, Yogyakarta, Indonesia, 2012.
Gonzalez-Guevara JL, Gonzalez-Lavaut JA, Pino-Rodriguez S, Garcia-Torres M, Carballo-Gonzalez MT, Echemendia-Arana OA, Molina-Torres J, Prieto-Gonzalez S. Phytochemical screening and in vitro antiherpetic activity of four Erythtroxylum species. Acta Farm Bonaer, 2004; 23(4):506–9.
Gorelick J, Rosenberg R, Smotrich A, Hanuš L, Bernstein N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry, 2015; 116:283–9.
Guo R, Liu Y, Xu ZP, Xia YG, Yang BY, Kuang HX. Withanolides from the leaves of Datura metel L. Phytochemistry, 2018; 155:136–46.
Handayani D, Rasyid W, Zainudin EN, Hertiani T. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: Hela, WiDr, T47D and Vero 055-058. J Appl Pharm Sci, 2018; 8(1):55–8.
Harbone J. Phytochernical methods. Chapman and Hall Ltd, London, UK, 2001.
Hossain MA, AL-Raqmi KAS, Al-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris’. Asian Pac J Trop Biomed, 2013a; 3(9):705–10.
Hossain MA, ALsabari KM, Weli AM, Al-Riyami Q. Gas chromatography–mass spectrometry analysis and total phenolic contents of various crude extracts from the fruits of Datura metel L. J Taibah Univ Sci, 2013b; 7(4):209–15.
Hossain MA, Nagooru MR. Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacogn J, 2011; 3(24):25–30.
Igbinosa O, Igbinosa E, Aiyegoro O. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). Afr J Pharm Pharmacol, 2009; 3(2):58–62.
Iranbakhsh A, Oshagi MA, Majd A. Distribution of atropine and scopolamine in different organs and stages of development in Datura stramonium L. (Solanaceae). Structure and ultrastructure of biosynthesizing cells. Acta Biol Crac Ser Bot, 2006; 48:13–8.
Javaid A, Saddique A. Control of charcoal rot fungus Macrophomina phaseolina by extracts of Datura metel. Nat Prod Res, 2012; 26(18):1715–20.
Jian W. Modern liquid chromatography and mass spectrometry for targeted biomarker quantitation. Wiley Online Books, Hoboken, NY, pp 45–63, 2017.
Khairunnisa K, Nahzi M, Diana S. Toxicity test of Dayak onion bulbs extract (Eleuthherine palmifolia (L) Merr) on Artemia Salina BSLT method (Preface study as root canal irrigation materials). Dentino, 2018; 3(1):91–5.
Marston A. Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A, 2011; 1218(19):2676–83.
McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals’. Drug Inf J, 1998; 32(2):513–524.
Mirzaei M, Mirzaei A. Comparison of the Artemia salina and Artemia uramiana bioassays for toxicity of 4 Iranian medicinal plants. Int Res J Biol Sci, 2013; 2(3):49–54.
Misico RI, Nicotra VE, Oberti JC, Barboza G, Gil RR, Burton G. Withanolides and related steroids’. In: Kinghorn A, Falk H, Kobayashi J (eds.). Progress in the chemistry of organic natural products, Springer, Vienna, Austria, pp 127–229, 2011.
Misra L, Mishra P, Pandey A, Sangwan RS, Sangwan NS, Tuli R. Withanolides from Withania somnifera roots. Phytochemistry, 2008; 69(4):1000–4.
Niessen W, Correa C. Interpretation of MS-MS mass spectra of drugs and pesticides. John Wiley & Sons, Hoboken, NJ, 2017.
Nugrahaningsih WH, Titi A, Dewi NK. Acute toxicity of papaya leaf extract on Artemia salina leach larvae. J Phys Conf Ser, 2019; 1321:32033.
Okwu D, Igara E. Isolation, characterization and antibacterial activity of alkaloid from Datura metel Linn leaves. Afr J Pharm Pharmacol, 2009; 3(5):277–81.
Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem, 2014; 2(5):115–9.
Ramachandran S, Vamsikrishna M, Gowthami KV, Heera B, Dhanaraju MD. Assessment of cytotoxic activity of Agave cantula using brine shrimp (Artemia salina) lethality bioassay. Asian J Sci Res, 2011; 4(1):90–4.
Rohmawati D, Sutoyo S. Steroid isolated from the dichlorometane extract of matoa’s stem bark (Pometia pinnata) and toxicity tests against Artemia salina Leach. Adv Eng Res, 2018; 171:103–105.
Rossing TD, Chiaverina CJ. Resource letter TLC-1: teaching light and color’. Am J Phys, 2000; 68(10):881–7.
Sandrawati N, Pariatno R, Suharti N, Handayani D. In vitro cytotoxic activity assay of bacteria extract derived marine sponge Haliclona fascigera toward Hela, WiDr, T47D, and Vero cell line ARTICLE INFO. J Appl Pharm Sci, 2019; 9:66–70.
Sarker S, Nahar L. Steroid dimers: chemistry and applications in drug design and delivery. John Wiley & Sons, Hoboken, NJ, 2012.
Schröder D. Applications of electrospray ionization mass spectrometry in mechanistic studies and catalysis research. Acc Chem Res, 2012; 45(9):1521–32.
Sekar D, Kolanjinathan K, Saranraj P, Gajendiran K. Screening of Phyllanthus amarus, Acalypha indica and Datura metel for its antimicrobial activity against selected pathogens. Int J Pharm Biol Arch, 2012; 3(5):1231–6.
Singh A, Duggal S, Singh H, Singh J, Katekhaye S. Withanolides: phytoconstituents with significant pharmacological activities. Int J Green Pharm, 2010; 4(4):229–37.
Spangenberg B, Poole C, Weins C. Quantitative thin-layer chromatography: a practical survey. Springer, Berlin, Germany, 2011.
Subhadra S, Kanacharalapalli V, Ravindran V, Parre S, Chintala S, Thatipally R. Comparative toxicity assessment of three Tephrosia species on Artemia salina and animal cell lines’. J Nat Pharm, 2011; 2(3):143–8.
Sunic K, Kovac T, Loncaric A, Babic J, Sulyok M, Krska R, Spanic V. Fusarium secondary metabolite content in naturally produced and artificially provoked fhb pressure in winter wheat. Agronomy, 2021; 11(11):2239.
Suresh S, Nagarajan N. Preliminary phytochemical and antimicrobial activity analysis of Begonia malabarica Lam. J Basic Appl Biol, 2009; 3(1–2):59–61.
Suzery M, Cahyono B. Evaluation of cytotoxicity effect of Hyptis pectinata poit (Lamiaceae) extracts using BSLT and MTT methods. J Sains Dan Mat, 2014; 3(4)
Termopoli V, Famiglini G, Palma P, Piergiovanni M, Rocio-Bautista P, Ottaviani MF, Cappiello A, Saeed M, Perry S. Evaluation of a liquid electron ionization liquid chromatography–mass spectrometry interface. J Chromatogr A, 2019; 1591:120–30.
Thangapandi V, Pushpanathan T. Comparison of the Artemia salina and Artemia fransiscana bioassays for toxicity of Indian medicinal plants. J Coastal Life Med, 2014; 2:453–7.
Thitilertdecha N, Teerawutgulrag A, Rakariyatham N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT Food Sci Technol, 2008; 41(10):2029–35.
Tiwari S, Sirohi B, Shukla A, Bigoniya P. Phytochemical screening and diuretic activity of Allium sativum steroidal and triterpenoid saponin fraction. Int J Pharm Sci Res, 2012; 3(9):33–54.
Trivedi M, Branton A, Trivedi D, Nayak G, Plikerd WD, Surguy PL, Kock RJ, Piedad RB, Callas RP, Ansari SA, Barrett SL, Friedman S, Christie SL, Liu SC, Starling SE, Jones S, Allen SM, Wasmus SK, Benczik TA, Slade TC, Orban T, Vannes VL, Schlosser VM, Albino YSY, Sethi KK, Panda P, Jana S. Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania somnifera (Ashwagandha) root extract. Eur J Biophys, 2017; 5:38–47.
Vadlapudi V, Kaladhar DSVGK. Antimicrobial study of plant extracts of Datura metel L. against some important disease causing pathogens. Asian Pac J Trop Dis, 2012; 2:S94–7.
Waksmundzka-Hajnos M, Sherma J, Kowalska T. Thin layer chromatography in phytochemistry. CRC Press, London, UK, 2008.
Wall PE. Thin-layer chromatography. Royal Society of Chemistry, London, UK, 2005.
Wang J, Yue YD, Tang F, Sun J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa. textilis McClure’. Molecules (Basel, Switzerland), 2012; 17(10):12297–311.
Wasnik NG, Muthusamy M, Chellappan S, Vaidhyanathan V, Pulla R, Senthil K, Yang DC. Establishment of in vitro root cultures and analysis of secondary metabolites in Indian ginseng—Withania somnifera. Korean J Plant Res, 2009; 2(6):584–91.
Wei JN, Belanger D, Adams RP, Sculley D. Rapid prediction of electron–ionization mass spectrometry using neural networks’. ACS Cent Sci, 2019; 5(4):700–8.
Wu J, Zhang T, Yu M, Jia H, Zhang H, Xu Q, Zou Z. Anti-inflammatory withanolides from Physalis minima. ACS Omega, 2020; 5(21):12148–53.
Xia C, Liu Y, Qi H, Niu L, Zhu Y, Lu W, & Wang Q. Characterization of the metabolic fate of Datura metel seed extract and its main constituents in rats. Front Pharmacol, 2019; 10:571.
Xu YM, Wijeratne EK, Brooks AD, Tewary P, Xuan LJ, Wang WQ, Gunatilaka AL. Cytotoxic and other withanolides from aeroponically grown Physalis philadelphica. Phytochemistry, 2018; 152 :174–81.
Yang BY, Guo R, Li T, Wu JJ, Zhang J, Liu Y, Kuang HX. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids, 2014; 87:26–34.
Yang SH, Liu Y, Wang Q, Sun YP, Guan W, Liu Y, Kuang HX. UPLC-MS/MS identification and quantification of withanolides from six parts of the medicinal plant Datura Metel L. Molecules (Basel, Switzerland), 2020; 25(6):1260.
Yu K, Powell M, Maziarz M, Patel DN. Analysis of an adulterated herbal medicinal product using ultra-performance liquid chromatography coupled with QTOF mass spectrometry. World J Tradit Chin Med, 2016; 3(1):1–13.