INTRODUCTION
Exopolysaccharides (EPS), a class of useful polysaccharides that bacteria produce and exude into the environment, are made up of homopolymers or heteropolymers with a range of molecular weights (Moradali and Rehm, 2020). EPS from bacteria have been the subject of growing investigation in recent years due to the widespread use of natural polymers in various sectors. Microbial EPS offers promising potential medical applications, particularly as a source of cancer treatment, given that existing medicines include adverse effects and drug resistance (Abdelhamid et al., 2020; Li et al., 2021). These EPS have numerous recognized bioactivities, including antioxidant, antimicrobial, anticancer, antiviral, prebiotic, and immunomodulatory properties (Guo et al., 2022).
The biotechnological benefits of microbial polysaccharides include a quick fermentation process, form stable emulsions, often exhibit effective anti-cancer characteristics with fewer side effects. The EPS derived from Bacillus subtilis xztubd1 isolated from house fly showed antioxidant, and cytotoxic activity against HeLa cells (Zhang and Yi, 2022). The antiproliferative effects of EPS can be attributed to its heterogeneous monosaccharide composition and sulfur and uronic acids they contain (Angelin and Kavitha, 2020). A recent finding reported that a mannose containing EPS from novel Bifidobacterium breve lw01 induced caspase 3, and PARP-mediated apoptosis in head and neck cancer cell lines (Wang et al., 2019).
The microbiome contains a wide variety of microorganisms that are found in various human organs (the digestive system, the respiratory system, the reproductive system, and so on), and have an important effect on human health. Microbiomes are considered game changers in cancer research, as the metabolite produced by the tumor microbiome can readily reach into the tissues and cells and thus modifying cancer and immune cell signaling pathways (Zhou et al., 2022). Targeting the metabolism of the microbiome could offer a fresh perspective not just on the evolution of cancer, but also on the creation of new therapeutic methods for a wide variety of cancers, including ovarian cancer (Cheng et al., 2020). The most prevalent types of microbiota in both humans and animals are Firmicutes and Bacteroidetes, which are also known to produce and excrete polysaccharides (Oerlemans et al., 2021). Recent microbiome studies have reported the presence of Bacillus cereus from the ovaries of broiler hens (Mohanta et al., 2017), Firmicutes and Bacteroidetes from the healthy lungs (Paudel et al., 2020), and bacteria from different organs (liver, lung, spleen, brain, and kidney) of rats (Ayyal et al., 2019). In support of the notion that ovaries have their unique microbiota, a different investigation compared and revealed the presence of microflora in the ovaries of women with and without epithelial ovarian carcinoma (Brewster et al., 2022). In a previous study, we isolated five bacillus strains from the ovaries of 8-week-old Swiss albino mice. Among the isolates, an EPS-producing strain was identified as Bacillus velezensis OM03 through 16S rDNA sequencing (GenBank accession number MW882947) (Chirakkara and Abraham, 2022). In this study, the synthesis of EPS was optimized, and its structural features were characterized using Fourier Transform Infra-Red (FTIR), X-ray diffraction (XRD), and Gas chromatography-Mass (GC-MS).. The anticancer potential of EPS from OM03 was then evaluated toward human ovarian carcinoma cell lines such as PA-1 and SKOV-3.
MATERIALS AND METHODS
Bacterial strain and production conditions for EPS
For the mass production of EPS, the B. velezensis OM03 strain isolated from the ovaries of an 8-week-old Swiss albino mouse was utilized. The submerged fermentation and optimization of culture conditions for EPS formation were conducted with modifications to the approved procedure (Gangalla et al., 2021). Briefly, OM03 was cultured in the EPS Basal (EPSB) medium ((NH4)2SO4 1.5 g, sodium chloride (2.5 g), K2HPO4 2.5 g, MgSO4 0.3 g, yeast extract 2.0 g pH 7.5 per liter). The EPS production process was carried out in a rotary shaker incubator at 150 rpm at 40°C.
Optimization of EPS production conditions
To enhance EPS production from strain OM03 (EPS-OM03), various modifications were made to the EPSB medium. Using glucose, fructose, and sucrose (25, 50, and 100 g/l, w/v), the impact of carbon sources was investigated. The effects of variables, including culture temperature (25°C, 30°C, 35°C, up to 50°C), pH (5, 5.5, 6, up to 9), and time (24, 48, 72, 96, and 120 hours) were successively assessed.
Extraction, purification, and quantification of EPS
The fermentation broth was spun in a centrifuge for 10 minutes, at 1,000 rpm, and 4°C. About 5% TCA was then added to the cell-free supernatant and maintained overnight at 4°C. After that, the protein precipitate was removed by centrifuging it at 10,000 rpm for 15 minutes. After adjusting the pH to 7.0, the EPS-OM03 was precipitated with three volumes of 95% ethanol. EPS was further purified using acetone and ether according to the methods described previously (Vidhyalakshmi et al., 2016). Dialysis was used to further purify the EPS, which was subsequently lyophilized (Innova INOFD, China). The total carbohydrate concentration present in the samples was analyzed by the phenol sulfuric acid method, and glucose was used as the standard (Oleksy-Sobczak and Klewicka, 2020).
FTIR spectroscopy
The purified and freeze-dried EPS-OM03 was treated with KBr and made into pellets using hydraulic press, following that, the pellet was placed in the sample holder and FTIR spectra were taken using an FTIR spectrometer (ALPHA, Bruker, Germany). The range of operation was done between 4,000 and 400 cm−1.
XRD analysis
XRD experiment was performed on the Empyrean - Malvern Panalytical using a PIXcel 3D detector and Ni-filtered CuK radiation (=1.54060) at 40 mA and 45 kV. To investigate the properties, a scan was performed at various 2? angles (10–80°) with scattering angle intervals (°2?). The percentage crystallinity index (CIxrd) of EPS was later derived using Originpro software (Mathivanan et al., 2021).
Scanning Electron Microscopy (SEM) analysis of EPS
The surface structure and morphology of EPS-OM03 were examined using a scanning electron microscope (TM3030, Hitachi, Tokyo) with a 15,000 V acceleration voltage.
GC-MS spectra of EPS
To ascertain the monosaccharides content, EPS-OM03 (1 mg) was subjected to Trifluoracetic acid (TFA) (2.5M) hydrolysis for 5 hours at 100°C, then dried in the speedvac vacuum concentrators (Thermo Fisher Scientific, USA). When the sample was fully dried the silylation was done to improve the volatility by adding N-Methyl-N-(trimethylsilyl) trifluoroacetamide and 100 µl of pyridine, incubating at 65°C for 1 hour at 300 rpm. The sample was centrifuged, and the supernatant was put into a GC container. The monosaccharide components of the EPS-OM03 were evaluated at the Centre for Cellular and Molecular Platforms (Bengaluru, India) using a triple quadrupole GC-MS/MS done on an Agilent 7890B GC system fitted 7,000C MS model (Agilent technologies, USA). The DB-5MS+DG GC capillary column having a length (of 30 m), inner diameter (0.25 mm), and thickness of 1 mm were utilized in the GC system. Hydrolyzed EPS (1 µl) was introduced in a split mode with a ratio of 5:1, and 1 ml/minute of helium was used as the carrier. During the analysis, the temperature of the column started at 60°Cfor 1 minute, then went up to 190°C at a rate of 20°C/minutes for 8.5 minutes, and then went up to 280°C at a rate of 5°C/minutes, where the run time was kept for 29.5 minutes. The retention times of the monosaccharides were used to identify them in comparison to standards, and the carbohydrate nature of the monosaccharides was confirmed by their mass spectra.
Cell culture
Human ovarian carcinoma cell lines SKOV-3 and PA-1, and normal human ovarian surface epithelial cell line T1074 were purchased from National Centre for Cell Science, India. The cells were grown in Dulbecco’s Modified Eagle Medium enriched by fetal bovine serum (10%) and 2.5 ml of an antibiotic and antimycotic solution at 37°C in a humidified incubator with 5% CO2 (NuAire, USA).
MTT assay
EPS-OM03 was tested for cytotoxicity against T1074, PA-1, and SKOV-3 cell lines using the conventional microplate MTT assay technique (Koroth et al., 2019). Briefly, logarithmically growing cells (1 × 104 cells/well) were inoculated in a 96-well plate. After 24 hours growth period, cells were exposed to various concentrations of EPS-OM03 (6.25–1,600 µg/ml) and then allowed to grow at 37°C for another 24 and 48 hours, respectively. Then EPS-containing media was aspirated, 50 µl of MTT was added, and the plates were then incubated for 3 hours. After the removal of the growth media, the violet formazan crystals were subsequently dissolved in 100 µl of Dimethyl sulfoxide (DMSO). The absorbance was then determined by using a microplate reader at 570 nm.
Assessment of apoptosis by using AO/EtBr double staining
The PA-1 cells (3 × 105 cells/2 ml) were treated with IC50 concentration of EPS-OM03 and grown for 24 hours at 37°C. Untreated cells are considered a negative control and cells treated with cisplatin (10 µg/ml) as positive control. Following treatment, cells were washed with 1× Dulbecco’s Phosphate Buffered Saline to remove the medium and stained with 500 µl of AO/EtBr staining solution for 5 minutes. Micrographs were recorded immediately using a fluorescence microscope.
Clonogenic assay
The assay was carried out in essentially the same way as reported previously with a few tweaks (Brix et al., 2020). Briefly stated, PA-1cells were grown in T-25 flasks for 24 hours and then challenged to EPS-OMO3 (153.5, 307, and 614 µg/ml) for another 6 hours. Afterwards, cells are trypsinized, enumerated, and plated on a 6-well plate (200 cells/2 ml per well) for 8 days of growth. Crystal violet at 0.04% is used to stain the colonies after they have been fixed in methanol. Photographed, counted, and analyzed, the colonies using ImageJ (version 1.48) software. The plating efficiency (PE) and surviving cell fraction (SF) were then calculated.
Annexin V apoptosis assay by flow cytometry
PA-1 cells were grown on 6-well plate (3 × 105 cells/2 ml) in both the presence and absence of EPS-OM03 at 37°C for 24 hours. An apoptosis assay was performed using Annexin V- AbFlour™ 488 Apoptosis Detection Kit (KTA0002, Abbkine, Inc., China). After staining the cell population with AbFlour™ 488 annexin V and propidium iodide in the provided binding buffer, early apoptotic cells show green fluorescence of the cellular membrane, dead cells show red fluorescence of the nucleus, and green fluorescence of the cellular membrane, and live cells with no or little fluorescence. The data were collected using a flow cytometer, Cytomics FC500 (Beckman Coulter, USA). The results were analyzed by FlowJo™ version 10.8 Software (BD Life Sciences).
Detection of DNA damage by transferase dUTP nick end labeling (TUNEL) assay
PA-1 cell lines (3 × 105 cells/2 ml) were grown in the presence and absence of IC50 concentration of EPS-OM03 for 24 hours. Then, terminal deoxynucleotidyl transferase dUTP nick end labeling reaction was performed with APO-DIRECT™ Kit (Pharmingen, BD Biosciences). Briefly, approximately 1 × 106 cells/ml were treated with 50 µl DNA labeling mixture at 37°C for 1 hour. The cells were then reconstituted in PI/RNase staining solution (0.5 ml), kept at room temperature in dark for 30 minutes, and examined using a flow cytometer (Cytomics FC500, Beckman Coulter, USA).
Caspase-3 assay
PA-1 cells (3 × 105 cells/2 ml) were seeded in a 6-well plate and cultured for 24 hours at 37°C. Cells were then treated with the IC50 value of EPS-OM03 for 24 hours. Caspase-3 activity was measured according to the manufacturer’s instructions utilizing Fluorescein isothiocyanate (FITC) Rabbit Anti-Active Caspase-3 IgG Antibody (BD Biosciences, Asia Pacific). The bandpass filter set at 525 nm allowed green fluorescence from FITC to be detected in the FL1 detector of a Cytomics FC500 flow cytometer (Beckman Coulter, USA).
Chorioallantoic membrane (CAM) assay in fertilized chicken eggs
The influence of EPS-OM03 on ex-ovo angiogenesis was ascertained by CAM assay as described before (Naik et al., 2018). The fertilized chicken egg was collected on the 0th day from Souza Hatcheries, Mangalore, Karnataka and was disinfected using 70% ethanol and then incubated in a horizontal position at 37°C. In brief, EPS-OM03 at 50, 100, 200, and 400 µg/ml was made in 0.5% DMSO and air-dried on sterile paper discs with a diameter of 6 mm (Himedia, India). Discs were then transferred aseptically over the CAM and the eggs were returned to the incubator for 48 hours. After incubation, the anti-angiogenic potential of EPS was calculated by measuring the inhibition of blood vessel branch points in the area of the disc under a microscope, and images were captured. Dexamethasone (50 mg/ml) served as the positive control whereas 0.5% DMSO was the negative control.
Statistical analysis
Data analysis was carried out using the Statistical Package for the Social Sciences software suite version 23.0, IBMR Co., USA. The significant differences between the treatment groups were determined using the one-way ANOVA and Tukey’s honestly significant difference (HSD) test at the p < 0.05 level.
RESULTS AND DISCUSSION
Optimizing the cultural conditions to produce EPS
The influence of different sources of carbon and their concentration on EPS production by B. velezensis OM03 were examined using glucose, fructose, and sucrose. As presented in Figure 1, the highest yield of EPS was obtained in a medium containing glucose (50 g/l), and the yield was 59.4 mg/100 ml after 96 hours of fermentation (p < 0.001). These findings contradict those of B. velezensis SN-1, which generated the maximum EPS when sucrose was employed as source carbon (Cao et al., 2020). Furthermore, the role of time, pH, and temperature on the fermentation of EPS-OM03 was analyzed. The optimum temperature, pH, and time of incubation were found to be 40°C, pH 7.5, and 96 hours, respectively (Fig. 1).
EPS sample after deproteinization showed the maximum absorption at 220 nm (Fig. 2A). Also, the lack of absorption peaks around 260, as well as 280 nm, demonstrated that the sample is pure or devoid of DNA and proteins.
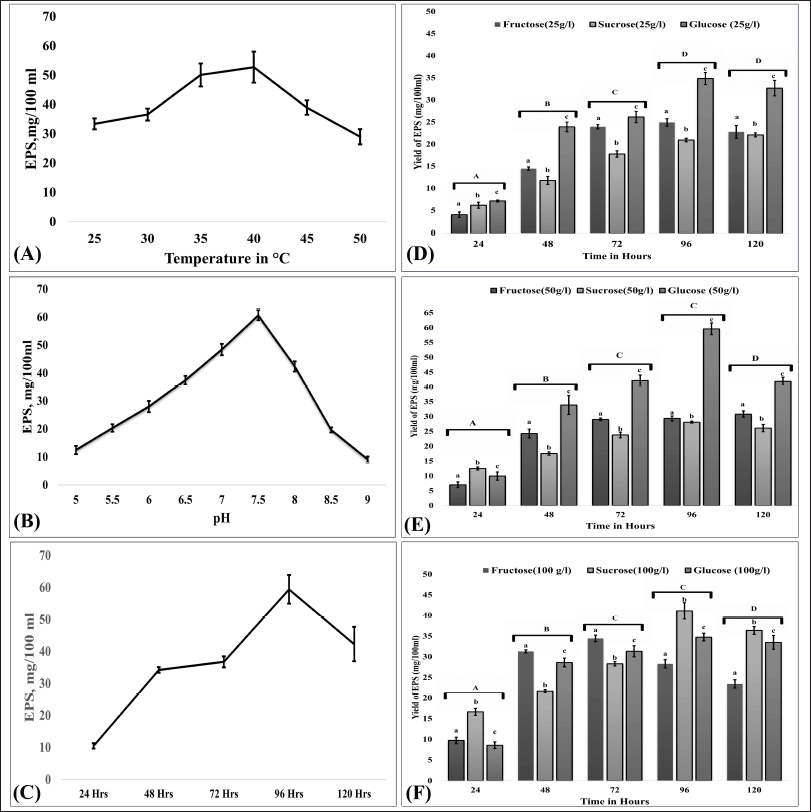 | Figure 1. Optimization of cultural conditions for EPS production. (A), (B), and (C) represent the effects of temperature, pH, and time, respectively. The effect of concentration of carbon sources on EPS production is as follows: (D) carbon sources at 25 g/l, (E) carbon sources at 50 g/l, and (F) carbon sources at 100 g/l. Error bars indicate ± SEM. For pH, temperature, and time, one-way ANOVA and Tukey’s HSD (n = 3) were used, while for the concentration of carbon sources on EPS production, two-way ANOVA and Tukey’s HSD (n = 3) were used. Different letter labels denote significant differences (p < 0.05) while identical letter labels denote non-significant differences. [Click here to view] |
FTIR analysis of EPS-OM03
EPS-OM03 when scanned in the range of 4,000–400 cm−1 indicated the typical absorption peak of a polysaccharide (Fig. 2B). The sample exhibited a strong peak at 3,439.50 cm−1 representing stretching vibrations of hydroxyl moieties of polysaccharides. The poor absorption at 2,931 cm−1 may represent the C-H stretching vibration of CH2 groups (Kavitha et al., 2018). Asymmetric intermediate stretching peak at 1,587.86 cm−1 (1,593–1,662 cm−1) could be caused by mannose ring stretching (Angelin and Kavitha, 2020) or by the stretch vibration of the carbonyl group (Mohite et al., 2019). The medium absorption peak around 1,405.54 cm−1 represents –OH bend vibration by carboxylic groups present in EPS (Castellane et al., 2015). A peak between 1,000–1,200 cm−1 indicates stretching vibration of C-O-C and C-C of the glucose ring (Smith, 2018). The shoulder peak appeared near 1,120 cm−1 a fingerprint of the stretching vibration in C=O (Cao et al., 2020). The band at 615.21 cm−1 and 470.21 cm−1 may represent β(OCO) of glucose or mannose (Wiercigroch et al., 2017). The bands at 920 cm−1 as well as 890 cm−1was contributed by the C-O-C vibration of α-1,4- glycosidic bonds (Wei et al., 2021) and β-1,4 glycosidic linkage (Hong et al., 2021), respectively.
XRD analysis of EPS-OM03
EPS displayed two distinctive XRD peaks at 15.9° as well as 26.1° with an inter-planar spacing of 5.56958 and 3.42010 Å respectively (Fig. 2C). This pattern of XRD spectra of EPS explained the amorphous characteristics, with a crystalline phase in the structure. The percentage crystallinity and the crystalline index (CIxrd) were calculated using Originpro software and were found to be 40.31% and 0.58, respectively. The percentage crystallinity was found to be higher than the EPS from Leuconostoc lactis KC117496 (33.4%) (Saravanan and Shetty, 2016). Crystallization of EPS can be hampered by the uneven arrangement of monomers and their composition (Wang et al., 2021).
SEM analysis of EPS
The SEM analysis has revealed the aggregated and irregular-shaped structural elements of EPS-OM03, which clearly describe their crystalline/amorphous nature. EPS with a similarly shaped irregular and uneven surface was demonstrated by B. cereus KMS3 (Krishnamurthy et al., 2020) and Bacillus aerophilus rk1 (Ravi et al., 2021).
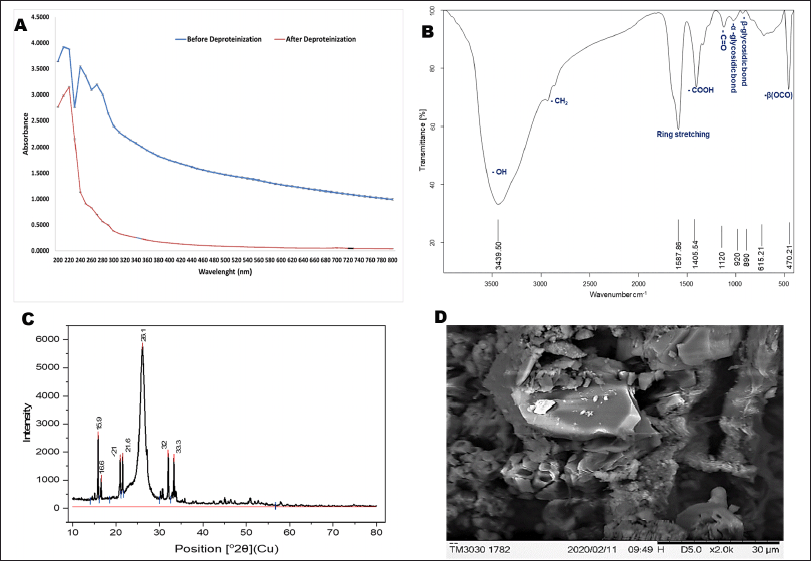 | Figure 2. Characterization of EPS- OM03 (A) UV-Vis spectrum of EPS before and after deproteinization. (B) FTIR spectra of EPS-OM03 in the range of 500–4,000 cm-1. (C) XRD pattern of EPS. (D) SEM images of EPS-OM03. [Click here to view] |
GC-MS spectra of EPS
The GC-MS spectrum of the hydrolyzed EPS-OM03 revealed five main peaks with retention times of 5.759, 6.853, 12.033, 13.456, and 14.522. The monomeric units present in the EPS were deciphered using the analysis and were found to be glucose and mannose (Table 1). The Thin layer chromatography analysis of TFA hydrolyzed EPS-OM03 also supported the presence of mannose and glucose in the sample. Mannose was the most prevalent monosaccharide, accounting for 63.52% of all monosaccharides found. On other hand, the EPS from B. velezensis KY471306 has a molecular weight of 1.14 × 105 D and is made up of glucose, mannose, and galactose, according to a prior study (Moghannem et al., 2018). A Levan-type EPS isolated from Bacillus amyloliquefaciens JN4 was found to have a molar composition of 46:1 and was made up of fructose and glucose (Cai et al., 2019). From the previous studies, it is proved that the chemical makeup and linking bonds, the composition of polysaccharides varies upon the environmental conditions (Nguyen et al., 2021), type of carbon source in the media (Zhang et al., 2021), biosynthetic pathways, enzymes behind its synthesis and the site of production (Schmid et al., 2015).
Cytotoxicity assay
The assay showed that EPS-OM03 has significantly (p < 0.05) hindered the proliferation of cell lines in a way that was affected by concentration and time (Fig. 3). Among the cell lines tested, the lowest IC50 values were observed for PA-1 cells. The IC50 value of EPS-OM03 on PA-1 dropped by a significant amount after 48 hours of treatment and was found to be 123 ± 2.5 μg/ml. After 24 hours of treatment, it was 238 ± 1.25 μg/ml. Meanwhile, the tested EPS was found to be moderately toxic to SKOV-3 cells. The IC50 was determined to be 620 ± 2.52 μg/ml on SKOV-3 after 24 hours of treatment; however, this value fell to 375 ± 2.25 μg/ml after 48 hours of therapy. Normal human ovarian cells-T1074, on the other hand, were not affected by EPS-OM03 at concentrations between 6.25 and 800 μg/ml, hence are nontoxic. It has been demonstrated that EPS-OM03 has a more pronounced effect on PA-1 cell lines; hence, these cell lines were utilized to further investigate the mechanism of antiproliferation and cytotoxicity. Numerous natural compounds, from microbial sources, have demonstrated anti-cancer effects against ovarian cancer without any harmful impact on healthy cells. For instance, polysaccharides can prevent ovarian cancer cells from migrating and invading. Particularly, Pyracantha fortuneana, selenium-enriched polysaccharides prevent growth of ovarian cancer cells by blocking the β-catenin signaling (Sun et al., 2018). Additionally, a functional polysaccharide Lentinan obtained from Lentinus edodes was cytotoxic, immunomodulating, and anti-tumor in the treatment of different malignancies including ovarian carcinoma (Trivedi et al., 2022).
 | Table 1. Mass spectrum of EPS from B. velezensis OM03. [Click here to view] |
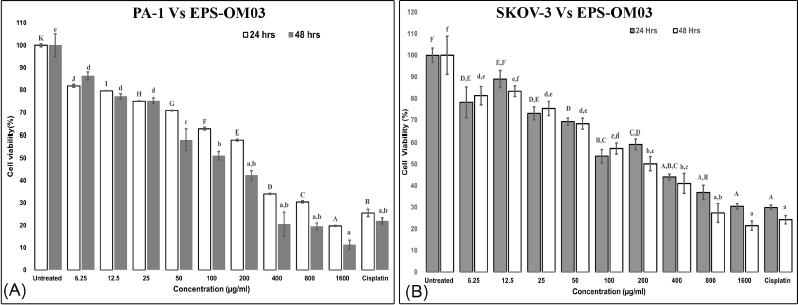 | Figure 3. (A). In vitro cytotoxic activity of the EPS-OM03 on PA-1 cells. (B). In vitro cytotoxic activity of the EPS-OM03 on SKOV-3 cells. Results are presented as mean ± SEM (n = 4). Cisplatin= Cisplatin 10 μg/ml. Different letter labels denote significant differences (p < 0.05) while identical letter labels denote non-significant differences, according to the one-way ANOVA and Tukey’s HSD. [Click here to view] |
Assessment of apoptosis by using AO/EtBr double staining
The preferred form of cell death for the treatment of malignancies is by induction of apoptosis. After 24 hours of exposure to EPS-OM03, PA-1 cell lines showed notable morphological alterations, including cell shrinkage, membrane blebbing, and the production of apoptotic bodies (Fig. 4). A change in shape with granular yellow-green nuclei was noticed in the PA-1 cells after treatment with the IC50 concentration of EPS-OM03, which may indicate the existence of early apoptotic cells. A few numbers of dead or late apoptotic cells were also seen to have dense and asymmetrically localized orange nuclear ethidium bromide staining. Similarly, in A549 cancer cells, an EPS from the Bacillus albus DM-15 triggered apoptotic features including condensation of chromatin, cell shrinkage (early apoptosis), and cell disintegration (Vinothkanna et al., 2022).
EPS-OM03 inhibits colony formation in PA-1 cell lines
When PA-1 cells were treated with EPS-OM03, their capacity to proliferate clonally was completely suppressed at all doses used for testing (119, 238, and 476 µg/ml). The PE and SF of the untreated control cells were 104.75 and 1, respectively. The absence of colonies after EPS-OM03 treatment is strong evidence that it has an antiproliferative effect on PA-1 ovarian carcinoma, supporting the results of MTT assays. Correspondingly, the polysaccharide isolated from Polygonatum sibiricum demonstrated a concentration-dependent reduction in the colony formation of HepG2 cells (Li et al., 2022).
Annexin V apoptosis assay by flow cytometry
Most chemotherapy drugs are designed to induce apoptosis (Chen et al., 2018). The number of apoptotic PA-1 cells upon being treated with an IC50 dose of EPS-OM03 for 12–16 hours was quantified with flow cytometry. EPS-OMO3 and the standard drug Cisplatin both increased the population of early apoptotic cells in comparison to untreated control cells (Table 2). In these interventions, a rise in the overall intensity of fluorescence by Annexin V-AbFlour 488 was detected. It resulted in a >5-fold rise in the percentage of early apoptotic cells and a 1.4-fold of raise in dead cells. As illustrated in Figure 5, EPS-OM03 induced apoptosis in PA-1 cells at various levels when compared to untreated control cells.
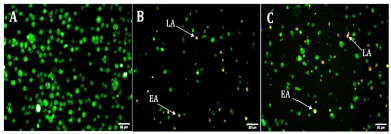 | Figure 4. Fluorescent micrographs of AO/EtBr double-stained PA-1 cell lines after 24 hours (20× magnification). A: untreated PA-1, B: Cisplatin . 10 μg/ml, C: EPS 238 μg/ml.; EA indicates early apoptotic cells and LA indicates late apoptotic cells. [Click here to view] |
Detection of EPS-OM03 triggered DNA damage by TUNEL assay
An increase in the DNA fragmentation index, calculated as the percentage of cells with damaged DNA, and an increase in overall FITC mean fluorescence intensity (MFI), were perceived in the EPS-OMO3 and Cisplatin treated PA-1 ovarian carcinoma as compared to the untreated cells (Fig. 6A). This study has shown that the IC50 concentration of EPS-OM03 caused 44.3% DNA fragmentation in treated PA-1 cell lines (Table 3). This mechanism was also demonstrated in human hepatocellular carcinoma (HepG2) treated with polysaccharide from Nepeta septemcrenata (Nasr and Saad, 2021). DNA fragmentation, a defining feature of apoptosis, is brought on by apoptotic triggers in different cell types. Apoptosis is characterized by DNA fragmentation by an endonuclease, which is triggered via a signaling pathway coordinated by the family of cysteinyl aspartate-specific protease caspases (Vigneswara and Ahmed, 2020).
Caspase-3 assay
The results indicated that the EPS-OM03 induces intrinsic apoptosis, which results in an upregulation of caspase 3 expressions. In the intrinsic apoptotic pathway, initiator caspases like caspase-9 activates executor caspase-3 proteins. These molecules are activated in a cascade that causes cytoskeleton protein breakage, activation of poly (ADP-ribose) polymerase, and caspase-activated DNAse, and chromatin fragmentation that triggers apoptosis (Obeng, 2021). We observed a >10-fold raise in the concentration of active form of caspase-3 in PA-1 cell lines treated with EPS-OMO3 as compared to the untreated group of cells (Table 4). A large increase in the FITC MFI was also observed (Fig. 6B). Numerous polysaccharides have been found to reduce mitochondrial membrane potential, which causes cytochrome C to move from the mitochondria to the cytoplasm, activates a series of caspases, and finally cause apoptosis (Wu et al., 2021). In such apoptotic cells, the active Caspase-3 causes cell shrinkage, DNA degradation, and condensation of chromatin without impacting the surrounding healthy tissue (Hu et al., 2021). According to the results of the AO/EtBr double labeling and annexin V apoptosis studies, EPS-OM03 caused early and late apoptosis in the ovarian cancer PA-1 cell lines. Additionally, it fragments DNA and upregulates caspase 3 expression. This demonstrates that the potential anticancer activity of EPS-OM03 is caused by caspase-3-induced apoptosis. These observations are in line with the previous reports that explained how human breast cancer cell line MCF-7 underwent apoptosis when exposed to EPS from B. velezensis MHM3, which was linked to caspase-3 activation (Mahgoub et al., 2018). It has been discovered that the activation of effector caspase such as caspase-3 is necessary for apoptotic chromatin condensation and DNA fragmentation, and its deficiency may be a potential factor for chemoresistance in breast cancer cell line MCF-7 (Pu et al., 2017). Caspase-3 activation is frequently used as a marker to show that cancer cells have undergone apoptosis, meaning they have shrunk in size, their nuclei have fragmented, and their chromatin has condensed, without harming the healthy surrounding tissues (Jung et al., 2001).
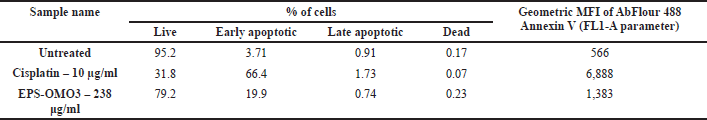 | Table 2. Annexin V apoptosis assay by flow cytometry. [Click here to view] |
Evaluation of antiangiogenic potential of EPS-OM03 using CAM assay
The CAM assay results indicated that EPS-OM03 has significantly (p < 0.001) been able to encumber the in vivo angiogenesis in a concentration-dependent manner. The representative images of CAMs of the chicken embryos indicating the reduced vascularization by treated concentration range are shown in Figure 7. The anti-angiogenic effect of EPS-OM03 was seen in all concentration ranges that were evaluated, it also showed that the greater the dosage, the lesser the branched points observed. At a concentration of 400 g/ml, 94.33% inhibition was seen. Our findings demonstrate a considerable reduction in the branching points, with an IC50 value of 146.0 ± 3.56 µg/ml. The presence of glucose and mannose in the EPS-OM03 may account for its ability to inhibit vascularization. According to the earlier findings, STPC2 polysaccharide from Sargassum thunbergia, which belongs to the fucoidan group, has exhibited strong anti-angiogenesis by preventing VEGF from interacting with VEGFR2 (Hu et al., 2017).
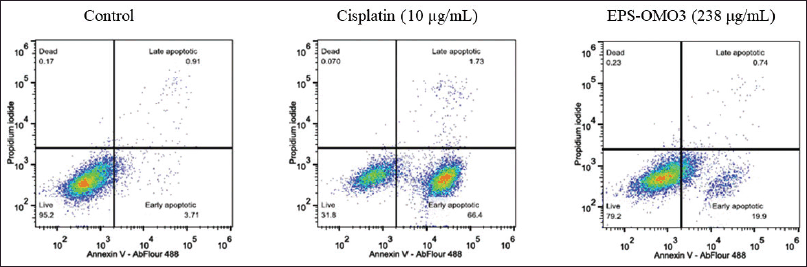 | Figure 5. Flow cytometry analysis with annexin V/PI. [Click here to view] |
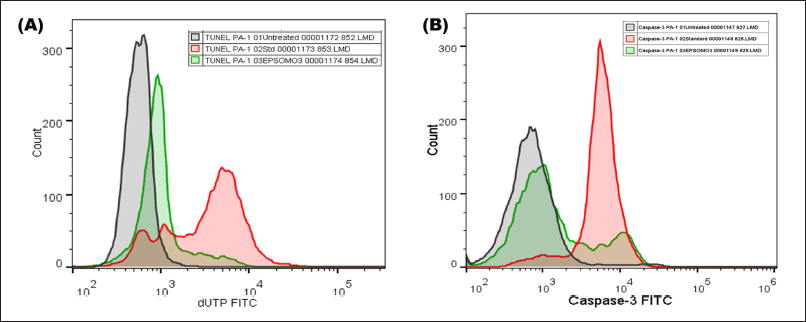 | Figure 6. EPS-OM03 treatment induced DNA fragmentation and caspase-3 activity in human ovarian cancer cell lines. PA-1 cells were subjected to treatment with EPS-OM03 (238 μg/ml), cisplatin (10 μg/ml), and an untreated control group was established. (A). Quantification of DNA fragmentation by TUNEL assay. (B). EPSOM03 stimulates PA-1 cell apoptosis by upregulating the activity of caspase-3. [Click here to view] |
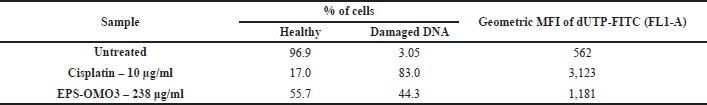 | Table 3. TUNEL assay showing DNA fragmentation in EPS-OM03 treated PA-1 cell lines. [Click here to view] |
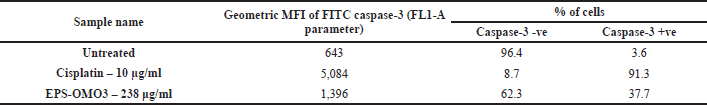 | Table 4. Flow cytometry assay showing increased caspase 3 activity in PA-1 cells after treatments with EPS-OM03 (238 μg/ml), and cisplatin (10 μg/ml). [Click here to view] |
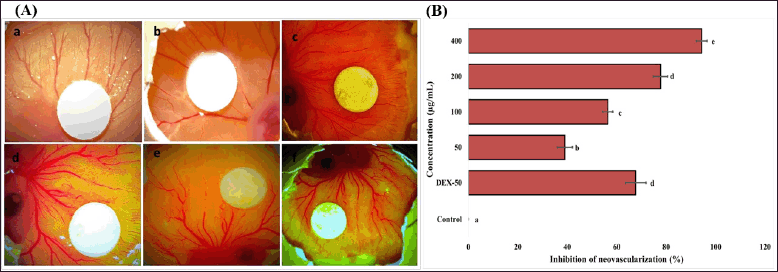 | Figure 7. Effect of EPS-OM03 on neovascularization in chick embryo CAM assays. A. Representative photographs show the inhibition of angiogenesis in the presence of EPS in which: a. Control (DMSO), b. CAM + 50 μg/ml of EPS, c. CAM + 100 μg/ml of EPS, d. CAM + 200 μg/ml of EPS, e. CAM + 400 μg/ml of EPS, f. Dexamethasone (DEX-50 μg/ml). B. Quantitative comparison of inhibition of neovascularization (%) surrounding the EPS-containing filter paper discs. Different letter labels denote significant differences (p < 0.05) while identical letter labels denote non-significant differences, according to the one-way ANOVA and Tukey’s HSD. [Click here to view] |
CONCLUSION
According to the study, the ability of B. velezensis strain OM03 to produce EPS was influenced by source of carbon and environmental conditions (pH, Temp, and Time). The highest yield of 59.4 ± 1.97 mg/100 ml was attained when B. velezensis OM03 was cultured on EPSB medium (pH 7.5) having 50 g/l of glucose at 40°C for 96 hours. The FTIR and GC-MS study of the ESP-OM03 revealed that it’s a novel heteropolysaccharide made up of 63.52% mannose and 36.45% glucose linked through α-1,4 and β-1,4 glycosidic bonds. This chemical makeup probably determines the reported bioactivity of EPS-OM03 to hinder the proliferation of ovarian carcinoma cells. EPS-OM03 boosted DNA fragmentation and caspase-3 activity, which in turn caused PA-1 cells to undergo apoptosis. Additionally, EPS- OM03 was able to reduce angiogenesis in chick CAMs. When seen as a whole, our findings unmistakably demonstrate EPS-OM03 as a promising antitumor medication for ovarian cancer. We, therefore, anticipate that EPS-OM03, which had no significant side effects, might become a new alternative medication. In the meanwhile, further investigation into anticancer mechanisms in animal models is required.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research work was funded by the Mangalore Jesuit Educational Society (MJES), St. Aloysius College (Autonomous), Mangaluru, Karnataka, India.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelhamid SA, Mohamed SS, Selim MS. Medical application of exopolymers produced by marine bacteria. Bull Natl Res Cent, 2020; 44:1–14.
Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol, 2020; 162:853–65.
Ayyal NM, Abbas ZMA, Karim AJ, Abbas ZMA, Al-Salihi KA, Khalaf JM, Mahmood DD, Mohammed EA, Jumaa RS, Abdul-Majeed DI. Bacterial isolation from internal organs of rats (Rattus rattus) captured in Baghdad city of Iraq. Vet World, 2019; 12:119–25.
Brewster WR, Burkett WC, Ko EM, Bae-Jump V, Nicole McCoy A, Keku TO. An evaluation of the microbiota of the upper reproductive tract of women with and without epithelial ovarian cancer. Gynecol Oncol Reports, 2022; 42:101017.
Brix N, Samaga D, Hennel R, Gehr K, Zitzelsberger H, Lauber K. The clonogenic assay: robustness of plating efficiency-based analysis is strongly compromised by cellular cooperation. Radiat Oncol, 2020; 15:1–12.
Cai G, Liu Y, Li X, Lu J. New levan-type exopolysaccharide from Bacillus amyloliquefaciens as an antiadhesive agent against enterotoxigenic Escherichia coli. J Agric Food Chem, 2019; 67:8029–34.
Cao C, Liu Y, Li Y, Zhang Y, Zhao Y, Wu R, Wu HJ. Structural characterization and antioxidant potential of a novel exopolysaccharide produced by Bacillus velezensis SN-1 from spontaneously fermented Da-Jiang. Glycoconj J, 2020; 37:307–17.
Castellane TCL, Otoboni AMMB, de MacedoLemos EG. Characterization of exopolysaccharides produced by Rhizobia species. Rev Bras Ciênc Solo, 2015; 39:1566–75.
Chen L, Zeng Y, Zhou SF, Chen L, Zeng Y, Zhou SF. Role of apoptosis in cancer resistance to chemotherapy. Current understanding of apoptosis—Program Cell Death, IntechOpen, Paris, France, 2018; 2018:126–36.
Cheng H, Wang Z, Cui L, Wen Y, Chen X, Gong F, Yi H. Opportunities and challenges of the human microbiome in ovarian cancer. Front Oncol, 2020; 10:163.
Chirakkara SP, Abraham A. Bacillus strains from the ovaries of Swiss albino mice (Mus musculus): deciphering of probiotic potential through an in vitro approach. Biomedicine, 2022; 42:1200–8.
Gangalla R, Gattu S, Palaniappan S, Ahamed M, Macha B, Thampu RK, Fais A, Cincotti A, Gatto G, Dama M, Kumar A. Structural characterisation and assessment of the novel Bacillus amyloliquefaciens RK3 exopolysaccharide on the improvement of cognitive function in Alzheimer’s disease mice. Polymers (Basel), 2021; 13(17):2842.
Guo R, Chen M, Ding Y, Yang P, Wang M, Zhang H, He Y, Ma H. Polysaccharides as potential anti-tumor biomacromolecules —a review. Front Nutr, 2022; 9:838179.
Hong T, Yin JY, Nie SP, Xie MY. Applications of infrared spectroscopy in polysaccharide structural analysis: progress, challenge and perspective. Food Chem X, 2021; 12:100168.
Hu M, Cui N, Bo Z, Xiang F. Structural determinant and its underlying molecular mechanism of STPC2 related to anti-angiogenic activity. Mar Drugs, 2017; 15(2):458.
Hu XM, Li ZX, Lin RH, Shan JQ, Yu QW, Wang RX, Liao LS, Yan WT, Wang Z, Shang L, Huang Y, Zhang Q, Xiong K. Guidelines for regulated cell death assays: a systematic summary, a categorical comparison, a prospective. Front Cell Dev Biol, 2021; 9:368.
Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-HEP-1 hepatocarcinoma cells involves bcl-2 downregulation and caspase-3 activation. Cancer Lett, 2001; 165:139–45.
Kavitha M, Raja M, Perumal P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac Rep, 2018; 11:59–69.
Koroth J, Nirgude S, Tiwari S, Gopalakrishnan V, Mahadeva R, Kumar S, Karki SS, Choudhary B. Investigation of anti-cancer and migrastatic properties of novel curcumin derivatives on breast and ovarian cancer cell lines. BMC Complement Altern Med, 2019; 19:1–16.
Krishnamurthy M, Jayaraman Uthaya C, Thangavel M, Annadurai V, Rajendran R, Gurusamy A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr Polym, 2020; 227:115369.
Li M, Liu Y, Zhang H, Liu Y, Wang W, You S, Hu X, Song M, Wu R, Wu J. Anti-cancer potential of polysaccharide extracted from Polygonatum sibiricum on HepG2 cells via cell cycle arrest and apoptosis. Front Nutr, 2022; 9:1–12.
Li N, Wang C, Georgiev MI, Bajpai VK, Tundis R, Simal-Gandara J, Lu X, Xiao J, Tang X, Qiao X. Advances in dietary polysaccharides as anticancer agents: structure-activity relationship. Trends Food Sci Technol, 2021; 111:360–77.
Mahgoub AM, Mahmoud MG, Selim MS, El Awady ME. Exopolysaccharide from marine Bacillus velezensis MHM3 induces apoptosis of human breast cancer MCF-7 cells through a mitochondrial pathway. Asian Pac J Cancer Prev, 2018; 19:1957–63.
Mathivanan K, Chandirika JU, Mathimani T, Rajaram R, Annadurai G, Yin H. Production and functionality of exopolysaccharides in bacteria exposed to a toxic metal environment. Ecotoxicol Environ Saf, 2021; 208:111567.
Moghannem SAM, Farag MMS, Shehab AM, Azab MS. Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz J Microbiol, 2018; 49:452–62.
Mohanta M, Khanam S, Islam MS, Mohanta MK. Isolation, characterization and identification of bacterial isolates from the poultry environment at Rajshahi Metropolis, Bangladesh. Artic J Entomol Zool Stud, 2017; 5:918–26.
Mohite BV, Koli SH, Rajput JD, Patil VS, Agarwal T, Patil SV. Production and characterization of multifacet exopolysaccharide from an agricultural isolate, Bacillus subtilis. Biotechnol Appl Biochem, 2019; 66:1010–23.
Moradali MF, Rehm BHA. Bacterial biopolymers: from pathogenesis to advanced materials. Nat Rev Microbiol, 2020; 18(4):195–210.
Naik M, Brahma P, Dixit M. A cost-effective and efficient chick Ex-Ovo CAM assay protocol to assess angiogenesis. Methods Protoc, 2018; 1:1–9.
Nasr SA, Saad AAEM. Evaluation of the cytotoxic anticancer effect of polysaccharide of Nepeta septemcrenata. Beni-Suef Univ J Basic Appl Sci,2021; 10:1–11.
Nguyen PT, Nguyen TT, Vo TNT, Nguyen TTX, Hoang QK, Nguyen HT. Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium chloride stresses. Sci Rep, 2021; 11:1–9.
Obeng E. Apoptosis (Programmed cell death) and its signals-a review. Braz J Biol, 2021; 81:1133–43.
Oerlemans MMP, Akkerman R, Ferrari M, Walvoort MTC, de Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J Funct Foods, 2021; 76:104289.
Oleksy-Sobczak M, Klewicka E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob Proteins, 2020; 12:774–83.
Paudel KR, Dharwal V, Patel VK, Galvao I, Wadhwa R, Malyla V, Shen SS, Budden KF, Hansbro NG, Vaughan A, Yang IA, Kohonen-Corish MRJ, Bebawy M, Dua K, Hansbro PM. Role of lung microbiome in innate immune response associated with chronic lung diseases. Front Med, 2020; 7:554.
Pu X, Storr SJ, Zhang Y, Rakha EA, Green AR, Ellis IO, Martin SG. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis, 2017; 22:357–68.
Ravi G, Sampath G, Srinivas B, Sarika K, Govindarajan RK, Ameen F, Alwakeel S, Thampu RK. Erratum: optimization and characterization of exopolysaccharide produced by Bacillus aerophilus RK1 and its in vitro antioxidant activities. J King Saud Univ Sci, 2021; 33(5):101571.
Saravanan C, Shetty PKH. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol, 2016; 90:100–6.
Schmid J, Sieber V, Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol, 2015; 6:496.
Smith BC. The C=O bond, part III: carboxylic acids. Spectroscopy, 2018; 33:14–20–14–20.
Sun B, Straubinger RM, Lovell JF. Current taxane formulations and emerging cabazitaxel delivery systems. Nano Res, 2018; 11:5193–218.
Trivedi S, Patel K, Belgamwar V, Wadher K. Functional polysaccharide lentinan: role in anti-cancer therapies and management of carcinomas. Pharmacol Res Mod Chinese Med, 2022; 2:100045.
Vidhyalakshmi R, Valli Nachiyar C, Narendra Kumar G, Sunkar S. Bacillus circulans exopolysaccharide: production, characterization and bioactivities. Int J Biol Macromol, 2016; 87:405–14.
Vigneswara V, Ahmed Z. The role of caspase-2 in regulating cell fate. Cells, 2020; 9(5):1259.
Vinothkanna A, Sathiyanarayanan G, Rai AK, Mathivanan K, Saravanan K, Sudharsan K, Kalimuthu P, Ma Y, Sekar S. Exopolysaccharide produced by probiotic Bacillus albus DM-15 isolated from ayurvedic fermented dasamoolarishta: characterization, antioxidant, and anticancer activities. Front Microbiol, 2022; 13:213.
Wang J, Salem DR, Sani RK. Two new exopolysaccharides from a thermophilic bacterium Geobacillus sp. WSUCF1: characterization and bioactivities. N Biotechnol, 2021; 61:29–39.
Wang L, Wang Y, Li Q, Tian K, Xu L, Liu G, Guo C. Exopolysaccharide, isolated from a novel strain Bifidobacterium breve lw01 possess an anticancer effect on head and neck cancer - genetic and biochemical evidences. Front Microbiol, 2019; 10:1–10.
Wei L, Ma F, Du C. Application of FTIR-PAS in rapid assessment of rice quality under climate change conditions. Foods, 2021; 10:159.
Wiercigroch E, Szafraniec E, Czamara K, Pacia MZ, Majzner K, Kochan K, Kaczor A, Baranska M, Malek K. Raman and infrared spectroscopy of carbohydrates: a review. Spectrochim Acta Part A Mol Biomol Spectrosc, 2017; 185:317–35.
Wu J, Lin C, Chen X, Pan N, Liu Z. Polysaccharides isolated from Bangia fuscopurpurea induce apoptosis and autophagy in human ovarian cancer A2780 cells. Food Sci Nutr, 2021; 9:6707–19.
Zhang L, Yi H. Potential antitumor and anti-inflammatory activities of an extracellular polymeric substance (EPS) from Bacillus subtilis isolated from a housefly. Sci Rep, 2022; 12:1–10.
Zhang Y, Dai X, Jin H, Man C, Jiang Y. The effect of optimized carbon source on the synthesis and composition of exopolysaccharides produced by Lactobacillus paracasei. J Dairy Sci, 2021; 104:4023–32.
Zhou X, Kandalai S, Hossain F, Zheng Q. Tumor microbiome metabolism: a game changer in cancer development and therapy. Front Oncol, 2022;12:3680.