INTRODUCTION
The mechanism of the disease and the drug target is substantial in drug discovery. Diabetes mellitus is a metabolic disorder marked by a prolonged period of high blood sugar levels (Pathak and Pathak, 2012). There were 536.6 million diabetes mellitus patients (20–79-year olds) worldwide in 2021, and it was estimated to rise to 783.2 million by 2045 (Sun et al., 2022). There are two types of diabetes mellitus, type-1 for insulin diabetes and type-2 for noninsulin diabetes. Type-1 diabetes is generally risked by genetic susceptibility (Krischer et al., 2017), while type-2 diabetes is generally risked by unhealthy lifestyles (Pathak and Pathak, 2012). One drug mechanism that is used to treat diabetes mellitus, especially type-2 diabetes, is an α-glucosidase inhibitor. The α-glucosidase inhibitor acts by inhibiting digestion, delaying the absorption of glucose, and is followed by decreasing the glycemic level (Kalra, 2014). Targeting drugs on α-glucosidase inhibition show its prevention of type-2 diabetes development (Moelands et al., 2018).
For years, researchers have focused on drug discovery from herbal medicines that can prevent or treat diseases with little or no side effects (Thomford et al., 2018). The biodiversity of Indonesia shows an immense potency for herbal medicine. Garcinia mangostana (Gm) (family Clusiaceae) is booming as a natural product that is rich in antioxidants (Rohman et al., 2020; Tjahjani et al., 2014). Gm also shows antidiabetic activity (Taher et al., 2016). Traditionally, people drink this natural product by decoction or infusion to gather its benefit. Unfortunately, Gm pericarp extract has an unpleasant flavor. Thus, formulation to treat this unpleasant flavor needs to be done. On the other hand, Caesalpinia sappan (Cs) (family Leguminosae) is traditionally used as a beverage in Indonesia to improve health. This plant has a plain taste and shows several bioactivities including antioxidant, antigenotoxicity, and anticancer (Handayani et al., 2020; Jenie et al., 2017; Meiyanto et al., 2019; Nirmal et al., 2015; Nirmal and Panichayupakaranant, 2015; Rachmady et al., 2016). The flavonoid content in this extract inhibits colon cancer cell growth (Handayani et al., 2017). The α-glucosidase inhibitor also decreases the risk of gastrointestinal and colorectal cancer (Zhao et al., 2017). Interestingly, Cs is not traditionally used to treat diabetes mellitus, but this plant also shows antidiabetic activity through the inhibition of α-glucosidase (Nirmal et al., 2015). Additionally, in vivo toxicological studies demonstrate the safety of Cs extract (Athinarayanana et al., 2017). Then, the antidiabetic activity of the combination of Cs and Gm was interesting to be observed.
Combination therapy is commonly used in herbal medicine (Zhou et al., 2016). Combination therapy would be effective while interactions of the compounds inside the combination show synergistic effects. Investigating synergistic effects on drug interaction is essential while studying a combination of herbal medicine. The combination index (CI) was used to measure the level of synergism (Huang et al., 2017). The synergistic effect of brazilin and brazilein from Cs was shown when combined with chemotherapeutic agents (Handayani et al., 2022, 2020, 2016; Jenie et al., 2018). Combinations of Gm with Cs would be helpful to maintain both its antidiabetic activity and people’s acceptance. Then, this study aimed to analyze the synergistic effect of in vitro α-glucosidase activity of the combination of Gm pericarp and Cs heartwood extracts.
MATERIALS AND METHODS
Preparation of extracts
The Cs heartwood and Gm pericarp were determined by Medicinal Plant and Traditional Medicine Research and Development Centre, Ministry of Health, Republic of Indonesia. The ethanol extract of each sample was prepared by maceration of dried powder of Cs L. heartwood (Cs_EtOH) or Gm pericarp (Gm_EtOH) with 70% ethanol-water (1:10) for 24 hours. Then, supernatants were evaporated using a rotary evaporator followed by a vacuum oven to make ethanol extracts. The aqueous extract of each sample was prepared by decoction or boiling of dried powder of Cs (Cs_W) or Gm (Gm_W) with water (1:10) for 1 hours. Each sample was filtered to separate cellular debris from the extract. Then, a freeze-drying method was used to get aqueous extracts.
Total flavonoid content
The AlCl3 colorimetric assay (Chandra et al., 2014; Sembiring et al., 2018) with slight modifications was used to measure total flavonoid content. Initially, standard or extracts were added to 96 well-plates and NaNO2 (5%, w/v) was added. After 5 minutes of incubation, AlCl3 (10%, w/v) was added and gently shaken for 6 minutes in the dark. Then, 1M NaOH was added and absorbance was measured by a microplate reader (Varioskan Flash, Thermo) at 420 nm. The results were shown as percentage total flavonoid content [G quercetin (Qe) (Sigma-Aldrich) equivalent /100 G extract].
α-Glucosidase inhibition assay
The inhibitory activity of α-glucosidase was measured following Zhang et al. (2017) procedure with slight modifications. The α-glucosidase was tested using 50 μl of various concentrations of sample extracts (single and combination) and 25 μl of 5 mM p-nitrophenyl-α-d-glucopyranoside (Wako, Japan) solution in 50 mM sodium phosphate buffer (pH 7). After a 5 min preincubation period, 25 μL of 50 mM sodium phosphate buffer (pH 7) containing α-glucosidase (Wako, Japan) solution (10 μg/ml) was added. The reaction mixtures were incubated at 37°C for 15 minutes and stopped by adding 100 μl of 200 mM Na2CO3. The absorbance was measured by a microplate reader (Varioskan Flash, Thermo) at 405 nm.
Sample extraction for compound analysis
The extraction method was carried out according to Windarsih et al. (2022) with slight modifications. The amount of 20 mg sample was weighed and extracted using 1 ml of Mass Spectrometry-grade (MS-grade) methanol (MeOH). After being vortexed for 30 seconds, samples were sonicated for 30 minutes (room temperature). Then, samples were centrifugated at 1,400 × g for 5 minutes, and the supernatant was collected. The supernatant was filtered using a 0.22 μm nylon filter and it was ready to be injected for liquid chromatography high-resolution mass spectrometry (LC-HRMS) analysis. MS-grade MeOH was used as a blank sample.
Compound analysis using LC-HRMS
The LC-HRMS condition was carried out according to Windarsih et al. (2022). Liquid chromatography (Thermo Scientific™ Vanquish™ UHPLC Binary Pump) and Orbitrap high-resolution mass spectrometry (Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap™ High-Resolution Mass Spectrometer) were used for compounds analysis. An analytical column of Thermo Scientific™ Accucore™ Phenyl-Hexyl 100 mm × 2.1 mm ID × 2.6 μm was used as a stationary phase. The MS-grade water (Fisher Scientific, USA) containing 0.1% formic acid (Merck, Germany) (A) and MS-grade acetonitrile (Fisher Scientific, USA) containing 0.1% formic acid (B) employing gradient technique (flow rate of 0.3 ml/minute) were used as mobile phases. The full MS/dd-MS2 acquisition mode at either positive or negative ionization polarities was used to analyze targeted compounds. The column temperature was set to 40°C. The injection volume was set to 3 μl. The system was performed using Xcalibur version 4.4 software (Thermo Scientific, Germany).
Preparation of ligands and receptors
The two-dimensional (2D) and three-dimensional (3D) structures of test compounds and reference compounds were visualized using MarvinSketch software for molecular docking studies with the selected enzymes. Meanwhile, the 3D structure of receptors, i.e., α-glucosidase [Protein Data Bank (PDB) ID 3W37], was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) protein database (http://www.rcsb.org/pdb/home/home.do). The preparation of protein receptors and ligands was done using Yet Another Scientific Artificial Reality Application (YASARA) 20.8.23 software. Molecular docking was generated using Protein-Ligand Ant System (PLANTS) docking tool. Docking interaction was visualized by Ligplot+ version 2.2 software.
Docking protocol validation
The docking protocol was validated by calculating the root mean square deviation (RMSD) values between the native ligand isolated from the PDB file with its conformation and the docked ligand. The RMSD value was calculated using YASARA software. If the RMSD has a value less than 2 Å, the docking protocol can be used for further docking process.
Interaction of protein-ligand
The interaction of protein and ligand was done with a standard procedure of molecular docking using PLANTS (Meiyanto et al., 2014). The output data were shown as docking scores that showed the energy of the ligand in binding to the target protein. The affinity of the ligand binding to the protein is stronger when the docking score is more negative. Afterwards, the 2D docking interaction of protein receptor and ligand was visualized by Ligplot+ software.
Statistical analysis
The IC50 value was calculated using a linear regression depending on the various concentrations versus activity (α-glucosidase inhibition) and was performed by Excel 2013 software (Microsoft, Redmond, WA). Statistical analysis was performed using Student’s t-test (Excel 2013 software; Microsoft, Redmond, WA). Results were expressed as means ± standard deviation (n = 3). p values less than 0.05 were considered significant. The CI equation was developed by Reynold and Maurer (2005).
RESULTS AND DISCUSSION
Total flavonoid content
GM pericarp and Cs heartwood are rich in phenolic and flavonoid compounds (Aizat et al., 2019; Nirmal et al., 2015). While flavonoid is the active compound of the extract, the level of flavonoid content is usually related to their activity. Nevertheless, flavonoid contents in organic solvent (ethanol) and aqueous extract might be different. Then, analysis of the flavonoid content inside the extract is important. Table 1 showed that the ethanol extract of both Gm pericarp and Cs heartwood had similar results. The total flavonoid content of Cs_W was slightly lower than ethanol extracts (Cs_EtOH and Gm_EtOH) but higher than Gm_W (Table 1). Thus, the total flavonoid content of the aqueous extract in both plants was less than that in the ethanol extract. It possibly occurred because the dissolution ability of the compounds inside the extracts was different. Flavonoid with glycoside structure is usually more water-soluble than aglycone structure (Slámová et al., 2018). Nevertheless, all of the extracts used in this study were rich in flavonoids. Flavonoid compounds have a role in α-glucosidase inhibition (Proença et al., 2017; ?öhreto?lu and Sari, 2019). Then, we compared the synergistic effect of the α-glucosidase activity of the combination of Gm pericarp and Cs heartwood in both ethanol and aqueous extracts.
α-Glucosidase inhibition
Flavonoids are promising modulators of α-glucosidase activity (Proença et al., 2017). Flavonoid content in Gm pericarp and Cs heartwood extracts is possibly active as α-glucosidase inhibitors. The results showed that both Gm_EtOH and Cs_EtOH almost had a similar α-glucosidase inhibitory effect, while Cs_W showed two folds higher α-glucosidase inhibitory activity than Gm_W. The α-glucosidase inhibitory effect of Cs_EtOH was five folds higher than its aqueous extract, while Gm_EtOH was ten folds higher than its aqueous extract (Fig. 1; Table 1). Qe as a flavonoid standard showed the highest α-glucosidase inhibitory effect (Table 1). The α-glucosidase inhibitory activity of both extracts was in line with their total flavonoid content. Cs contained flavonoids, i.e., protosappanin A, protosappanin B, sappanchalcone, and homoisoflavone (brazilin and brazilein) (Nirmal et al., 2015), while anthocyanidin, proanthocyanidin A, proanthocyanidin B, epicatechin, and epigallocatechin are examples of flavonoids contained in Gm (Fu et al., 2007; Rohman et al., 2020; Yoshimura et al., 2015). Free hydroxyl groups at C-4’ in the B ring of brazilin and brazilein possibly have a role in α-glucosidase inhibition. ?öhreto?lu and Sari (2019) reported that the free hydroxyl group at C-4’ in the B ring of isoflavone compound is important for α-glucosidase inhibition (?öhreto?lu and Sari, 2019).
Interestingly, the α-glucosidase inhibitory effect of Cs_W and Gm_W was less than the ethanol extract of Cs and Gm. We suggest that flavonoid content in aqueous extract might be dominated by glycoside flavonoid (flavonoid-containing sugar). Sugar substitution at any position on the aglycon reduced the inhibitory effect of flavonoids (?öhreto?lu and Sari, 2019). Then, the α-glucosidase inhibitory activity of glycoside flavonoid was less than its aglycon. Anyhow, the utility of those plants in the community generally used decoction with water rather than ethanol extract. This study proved that the decoction of Gm pericarp and Cs heartwood extract also revealed its α-glucosidase inhibitory activity.
 | Table 1. Total flavonoid contents and α-glucosidase inhibition (IC50 value) of Cs and Gm extracts. [Click here to view] |
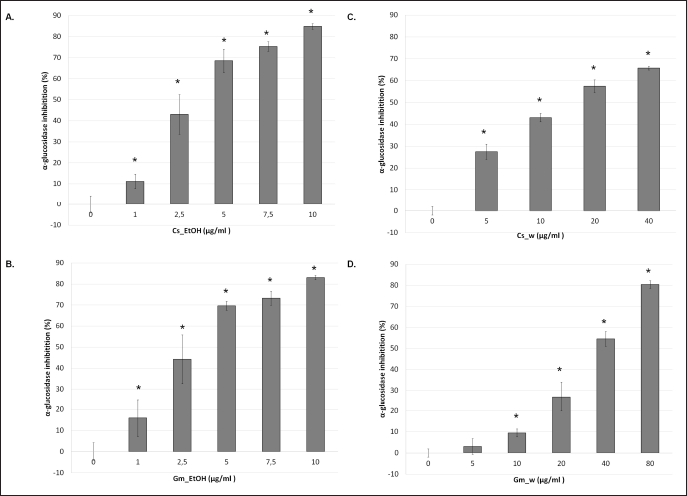 | Figure 1. Inhibition of α-glucosidase of Cs and Gm extracts. Ethanol extract (A–B) and aqueous extract (C–D) of Cs and Gm were presented respectively. Results were collected according to the description in Methods. Results were expressed as means ± standard deviation (n = 3, * p < 0.05). [Click here to view] |
Compounds analysis using LC-HRMS
As a susceptible and selective technique for determining polyphenols in food matrices, LC-HRMS has gained wide acceptance (Lucci et al., 2017). The LC-HRMS analysis of targeted compounds from ethanol and aqueous extracts of Cs according to their compound area was shown in Table 2. Brazilein, protosappanin A, and sappanchalcone from Cs heartwood showed higher areas in ethanol extract compared to its aqueous extract. While protosappanin B showed a slightly higher area in the aqueous extract compared to its ethanol extract. Interestingly, brazilin showed a higher area in aqueous extract. Brazilin is one of the major compounds in Cs heartwood (Nirmal et al., 2015). Nevertheless, brazilin was identified in this study as the lowest area in both extracts. Brazilin probably hydrolyzed to brazilein. Because of exposure to air and light, brazilin can be hydrolyzed to brazilein (de Oliveira et al., 2002). Then, brazilein in both extracts appeared in high areas (Fig. 2; Table 2).
On the other hand, the LC-HRMS analysis of targeted compounds from ethanol and aqueous extracts of Gm pericarp was also shown in Table 2. According to Yoshimura et al. (2015), flavonoids epicatechin and proanthocyanidin B2 are abundant in Gm pericarp. This study showed that catechin and proanthocyanidin B2 composed higher areas in the ethanol extract of Gm compared to its aqueous extract, while gallocatechin and proanthocyanidin A2 composed higher areas in the aqueous extract compared to its ethanol extract (Fig. 2; Table 2). This study also analyzed the presence of mangostin, a xanthone compound that is major in Gm pericarp. Mangostin is poorly soluble in water (Bumrung et al., 2020). This study revealed that mangostin was only detected in ethanol extract of Gm (Fig. 2; Table 2). Thus, mangostin in the aqueous extract was probably in a very low concentration and was not detected in LC-HRMS.
Protein-ligand interaction
Molecular docking effectively describes the interaction between enzymes and small molecules (Zhu et al., 2019). Then, the interaction of α-glucosidase enzyme (3W37) with several flavonoid contents from Cs heartwood and Gm pericarp in this study was described using a molecular docking study. The RMSD parameter was used for the validation of the docking protocol. The result showed that the RMSD value of docking validation of α-glucosidase (3W37) was 1.5 Å. Then, the docking protocol of α-glucosidase (3W37) used in this study meets the required RMSD value (≤2 Å). Qe performed a good inhibition toward the α-glucosidase enzyme (Table 1). Then, the affinity of Qe on α-glucosidase was also tested with this method. Interestingly, Qe had a higher docking score compared to acarbose (Table 3). The higher the docking score, the lower the affinity of the protein. This phenomenon was explained by ?öhreto?lu et al. (2018). ?öhreto?lu et al. (2018) found that Qe docked to several predicted allosteric sites of the α-glucosidase enzyme and was found to be a noncompetitive inhibitor.
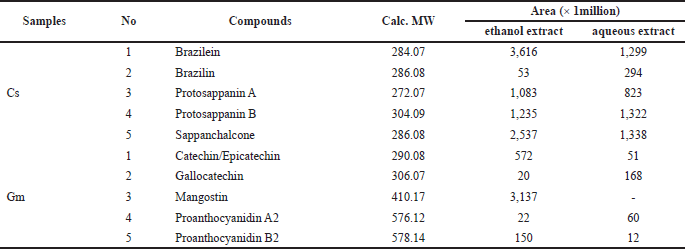 | Table 2. The LC-HRMS analysis of targeting compounds from Caesalpinia sappan heartwood (Cs) and Garcinia mangostana pericarp (Gm) extracts. [Click here to view] |
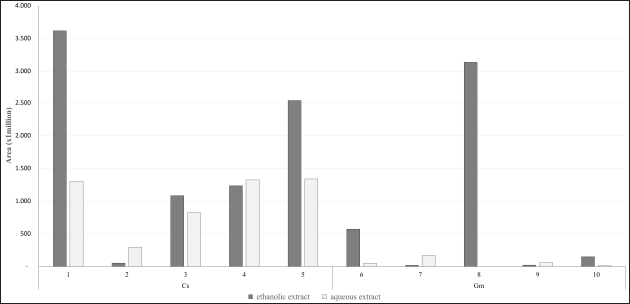 | Figure 2. The area of targeting compounds from Cs heartwood and Gm pericarp extracts according to LC-HRMS analysis. 1. Brazilein, 2. Brazilin, 3. Protosappanin A. 4. Protosappanin B. 5. Sappanchalcone. 6. Catechin. 7. Gallocatechin. 8. Mangostin. 9. Proanthocyanidin A2. 10. Proanthocyanidin B2. [Click here to view] |
The interaction of α-glucosidase (3W37) with several flavonoid compounds from Cs heartwood, namely brazilein, brazilin, protosappanin A, protosappanin B, and sappanchalcone (Nirmal et al., 2015), was also tested using this docking method. The results revealed that sappanchalcone showed the lowest energy. All of the target compounds from Cs heartwood performed their affinity toward α-glucosidase (Fig. 3; Table 3). Nevertheless, it showed higher docking scores compared to acarbose as a native ligand.
Although α-mangostin is not a flavonoid compound, its presence as a major compound in Gm pericarp cannot be ignored. This study revealed that the affinity of α-mangostin toward α-glucosidase was as lower as Qe. Epicatechin and epigallocatechin, phenolic flavonoid compounds of Gm pericarp used in this study, showed higher affinity toward α-glucosidase compared to Qe and α-mangostin. However, it showed lower affinity compared to acarbose as a native ligand. The lower affinity of several compounds toward α-glucosidase in this study probably occurred because of the different active sites with acarbose in the α-glucosidase enzyme. Nevertheless, further investigation needs to be done.
On the other hand, two flavonoid compounds of Gm pericarp used in this study, namely, proanthocyanidin A2 and proanthocyanidin B2, revealed good affinities compared to acarbose (Fig. 3; Table 3). Although proanthocyanidin A2 only had one hydrogen bond, the good affinity of proanthocyanidin A2 was probably performed because this compound also was bound with several hydrophobic and external bonds in the active site. The hydrophobic interaction allows stabilization for the compound in the active site and creates a very well-fit interaction in the enzyme’s active pocket (Sun et al., 2015). On the other hand, the good affinity of proanthocyanidin B2 was probably performed because of the bonding of its compound with four hydrogen bonds and several hydrophobic and external bonds in the active site (Fig. 3; Table 3). Proanthocyanidin A2 and proanthocyanidin B2 showed good affinities with α-glucosidase compared to the native ligand.
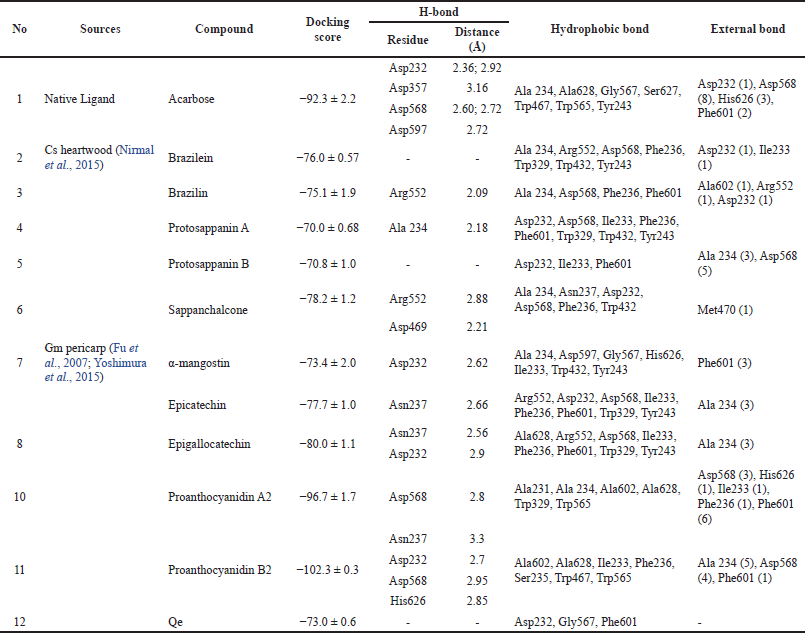 | Table 3. Protein-ligand interaction of α-glucosidase protein (3W37) with target compounds. [Click here to view] |
As shown in Table 3, the pharmacophore group of compounds that contributed to the interaction using this method can be found in the H-bond interaction (−OH group). Nevertheless, the hydrophobic structure of the compound also had an important role. Ur Rehman et al. (2019) reported that the active sites of the a-glucosidase enzyme are mainly the site for the stabilization of the substrate and the site for entrance and exit regulation of the substrate. However, the type of amino acid that has a role can vary depending on the substrate (Sakulkeo et al., 2022; Thao et al., 2021; Ur Rehman et al., 2019). In this study, the increase of affinity of the enzyme-substrate bond mostly depends on the number of interactions rather than the specific amino acid that is involved in this interaction. Even though targeted flavonoid compounds from GM had better affinity compared to Cs in the docking study, the inhibitory activity of Gm extracts was not better than Cs extracts in the in vitro study (Table 1). The phenomena can be explained using LC-HRMS results, whereas the abundance of the targeted compounds in the Gm extracts was lower than in the Cs extracts (Table 2). However, flavonoid compounds of Gm pericarp and Cs heartwood extracts used in this study revealed their affinity with the acarbose active site in the α-glucosidase enzyme.
Combination study
Traditional medicine using plant extract is generally prepared on beverage formulation. Gm pericarp extract has an unpleasant taste, while Cs heartwood extract tastes flavorless. Combinations of Gm and Cs extracts were expected to reduce the unpleasant taste of Gm and showed a higher α-glucosidase inhibition activity compared to its single extract. However, analysis of drug-drug interaction is important in combination studies. Drug interactions have been determined using in vitro studies. While computational approaches are used to analyze experimental data, i.e., synergistic, additive, or antagonistic (Zhao et al., 2010). The CI is a kind of parameter that is used for investigating synergistic drug combinations (Huang et al., 2017). Furthermore, we need to compare the effectiveness of both ethanol and aqueous extract of the combination of Gm pericarp and Cs heartwood on α-glucosidase inhibition. The results showed that the combination treatment of Cs and Gm ethanol extract under its IC50 concentration performed synergism to moderate-slight synergism of α-glucosidase inhibitory effect (CI = 0.6–0.9). In the higher concentration, the combination of 7.5 μg/ml Cs_EtOH with 2.5 and 5 μg/ml Gm_EtOH and the combination of 7.5 μg/ml Gm_EtOH with 5 μg/ml Cs_EtOH showed nearly additive effect (CI = 0.9–1.0). While the combination of a high concentration of Cs_EtOH with Gm_EtOH (7.5 μg/ml) showed a slight to moderate antagonism effect (CI = 1.2). A similar phenomenon also occurred in aqueous extract. Combination treatment of Cs and Gm aqueous extracts under its IC50 concentration performed synergism to moderate-slight synergism of α-glucosidase inhibitory effect (CI = 0.6–0.9). In comparison, the combination of 40 μg/ml Cs_W (above IC50) with all of Gm_W concentration treatments showed a slight to moderate antagonism effect. This study confirmed that even though performed in higher concentration compared to its ethanol extract, the decoction of both plants (aqueous extract) also showed a good performance on α-glucosidase inhibitory activity. The combination of both plants in ethanol and aqueous extracts revealed synergistic inhibitory effects on α-glucosidase activity especially under IC50 concentration, as shown by the CI values, which were less than 1 (Fig. 4). Nevertheless, to maintain both its synergistic antidiabetic activity and people’s acceptance, choosing of the combination of a high concentration of Cs extract with a low concentration of Gm extract would reduce the unpleasant taste of Gm and show a synergistic effect with higher α-glucosidase inhibition activity than its single application. Thus, the chosen combinations were a combination of 5 μg/ml Cs_EtOH–1 μg/ml Gm_EtOH and a combination of 20 μg/ml Cs_W–5 μg/ml Gm_W which synergistically inhibit α-glucosidase activity until we get 67.64% and 62.36%, respectively, with CI values less than 1 (0.74 and 0.7, respectively). These results were relevant to Sulastri et al. (2022) whereas polyherbal combination has a better α-glucosidase inhibitory activity than single herbs. These combinations also confirmed the LC-HRMS and docking results. According to the LC-HRMS and docking results, even though the abundance of the targeted compounds in the Gm extracts was lower than in the Cs extracts, the targeted compounds of Gm pericarp and Cs heartwood extracts used in this study revealed their affinity with the acarbose active site in the α-glucosidase enzyme (Tables 2–3). Thus, in vitro assay of the combination performed its synergistic activity on α-glucosidase inhibition.
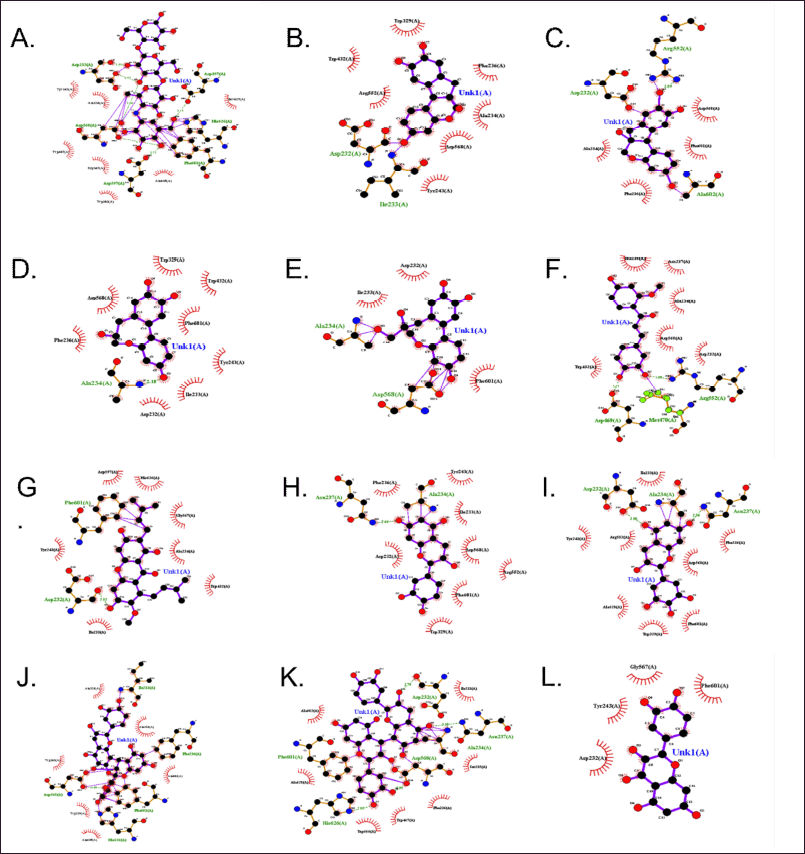 | Figure 3. The 2D molecular docking poses predicted for α-glucosidase (3W37). A. Native ligand Acarbose. B. Brazilein. C. Brazilin, D. Protosappanin A. E. Protosappanin B. F. Sappanchalcone. G. α-mangostin. H. Epicatechin. I. Epigallocatechin. J. Proanthocyanidin A2. K. Proanthocyanidin B2. L. Qe exhibiting different types of intermolecular interactions, i.e., hydrogen bond (green lines), external bond (light purple lines), hydrophobic interactions (red bricks). [Click here to view] |
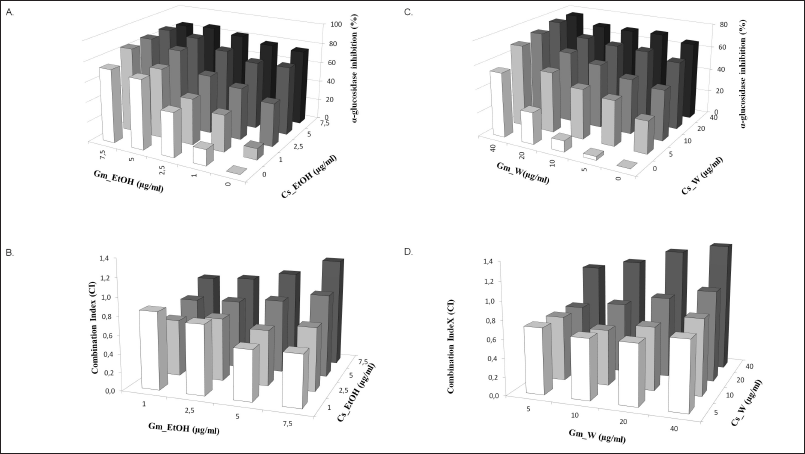 | Figure 4. The effect of combinations of Cs and Gm extracts on α-glucosidase inhibition. The α-glucosidase inhibition activity of combinations of Cs and Gm ethanol (A) and aqueous (C) extract. The CI value of combinations of Cs and Gm ethanol (B) and aqueous (D) extract. [Click here to view] |
Both ethanol and aqueous extracts of Gm pericarp and Cs heartwood inhibited α-glucosidase activity and had synergistic effects in combination treatment. Nevertheless, further investigation needs to be done to observe the synergistic mechanism of this combination.
CONCLUSION
The ethanol and aqueous extracts of Gm pericarp and Cs heartwood revealed their α-glucosidase inhibitory activity. Nevertheless, ethanol extract showed higher activity compared to its aqueous extract. Flavonoid compounds of Gm pericarp and Cs heartwood extracts that were used in this study indicated their affinity with the acarbose active site in the α-glucosidase enzyme. Analysis of the combination study showed that combinations of both Cs and Gm in ethanol and aqueous extracts inhibit α-glucosidase activity and show synergistic effects, especially under IC50 concentration. However, the limitation of this study is that it only covered the in vitro and in silico results. Thus, further investigations need to be done such as in vivo and clinical studies to strengthen the efficacy. Thus, the combination extract of Gm pericarp and Cs heartwood can be developed as a potential herbal supplement to prevent and manage diabetes mellitus.
AUTHORS’ CONTRIBUTION
S.H. and N.A. conceived and designed the project. N.A., I.D.D., A.W., S.S., and S.H. performed the experiments. D.M., N.A., and S.H. analyzed the results. N.A. and S.H. drafted, revised, and finalized the manuscript which was read and approved by all authors.
FINANCIAL SUPPORT
This study used materials and utilities from the National Research and Innovation Agency (BRIN). Authors have not received any financial support for this study.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not use experiments using animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aizat WM, Ahmad-Hashim FH, Syed Jaafar SN. Valorization of mangosteen, “The Queen of Fruits,” and new advances in postharvest and in food and engineering applications: a review. J Adv Res, 2019; 20:61–70; doi:10.1016/j.jare.2019.05.005
Athinarayanana G, Ranjitsingh AJA, Nanthini AUR, Padmalatha C. Toxicological studies of Caesalpinia sappan wood derived dye in Wister albino rats. Food Sci Hum Wellness, 2017; 6:34–8; doi:10.1016/j.fshw.2016.10.004
Bumrung J, Chanchao C, Intasanta V, Palaga T, Wanichwecharungruang S. Water-dispersible unadulterated α-mangostin particles for biomedical applications. R Soc Open Sci, 2020; 7:200543; doi:10.1098/rsos.200543
Chandra S, Khan S, Avula B, Lata H, Yang MH, Elsohly MA, Khan IA. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid-Based Complement Altern Med, 2014; 2014:253875; doi:10.1155/2014/253875
de Oliveira LFC, Edwards HGM, Velozo ES, Nesbitt M. Vibrational spectroscopic study of brazilin and brazilein, the main constituents of brazilwood from Brazil. Vib Spectrosc, 2002; 28:243–9; doi:10.1016/S0924-2031(01)00138-2
Fu C, Loo AEK, Chia FPP, Huang D. Oligomeric proanthocyanidins from mangosteen pericarps. J Agric Food Chem, 2007; 55:7689–94; doi:10.1021/jf071166n
Handayani S, Susidarti RA, Jenie RI, Meiyanto E. Two active compounds from Caesalpinia sappan L. in combination with cisplatin synergistically induce apoptosis and cell cycle arrest on WiDr cells. Adv Pharm Bull, 2017; 7:375–80; doi:10.15171/apb.2017.045
Handayani S, Susidarti RA, Lotulung PDN, Darmawan A, Meiyanto E, Jenie RI. Antimigratory activity of Brazilin-containing fraction from Caesalpinia sappan L. on MDAMB-231 cells. HAYATI J Biosci, 2020; 27:266–72; doi:10.4308/hjb.27.4.266
Handayani S, Susidarti RA, Udin Z, Meiyanto E, Jenie RI. Brazilein in combination with cisplatin inhibit proliferation and migration on highly metastatic cancer cells, 4T1. Indones J Biotechnol, 2016; 21:38–47; doi:10.22146/ijbiotech.26106
Handayani S, Susidarti RA, Utomo RY, Meiyanto E, Jenie RII. Synergistic cytotoxic and antimigratory effect of brazilein and doxorubicin on HER2-overexpressing cells. Asian Pac J Cancer Prev, 2022; 23:2623–32; doi:10.31557/APJCP.2022.23.8.2623
Huang L, Jiang Y, Chen Y. Predicting drug combination index and simulating the network-regulation dynamics by mathematical modeling of drug-targeted EGFR-ERK signaling pathway. Sci Rep, 2017; 7:40752; doi:10.1038/srep40752
Jenie RI, Handayani S, Susidarti RA, Udin LZ, Meiyanto E. The cytotoxic and antimigratory activity of brazilin-doxorubicin on MCF-7/HER2 cells. Adv Pharm Bull, 2018; 8:507–16; doi:10.15171/apb.2018.059
Jenie RI, Handayani S, Susidarti RA, Udin Z, Meiyanto E. Cytotoxic and antimetastasis effect of ethyl acetate fraction from Caesalpinia sappan L. on MCF-7/HER2 cells. Indones J Cancer Chemoprevent, 2017; 8:42–50; doi:10.14499/indonesianjcanchemoprev8iss1pp42-50
Kalra S. Incretin enhancement without hyperinsulinemia: α-glucosidase inhibitors. Expert Rev Endocrinol Metab, 2014; 9:423–5; doi:10.1586/17446651.2014.931807
Krischer JP, Liu X, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B; TEDDY Study Group. The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes, 2017; 66:3122–9; doi:10.2337/db17-0261
Lucci P, Saurina J, Núñez O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal Chem, 2017; 88:1–24; doi:10.1016/j.trac.2016.12.006
Meiyanto E, Lestari B, Sugiyanto RN, Jenie RI, Utomo RY, Sasmito E, Murwanti R. Caesalpinia sappan L. heartwood ethanolic extract exerts genotoxic inhibitory and cytotoxic effects. Orient Pharm Exp Med, 2019; 19:27–36; doi:10.1007/s13596-018-0351-9
Meiyanto E, Putri DDP, Susidarti RA, Murwanti R, Sardjiman S, Fitriasari A, Husnaa U, Purnomo H, Kawaichi M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev, 2014; 15:179–84; doi:10.7314/APJCP.2014.15.1.179
Moelands SV, Lucassen PL, Akkermans RP, Grauw WJD, Laar FAV de. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev, 2018; doi:10.1002/14651858.CD005061.pub3
Nirmal NP, Panichayupakaranant P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm Biol, 2015; 53:1339–43; doi:10.3109/13880209.2014.982295
Nirmal NP, Rajput MS, Prasad RGSV, Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: a review. Asian Pac J Trop Med, 2015; 8:421–30; doi:10.1016/j.apjtm.2015.05.014
Pathak R, Pathak A. Study of life style habits on risk of type 2 diabetes. Int J Appl Basic Med Res, 2012; 2:92; doi:10.4103/2229-516X.106349
Proença C, Freitas M, Ribeiro D, Oliveira EFT, Sousa JLC, Tomé SM, Ramos MJ, Silva AMS, Fernandes PA, Fernandes E. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. J Enzyme Inhib Med Chem, 2017; 32:1216–28; doi:10.1080/14756366.2017.1368503
Rachmady R, Muntafiah L, Rosyadi F, Sholihah I, Handayani S, Jenie RI. Antiproliferative effect of secang heartwood ethanolic extract (Caesalpinia sappan L.) on HER2-positive breast cancer cells. Indones J Cancer Chemoprevent, 2016; 7:1–5; doi:10.14499/indonesianjcanchemoprev7iss1pp1-5
Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med, 2005; 110:173–83; doi:10.1385/1-59259-869-2:173
Rohman A, Arifah FH, Irnawati, Alam G, Muchtaridi, Rafi M. A review on phytochemical constituents, role on metabolic diseases, and toxicological assessments of underutilized part of Garcinia mangostana L. fruit. J Appl Pharm Sci, 2020; 10,:127–46; doi:10.7324/JAPS.2020.10716
Sakulkeo O, Wattanapiromsakul C, Pitakbut T, Dej-Adisai S. Alpha-glucosidase inhibition and molecular docking of isolated compounds from traditional Thai medicinal plant, Neuropeltis racemosa wall. Mol Basel Switz, 2022; 27:639; doi:10.3390/molecules27030639
Sembiring E, Elya B, Sauriasari R, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J, 2018; 10:123–7; doi:10.5530/pj.2018.1.22
Slámová K, Kapešová J, Valentová K. “Sweet Flavonoids”: glycosidase-catalyzed modifications. Int J Mol Sci, 2018; 19:2126; doi:10.3390/ijms19072126
?öhreto?lu D, Sari S. Flavonoids as alpha-glucosidase inhibitors: mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem Rev, 2019; doi:10.1007/s11101-019-09610-6
?öhreto?lu D, Sari S, Barut B, Özel A. Discovery of potent α-glucosidase inhibitor flavonols: insights into mechanism of action through inhibition kinetics and docking simulations. Bioorganic Chem, 2018; 79:257–64; doi:10.1016/j.bioorg.2018.05.010
Sulastri L, Simanjuntak P, Wahono Sumaryono RD, Ardiaynto D, Abdillah S. Antidiabetic formulation development based on natural materials as α-glucosidase enzyme inhibitor. J Hunan Univ Nat Sci, 2022; 49.
Sun H, Li Y, Zhang X, Lei Y, Ding W, Zhao X, Wang H, Song X, Yao Q, Zhang Y, Ma Y, Wang R, Zhu T, Yu P. Synthesis, α-glucosidase inhibitory and molecular docking studies of prenylated and geranylated flavones, isoflavones and chalcones. Bioorg Med Chem Lett, 2015; 25:4567–71; doi:10.1016/j.bmcl.2015.08.059
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract, 2022; 183:109119; doi:10.1016/j.diabres.2021.109119
Taher M, Tg Zakaria TMFS, Susanti D, Zakaria ZA. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement Altern Med, 2016; 16; doi:10.1186/s12906-016-1118-9
Thao TTP, Q. Bui T, Tu Quy P, Chi Bao N, Loc TV, Chien TV, Chi NL, Tuan NV, Sung TV, Nhung NTA. Isolation, semi-synthesis, docking-based prediction, and bioassay-based activity of Dolichandrone spathacea iridoids: new catalpol derivatives as glucosidase inhibitors. RSC Adv, 2021; 11:11959–75; doi:10.1039/D1RA00441G
Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A, Dzobo K. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci, 2018; 19; doi:10.3390/ijms19061578
Tjahjani S, Widowati W, Khiong K, Suhendra A, Tjokropranoto R. Antioxidant properties of Garcinia mangostana L (Mangosteen) rind. Procedia Chem, 2014; 13:198–203; doi:10.1016/j.proche.2014.12.027
Ur Rehman N, Rafiq K, Khan A, Ahsan Halim S, Ali L, Al-Saady N, Hilal Al-Balushi A, Al-Busaidi HK, Al-Harrasi A. α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris hoytii. Mar Drugs, 2019; 17:E666; doi:10.3390/md17120666
Windarsih A, Suratno, Warmiko HD, Indrianingsih AW, Rohman A, Ulumuddin YI. Untargeted metabolomics and proteomics approach using liquid chromatography-Orbitrap high resolution mass spectrometry to detect pork adulteration in Pangasius hypopthalmus meat. Food Chem, 2022; 386:132856; doi:10.1016/j.foodchem.2022.132856
Yoshimura M, Ninomiya K, Tagashira Y, Maejima K, Yoshida T, Amakura Y. Polyphenolic constituents of the pericarp of mangosteen (Garcinia mangostana L.). J Agric Food Chem, 2015; 63:7670–4; doi:10.1021/acs.jafc.5b01771
Zhang AJ, Rimando AM, Mizuno CS, Mathews ST. α-Glucosidase inhibitory effect of resveratrol and piceatannol. J Nutr Biochem, 2017; 47:86–93; doi:10.1016/j.jnutbio.2017.05.008
Zhao L, Au JLS, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci Elite Ed, 2010; 2:241–9.
Zhao Y, Wang Y, Lou H, Shan L. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Oncotarget, 2017; 8:81027–39; doi:10.18632/oncotarget.17515
Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A. Synergistic effects of chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol, 2016; 7:201; doi:10.3389/fphar.2016.00201
Zhu J, Zhang B, Tan C, Huang Q. α-Glucosidase inhibitors: consistency of in silico docking data with in vitro inhibitory data and inhibitory effect prediction of quercetin derivatives. Food Funct, 2019; 10:6312–21; doi:10.1039/c9fo01333d