INTRODUCTION
Type 2 diabetes mellitus (T2DM) is now recognized as a serious public health problem in the 21st century (Zimmet, 2000). As many as 463 million people were affected by T2DM in 2019 and it is predicted that the number will increase to 700 million (constituting 10.9% of the world population) by 2045 (Saeedi et al., 2019). T2DM is now a main cause of mortality and morbidity and imposes an important financial burden on society.
Several studies have demonstrated that T2DM is associated with dyslipidemia (Chehade et al., 2013; Mehta et al., 2021). Increased serum total cholesterol (TC), low-density lipoproteins (LDLs), triglycerides (TG), and/or a reduction in high-density lipoproteins (HDLs) are also associated with T2DM complications such as diabetic peripheral neuropathy (Al-Ani et al., 2011). Medication for T2DM without undesirable side effects remains a challenging question for medical practitioners, encouraging efforts to find novel treatments or strategies for the prevention and treatment of this morbid disorder.
There is much evidence that oxidative stress plays an important role in the pathogenesis of T2DM by inducing insulin resistance or decreasing insulin secretion, which further aggravates the development and progression of T2DM and its complications (Burgos-Morón et al., 2019). In addition to the currently available pharmaceutical treatments, various medicinal herbs, fruits, or plant components have been recommended for T2DM management (Bindu and Narendhirakannan, 2019). There is evidence that bioactive compounds present in fruits, herbs, and various parts of plants can lower blood glucose concentrations and inhibit or prevent oxidation processes, helping to preserve β-cell function by reducing β-cell apoptosis (Rahimi et al., 2005). Therefore, the exploration of management strategies in the form of natural food products with antioxidants and hypoglycemic effects for the treatment of T2DM should be encouraged because they have fewer or no side effects and may be more economic.
The large amount of fruit peel stemming from household kitchen waste and industrial processing is not only economically costly but also can cause environmental problems and need to be utilized or managed (Kumar et al., 2020). Furthermore, several studies have revealed that the peel of fruits such as pomegranate, Punica granatum, and Syzygium cumini exhibit antidiabetic and antioxidant effects (Salwe et al., 2015; Muttakin and Zulfajri, 2020). Thus, converting fruit peel waste into value-added natural antioxidant products to combat T2DM is an attractive challenge.
Clausena lansium (Lour.) Skeels or wampee peel (WP), a medicinal plant found in southern China and northern Thailand, belongs to the family Rutaceae (Fig. 1). Various parts of WP, such as the leaves, fruits, seeds, and roots, are used as a traditional medicine to prevent or alleviate symptoms including asthma, cough, bronchitis, hepatitis, malaria, dermatitis, and gastrointestinal disorders (Liu et al., 1996; Zhu et al., 2020). WP peel also possesses several biological and pharmacological properties such as antioxidant, antimicrobial, antiobesity, anti-inflammatory, antitumour, antifungal, hepatoprotective, neuroprotective, and antioxidant activities (Adebajo et al., 2009; Liu et al., 2019). In addition, it contains many phytochemically bioactive compounds such as flavonoids, polyphenols, coumarins, carbazole alkaloids, amides, and lactams (Xu et al., 2014). Interestingly, the ethanol extract from the peel of WP was found to have a high flavonoid content (49.73 ± 0.63 mg QE/100 g DW) and phenolic content (530.13 ± 0.07 mg GAE/100 g DW) and exhibited strong scavenging activity toward the 2,2-diphenyl-1-picrylhydrazyl radical (91.33% ± 0.27%) (Phachonpai and Tongun, 2021). However, to the best of our knowledge, there is no laboratory evidence of the effects of WP peel extract on oxidative stress in T2DM.
 | Figure 1. Clausena lansium (Lour.) Skeels or wampee from Nan province, Thailand. [Click here to view] |
Several studies have proposed that the combination of a high-fat diet (HFD) and a low-dose streptozotocin (STZ) injection induces an animal model for T2DM that closely mirrors the different stages of T2DM in humans (Barrière et al., 2018; Chao et al., 2021). An HFD initiates the insulin resistance that is one of the main pathophysiological features of T2DM (Lackey et al., 2016). At the same time, low-dose STZ has been reported to induce mild impairment of insulin secretion, which mimics the features of the later stages of T2DM (Sahin et al., 2007). Therefore, we investigated the antidiabetic and antihyperlipidemic properties of WP peel extract and its antioxidant effect in an animal model of T2DM induced by an HFD combined with a low-dose STZ injection.
MATERIALS AND METHODS
Drugs and chemicals
STZ, bovine serum albumin, trichloroacetic acid, thiobarbituric acid (TBA), and 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) were obtained from Sigma-Aldrich (St. Louis, MO). Rat ELISA kits for glutathione peroxidase (GPx) were purchased from Cayman (MI). All other chemical reagents used in this study were of analytical grade.
Wampee peel extract preparation
Mature WP fruits were collected from a local farm in Tanao district, in the central part of Nan province, Thailand. WP peels were removed and washed thoroughly twice under running tap water and dried in a hot air oven at 40°C for 48 hours. WP peel samples were homogenized with an electric blender and were macerated with 70% ethanol and distilled water for 72 hours, vacuum filtered, and evaporated using a rotary evaporator at 40°C. The final dry weight of WP peel extract was used to calculate the percentage yield, resulting in the extraction yield (10.93%). All samples were stored in an amber-colored reagent bottle at 4°C for further analysis.
Animals and experimental protocol
Experiments were conducted in adult male Sprague-Dawley rats, weighing between 220 and 250 g, purchased from Nomura Siam International Co., Ltd. (Bangkok, Thailand). They were placed in stainless steel cages (three rats per cage) under controlled ambient temperature (22°C ± 2°C), humidity (55%–65%), and a 12:12-hour light-dark cycle. The rats were fed with standard pellet diet and water ad libitum before any food-related treatment began. All the protocols in this study were carried out following the “Guide for the Care and Use of Laboratory Animals” of the National Institutes of Health (1985) and were approved by the Animal Ethics Committee of the University of Phayao, Thailand (approval number: 5801040006).
After 7 days of acclimatizing to laboratory conditions, all rats were fed an HFD (60% fat, 20% carbohydrates, and 20% protein) except for the uninduced control. Then, 4 weeks later, they received a low dose of STZ (30 mg/kg, intraperitoneally) to induce T2DM and 72 hours later; the fasting blood sugar (FBS) levels of all rats were determined using an Accu-Chek glucometer (Roche Diagnostic, Germany). Only rats with FBS levels ≥200 mg/dl were considered to be T2DM and enrolled in our study. According to the FBS concentration, 60 rats were randomly separated into the following six experimental groups (n = 10):
1) Normal control group (C): rats were fed on basal pelleted diet and not treated with STZ injection.
2) Diabetic control group (T2DM): diabetic rats were fed an HFD initially for 4 weeks before STZ injection. The rats were fed with an HFD for the rest of the experimental period.
3) (T2DM + WP peel 200): diabetic rats received WP peel extract in dosages of 200 mg/kg orally once daily for 4 weeks after induction of diabetes. The rats were fed with an HFD for the rest of the experimental period.
4) (T2DM + WP peel 400): diabetic rats received WP peel extract in dosages of 400 mg/kg orally once daily for 4 weeks after induction of diabetes. The rats were fed with an HFD for the rest of the experimental period.
5) (T2DM + WP peel 600): diabetic rats received WP peel extract in dosages of 600 mg/kg orally once daily for 4 weeks after induction of diabetes. The rats were fed with an HFD for the rest of the experimental period.
6) Positive control (T2DM + Met): diabetic rats received metformin in dosages of 500 mg/kg orally once daily for 4 weeks after induction of diabetes. The rats were fed with an HFD for the rest of the experimental period.
Food intake, water consumption, and body weight were recorded weekly. At the end of the experimental period (4 weeks), rats were deprived of food and water for 12 hours, anesthetized with chloral hydrate, and sacrificed by decapitation, and blood was collected to determine HbA1c, lipid profile, lipid peroxidation, and antioxidant enzyme activities.
Determination of glycosylated hemoglobin (HbA1c) and lipid profile
Whole blood was used in the measurement of glycosylated hemoglobin (HbA1c) with the aid of an automatic glycated hemoglobin HbA1c analyzer (HemoCue® HbA1c 501 System, HemoCue AB Co. Ltd., Sweden).
For lipid profile determination (TC; total triglyceride (TG); serum HDL cholesterol); and serum LDL cholesterol), blood was allowed to clot for 15 minutes before centrifuging at 5,000 rpm for 15 minutes to separate the serum. An automatic dry-chemistry analyzer (Fuji Dri-Chem 3,500, Fujifilm, Tokyo, Japan) was used to determine the amount of TG, TC, and HDL cholesterol. The LDL concentration in serum was calculated according to Friedewald’s formula (Friedewald et al., 1972).
Estimation of lipid peroxidation
Serum malondialdehyde (MDA) levels as a marker of lipid peroxidation were determined using a modification of Ohkawa et al. (1979) method. The MDA concentration was measured using a Thiobarbituric Acid Reactive Substances assay kit.
Evaluation of antioxidant enzyme activities
Serum superoxide dismutase (SOD) activity was evaluated using the SOD assay kit from Cayman Chemicals according to the manufacturer’s instructions. This technique was based on the generation of superoxide anion radicals produced by xanthine/xanthine oxidase. Absorbance of the reaction solution was detected at 505 nm and expressed as U/mg of protein.
Serum GPx activity was determined using a rat GPx ELISA kit (MBS727547, My BioSource, Inc., San Diego, CA), based on the method of Paglia and Valentine (1967). The activity of GPx was recorded spectrophotometrically at 450 nm and expressed as U/mg of protein.
Statistical analysis
All results were reported as mean ± SD. Statistical analysis of data was conducted by ANOVA followed by the Duncan multiple test using SPSS version 11.5 for Windows (SPSS, Chicago, IL). Differences were judged significant at p < 0.05.
RESULTS
The effects of WP peel extract on food consumption and water intake
Our results showed that the T2DM rats had a higher (p < 0.05) intake of food and water than that of control rats, which is consistent with the characteristics of T2DM. However, the increase in food and water intake was reversed by a high dose of WP peel extract as well as by metformin (Table 1).
The effect of WP peel extract on body weight
The mean body weight of all treated groups at the end of the experimental period is shown in Figure 2. At the beginning of the experiment, there was no significant difference in the mean body weight between experimental groups. However, it was observed that the mean body weight of the T2DM groups administered WP peel extract at doses of 200 and 400 mg/kg was significantly (p < 0.05) higher than that of the control group and showed characteristics of obesity after 1 week of treatment. Oral administration of a high dose of WP was able to reduce body weight when compared with control T2DM rats (p < 0.05). Moreover, rats treated with the highest concentration of WP peel extract showed no significant changes in body weight gain when compared with the control group after 2 weeks. This pattern continued throughout the experimental period. The results suggest that a high dose of WP peel extract was able to attenuate weight gain in T2DM rats.
 | Table 1. Effects of WP peel extract on food consumption and water intake in T2DM rats. [Click here to view] |
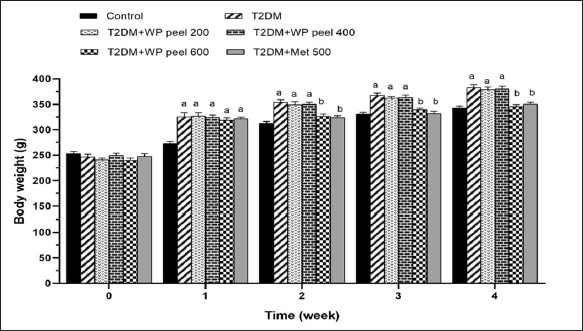 | Figure 2. Effect of WP peel extract on body weight in T2DM rats. ap < 0.05 versus control group, bp < 0.05 versus T2DM. T2DM: type 2 diabetic rat; WP: wampee peel extract; Met: Metformin. [Click here to view] |
The effect of WP peel extract on FBS concentration
According to the results presented in Figure 3, an HFD- and STZ-induced T2DM in rats, has indicated the significant increase in FBS concentration compared to control rats. Interestingly, rats subjected to WP peel extract at a dose of 600 mg/kg and metformin showed a significantly (p < 0.05) lower increase in FBS concentration in the 2nd week of treatment, and this effect continued until the end of the experiment. However, rats administered WP peel extract at doses of 200–400 mg/kg showed no significant change in this parameter.
The effect of WP peel extract on HbA1c and lipid profile
The HbA1c and lipid profile analysis of all rats in the treated groups is presented in Table 2. Our results showed that the rats fed an HFD and administered STZ experienced a significant (p < 0.05) increase in HbA1c levels compared to control rats. In addition, the control T2DM rats showed a significant (p < 0.05) increase in lipid profile concentrations at all times during the study as compared with all experimental groups. However, a significant (p < 0.05) reduction in HbA1c levels was observed in rats treated with both metformin and WP peel extract at a dose of 600 mg/kg compared with the control T2DM rats. Moreover, the one-way ANOVA revealed a significant (p < 0.05) reduction in the lipid profile concentrations as compared with the control T2DM rats. Unfortunately, low and medium doses of WP peel extract neither have any effect on HbA1c levels nor were the lipid profile concentrations significantly affected compared with the control T2DM rats.
The effect of WP peel extract on serum antioxidant enzyme activities and lipid peroxidation
Our data showed that rats treated with an HFD and low-dose STZ injection experienced a significant (p < 0.05) increase in serum MDA levels but a decrease (p < 0.05) in SOD and GPx activities compared to the control group. On the other hand, in the T2DM group that received WP peel extract at a dose of 600 mg/kg, there were significantly (p < 0.05) lower MDA levels, but significantly (p < 0.05) higher SOD and GPx activities when compared with the control T2DM rats (Table 3).
DISCUSSION
The possibility of developing nutraceutical agents from plants or fruit wastes has been receiving increased attention because of their biological and pharmacological properties that could aid in preventing and treating several diseases including T2DM. Our results indicate that the consumption of WP peel extract was quite effective at lowering blood glucose, HbA1c, lipid profile levels, body weight gain, and oxidative stress in a rat model of T2DM. In addition, treatment with various doses of WP peel extract did not produce any significant adverse effect or changes in behavior or mortality in the experimental rats.
One hallmark symptom of T2DM is chronic hyperglycemia, which results from the impaired metabolism of carbohydrates, lipids, and proteins and leads to uncontrolled lipolysis (Nolan and Prentki, 2019). Extreme hunger and excessive thirst are also related to hyperglycemia (Bibak et al., 2014). Uncontrolled high blood sugar concentrations are associated with the process of glucose autoxidation, protein glycation, and activation of the polyol pathway (Ighodaro, 2018). All these pathways lead to the generation of reactive oxygen species (ROS) and increase lipid peroxidation products in various tissues (Forbes and Cooper, 2013).
Owing to interactions between lipid metabolism and hyperglycemia, glycolipid metabolic disorder is becoming a main factor in T2DM (Perry et al., 2014). Several studies have confirmed that a low-dose STZ injection following HFD feeding in a rat model is a good choice for inducing impaired insulin secretion and insulin resistance, similar to the significant characteristic features of the later stages of T2DM (from insulin resistance to β-cell failure) (Gheibi et al., 2017; Srinivasan et al., 2005). A high single dose of STZ is widely used for experiments attempting to cause severe T1DM through direct toxicity to pancreatic islet β-cells (Schnedl et al., 1994). Reed et al. (2000) reported that when a low dose of STZ was used after high-fat feeding, the functions of the β-cell mass and β-cell capacity were moderately impaired without fully compromising insulin secretion, resulting in impaired insulin glucose tolerance. Similar to our study, a rodent model of T2DM was successfully induced with an HFD and a low-dose STZ (30 mg/kg) injection. All the T2DM rats developed signs of diabetes, that is, hyperglycemia, polydipsia, and hyperphagia and as a result, high levels of FBS and HbA1c, increased weight gain, and excessive thirst and eating. In addition, an increase in plasma levels of TG, TC, and LDL and a low level of HDL were seen in the T2DM rats.
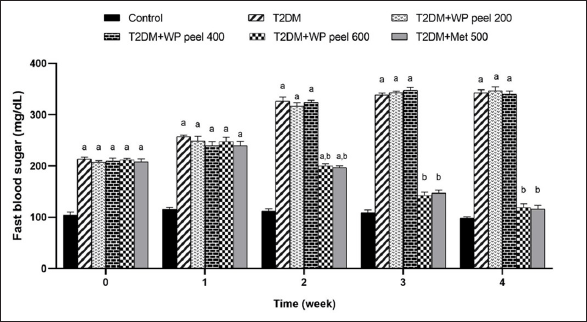 | Figure 3. Effect of WP peel extract on fasting blood glucose level in T2DM rats. ap < 0.05 versus. control group, bp < 0.05 versus T2DM. T2DM: type 2 diabetic rat; WP: wampee peel extract; Met: Metformin. [Click here to view] |
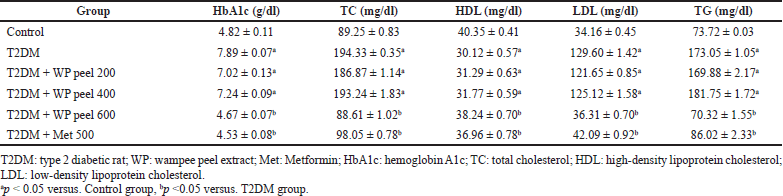 | Table 2. Effects of WP peel extract on HbA1c and lipid profile in T2DM rats. [Click here to view] |
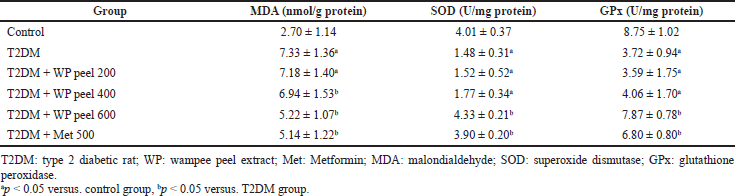 | Table 3. Effect of WP peel extract on serum lipid peroxidation and antioxidant enzymes activities in T2DM rats. [Click here to view] |
Our work clearly demonstrated that WP peel extract at a dose of 600 mg/kg has an antihyperglycemic effect by reducing FBS concentrations and HbA1c levels in experimental rats with T2DM. It was also observed that the antihyperglycemic effect of the WP peel extract given at a dose of 600 mg/kg was similar to that of metformin, which suggests that WP peel extract might have almost the same potential as metformin, a standard antidiabetic drug for T2DM management (Han et al., 2017).
The increment in food consumption and body weight is thought to be due to a decrease in the activity of endocrine regulators such as insulin and leptin, which mainly act on the hypothalamus, which in turn plays a crucial role in the regulation of energy balance and food intake (Amitani et al., 2013). In addition, excessive fluid intake following an insulin deficiency is promoted by a reduction in the hormones regulating satiety, such as cholecystokinin, peptide YY, and glucagon-like peptide-1, or a decrease in leptin receptor activity (German et al., 2010). However, T2DM rats that were treated with the highest dose of WP peel extract showed a significant attenuation in body weight gain and in the signs of polyphagia and polydipsia, which may be the result of better control of blood glucose level in diabetic rats.
Numerous epidemiological, clinical, and preclinical studies and in vivo and in vitro experiments have revealed that fruit peels are rich sources of bioactive compounds with antioxidative, antidiabetic, and hypolipidemic properties (Nanasombat et al., 2019; Sun et al., 2020). In our study, the treatment of T2DM rats with WP peel extract at a dose of 600 mg/kg led to a reduction in TC, TG, and LDL levels, suggesting that it may exert antihyperlipidemic effects. However, there was no difference in HDL levels between group receiving the high dose of WP peel extract and the control group, indicating that the WP peel extract may not affect HDL.
Hyperglycemia is a major cause of free radical formation, which can lead to increased lipid peroxidation and inadequate enzymatic antioxidant defense, which further aggravates impaired glucose tolerance in biological systems (Tangvarasittichai, 2015). An imbalance between antioxidant status and oxidation has been proposed to play a pivotal role in mediating insulin resistance (Singh et al., 2022). The primary antioxidant defense system against oxidative stress-induced cell damage consists of three antioxidant enzymes: SOD, CAT, and GSH-Px (Ighodaro and Akinloye, 2018). Usha et al. (2017) reported that various antioxidants, vitamins (A, B, C, E, and K), flavonoids, carotenoids, glycerophospholipids, polyphenols, zeaxanthin esters, and phytosterols can reduce oxidative stress and also have an antidiabetic effect (Usha et al., 2017), and our studies indicated the same. The T2DM rat model shows a buildup of oxidative stress due to the reduced activity of SOD and GPx. In addition, our team found that not only did the activities of SOD and GPx decrease in the serum, but also there was an increase in the concentrations of MDA in the T2DM rats. On the other hand, treatment of T2DM rats with WP peel extract at a dose of 600 mg/kg restored the levels of SOD and GPx in the serum. This was accompanied by a decrease in the plasma MDA concentration. Reports have shown that antioxidant supplementation could protect against the deleterious cellular and biomolecular effects that lead to islet cell dysfunction and subsequent development of T2DM (Karunakaran and Park, 2013). WP peel extract is a complex mixture that contains numerous active ingredients with a unique broad spectrum of pharmacological activities such as flavonoids, polyphenols, lactams, carbazole alkaloids, amides, and clausena coumarine, and in particular, coumarins, which are reported to possess antidiabetic and antioxidant activities (Mukherjee et al., 2006; Prasad et al., 2010). This suggests that the antidiabetic effect mediated by WP peel extract may be due to various bioactive compounds of this crude extract, which also possess the potential for additive, antagonistic, or synergistic activities. Further work is recommended to identify the unknown bioactive compound(s) in WP peel extract that may contribute to its antidiabetic activity.
Unfortunately, the low and medium doses of WP peel extract had no antidiabetic effect. The lack of effect may be related to insufficient concentrations of bioactive compounds in the WP peel extract, which may not have reached the therapeutic or plateau level of treatment. These possibilities will be addressed in the next phase of our research.
In summary, WP peel extract supplementation at a dose of 600 mg/kg attenuated hyperphagia and hyperdipsia, ameliorated hyperglycemia and body weight gain, and possessed hypolipidemic activity in T2DM rats. The reduction in oxidative stress caused by increased SOD and GPx activity could be due to the improvement in glycemia promoted by the WP peel extract. Therefore, the imbalance between the production of ROS and enzymatic antioxidant activity could be controlled in T2DM rats.
CONCLUSION
Our findings show that the crude extract of WP, a waste material generated from the consumption of wampee fruit, contains bioactive compounds that possess significant antidiabetic, antihyperlipidemic, and antioxidant properties in the HFD/STZ-induced T2DM rat model. WP peel extract thus shows promise as a nutraceutical in the management of T2DM.
ACKNOWLEDGMENTS
We are sincerely grateful to University of Phayao and would like to express our gratitude to all technical staffs for their assistance and support, as well as the National Research Council of Thailand (NRCT). This study was financially supported by the University of Phayao, Thailand, with grant no. R020058217047.
LIST OF ABBREVIATIONS
DPPH, 2,2-diphenyl-1-picrylhydrazyl; DW, Dry weight; FBS, Fasting blood sugar; GAE, Gallic acid equivalent; GPx, Glutathione peroxidase; HbA1c, Glycated hemoglobin; HDL, High-density lipoprotein; HFD, High-fat diet; LDL, Low-density lipoprotein; Met, Metformin; QE, Quercetin equivalents; SOD, Superoxide dismutase; STZ, Streptozotocin; T2DM, Type 2 diabetes mellitus; TC, Total cholesterol; TG, Triglyceride; WP, Wampee peel.
AUTHORS’ CONTRIBUTIONS
Wathita Phachonpai conceived and designed the study; Wathita Phachonpai, Prathakphong Riyamongkol, and Dej Mann conducted the experiments; Wathita Phachonpai and Terdthai Tongun analyzed all data; Wathita Phachonpai wrote the paper. All authors have read and approved the manuscript.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest regarding the publication of this paper.
ETHICAL APPROVAL
This study got ethical approval by the Animal Ethics Committee of the University of Phayao, Thailand (Approval Number: 5801040006).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adebajo AC, Iwalewa EO, Obuotor EM, Ibikunle GF, Omisore NO, Adewunmi CO, Obaparusi OO, Klaes M, Adetogun GE, Schmidt TJ, Verspohl EJ. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J Ethnopharmacol, 2009; 122:10–9.
Al-Ani FS, Al-Nimer MS, Ali FS. Dyslipidemia as a contributory factor in etiopathogenesis of diabetic neuropathy. Indian J Endocrinol Metab, 2011; 15:110–4.
Amitani M, Asakawa A, Amitani H, Inui A. The rolçe of leptin in the control of insulin-glucose axis. Front Neurosci, 2013; 7:51.
Barrière DA, Noll C, Roussy G, Lizotte F, Kessai A, Kirby K, Belleville K, Beaudet N, Longpré JM, Carpentier AC, Geraldes P, Sarret P. Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep, 2018; 8:424.
Bibak B, Khalili M, Rajaei Z, Soukhtanloo M, Hadjzadeh MA, Hayatdavoudi P. Effects of melatonin on biochemical factors and food and water consumption in diabetic rats. Adv Biomed Res, 2014; 3:173.
Bindu J, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. Biotech, 2019; 9:4.
Burgos-Morón E, Abad-Jiménez Z, Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, Salom C, Jover A, Mora V, Roldan I, Solá E, Rocha M, Víctor VM. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med, 2019; 8:1385.
Chao J, Cheng HY, Chang ML, Huang SS, Liao JW, Cheng YC, Peng WH, Pao LH. Gallic acid ameliorated impaired lipid homeostasis in a mouse model of high-fat diet-and streptozotocin-induced NAFLD and diabetes through improvement of β-oxidation and ketogenesis. Front Pharmacol, 2021; 11:606759.
Chehade JM, Gladysz M, Mooradian AD. Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs, 2013; 73:327–39.
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev, 2013; 93:137–88.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18:499–502.
German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky Jr GJ, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes, 2010; 59:1626–34.
Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother, 2017; 95:605–13.
Han X, Tao YL, Deng YP, Yu JW, Cai J, Ren GF, Sun YN, Jiang GJ. Metformin ameliorates insulitis in STZ-induced diabetic mice. Peer J, 2017; 5:e3155.
Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother, 2018; 108:656–62.
Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med, 2018; 54:287–93.
Karunakaran U, Park KG. A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes Metab J, 2013; 37:106–12.
Kumar H, Bhardwaj K, Sharma R, Nepovimova E, Ku?a K, Dhanjal DS, Verma R, Bhardwaj P, Sharma S, Kumar D. Fruit and vegetable peels: utilization of high value horticultural waste in novel industrial applications. Molecules, 2020; 25:2812.
Lackey DE, Lazaro RG, Li P, Johnson A, Hernandez-Carretero A, Weber N, Vorobyova I, Tsukomoto H, Osborn O. The role of dietary fat in obesity-induced insulin resistance. Am J Physiol Endocrinol Metab, 2016; 311:E989–97.
Liu YP, Guo JM, Liu YY, Hu S, Yan G, Qiang L, Fu YH. Carbazole alkaloids with potential neuroprotective activities from the fruits of Clausena lansium. J Agric Food Chem, 2019; 67:5764–71.
Liu GT, Li WX, Chen YY, Wei HL. Hepatoprotective action of nine constituents isolated from the leaves of Clausena lansium in mice. Drug Dev Res, 1996; 39:174–8.
Mehta RK, Koirala P, Mallick RL, Parajuli S, Jha R. Dyslipidemia in patients with type 2 diabetes mellitus in a tertiary care centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc, 2021; 59:305–9.
Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol, 2006; 106:1–28.
Muttakin M, Zulfajri M. Antioxidant activity of Syzygium Cumini fruit peel extract for diabetes mellitus treatment in alloxan-induced diabetic rats. Res J Chem Environ, 2020; 24:9–13.
Nanasombat S, Yansodthee K, Jongjaited I. Evaluation of antidiabetic, antioxidant and other phytochemical properties of Thai fruits, vegetables and some local food plants. WJST, 2019; 16:851–66.
Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab Vasc Dis Res, 2019; 16:118–27.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 1979; 95:351–8.
Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature, 2014; 510:84–91.
Phachonpai W, Tongun T. Cognition enhancing effects of Clausena lansium (Lour.) peel extract attenuate chronic restraint stress-induced memory deficit in rats. Heliyon, 2021; 7:e07003.
Prasad K, Xie H, Hao J, Yang B, Qiu S, Wei X, Chen F, Jiang Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem, 2010; 118:62–6.
Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother, 2005; 59:365–73.
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism, 2000; 49:1390–4.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract, 2019; 157:107843.
Sahin K, Onderci M, Tuzcu M, Ustundag B, Cikim G, Ozercan IH, Sriramoju V, Juturu V, Komorowski JR. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metabolism, 2007; 56(9):1233–40.
Salwe KJ, Sachdev DO, Bahurupi Y, Kumarappan M. Evaluation of antidiabetic, hypolipedimic and antioxidant activity of hydroalcoholic extract of leaves and fruit peel of Punica granatum in male Wistar albino rats. J Nat Sci Biol Med, 2015; 6:56–62.
Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes, 1994; 43:1326–33.
Singh A, Kukreti R, Saso L, Kukreti S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules, 2022; 27:950.
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res, 2005; 52:313–20.
Sun C, Liu Y, Zhan L, Rayat G, Xiao J, Jiang H, Li X, Chen K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci Technol, 2020; 117:3–14.
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes, 2015; 6:456–80.
Usha T, Middha SK, Narzary D, Brahma BK, Goyal AK. In silico and in vivo based scientific evaluation of traditional anti-diabetic herb Hodgsonia heteroclita. Bangladesh J Pharmacol. 2017; 12:165–6.
Xu X, Xie H, Wei X. Jasmonoid glucosides, sesquiterpenes and coumarins from the fruit of Clausena lansium. LWT Food Sci Technol, 2014; 59:65–9.
Zhu T, Zuo W, Yan J, Wen P, Pei Z, Lian H, Yang HC. Comparative assessment of the antioxidant activities among the extracts of different parts of Clausena lansium (Lour.) skeels in human gingival fibroblast cells. Evid Based Complement Alternat Med. 2020; 2020:3958098.
Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Intern Med, 2000; 247:301–10.