INTRODUCTION
Lactose is the most commonly used pharmaceutical diluent for the development of compressed solid dosage forms. However, poor flow property and compressibility of lactose limits its use as a direct compressible filler and binder (Patel et al., 2011; Ugoeze and Idris, 2020). Modification of shape and size of its particle usually improves flowability and compressibility of this excipient (Kudo et al., 2020). The compressibility of adjuvant developed by co-granulation of brittle and ductile material was found to be highly influenced by proportion of poorly fragmenting brittle material such as lactose (Gohel et al., 2003). Study on various techniques of granulation of lactose showed that freezing and subsequent drying of lactose with aqueous dispersions of starch can improve compressibility and exhibit lower ejection forces (Patel et al., 2011). However, a poor improvement in compressibility and dilution potential was observed in case of modified grades of lactose. Similarly, commercially available lactose-based co-processed excipients showed higher compactability, but with a poor tensile strength (TS)and high ejection force (Dominik et al., 2021; Hauschild and Picker, 2004; Rani and Begum, 2014).
Tablets compressed using developed excipient composite by co-granulation of highly compressible saccharides such as maltose with poorly compressible saccharides such as lactose showed marked improvement in mechanical strength without affecting disintegration, due to very high surface free energy and amorphous state of highly compressible saccharides (Mizumoto et al., 2005). Microgranules of lactose developed by coprocessing with microcrystalline cellulose and corn starch in a ratio of 7:2:1 gave excellent flow property, good compressibility with better binding property, and reduced disintegration time (Akram et al., 2011). Improved mechanical strength and disintegration property was observed for tablets compressed by using StarLac containing lactose and starch in the ratio of 85:15 (Hauschild and Picker, 2004; Schwarz et al., 2022). However, there was a negative impact on flowability of co-processed excipients observed with excipient formulation containing excess quantity of starch (Akram et al., 2011; Chang et al., 2008). A comparative study using fully gelatinized starch and pregelatinized starch showed that the tablet formulation compressed using pre-gelatinized starch exhibited better crushing strength and friability, which could be due to enlargement of the particles because of formation of agglomerates during process of pre-gelatinization (Olowosulu et al., 2011). Increase in swelling behavior was observed in case of pre-gelatinized starch. This could be due to increase in amylase content because of structural deterioration and molecular disarrangement during hydrothermal exposure of starch (Deshkar et al., 2019).
The study reported herein aims to develop COMBILOSE as a lactose-based composite excipient by coprocessing it with maltose and maize starch.
MATERIALS
Paracetamol IP was received as a gift sample from Wallace Pharmaceuticals Pvt Ltd Goa, India. Lactose monohydrate (SD Fine Chem, Mumbai, India), maize starch (Ozone International, Mumbai, India), maltose monohydrate (Molychem, Mumbai, India), purified talc (SD Fine Chem, Mumbai, India), and magnesium stearate (Molychem, Mumbai, India) were procured from Shri Venkatesh Enterprises, Dharwad, India.
Design and development of excipient composite: COMBILOSE
Aqueous solutions of maltose monohydrate in varying concentrations were prepared by dissolving the weighed quantity of maltose monohydrate (Table 1) in 100 ml of distilled water. The physical blends of lactose monohydrate and maize starch were prepared as per Table 1 and were added to respective solutions of maltose monohydrate with continuous stirring using a mechanical stirrer with rotational speed maintained at 500 rpm. The resultant dispersions were refrigerated overnight. The frozen dispersions were pre-heated for 15 minutes at 60°C to initiate pre-gelatinization of maize starch and then dried for 120 minutes at 50°C in a tray dryer. The dried composites were size reduced and sifted to obtain micro-fine granules of the required size. The developed granules were subjected to the assessment of various excipient functionality parameters.
Evaluation of pre-compression parameters
Flow properties
The rate of flow of sample of the developed COMBILOSE was determined using the angle of repose and Hausner’s ratio. A fixed funnel with a constant pressure head method was used to determine the angle of repose (Fuentes-Gonzalez and Villafuerte-Robles, 2014). Hausner’s ratio was calculated using tapped and untapped densities of COMBILOSE (Moondra et al., 2018). Carr index was determined as an indicative of compressibility of COMBILOSE. Ratio of difference in tapped density and untapped density against tapped density was determined (Moondra et al., 2018).
Moisture sorption capacity and swelling behavior
The moisture content of developed COMBILOSE was determined by exposing weighed quantity of COMBILOSE in a Petri plate to 60°C in a tray dryer till a constant weight was obtained and the percentage moisture loss against the total weight of COMBILOSE was recorded (Olorunsola et al., 2017). Moisture sorption capacity was determined by exposing the weighed amount of COMBILOSE to 75%RH for 120 hours at room temperature. The percent weight of water absorbed against the dry weight of the sample was determined (Olorunsola et al., 2017). The percent rise in the volume of wet powder of COMBILOSE due to swelling was used to calculate the percentage swelling index (Olorunsola et al., 2017).
True density and porosity
The true density of COMBILOSE was determined using a specific gravity bottle. Ortho-Xylene was used as displacement fluid (Hasan et al., 2012). The obtained values of bulk volume and true volume were used to calculate porosity of the sample of COMBILOSE (Hasan et al., 2012).
Evaluation of post-compression parameters
Evaluation of mechanical properties
Mechanical properties of the developed COMBILOSE were evaluated by compressing the blend of COMBILOSE with 2% magnesium stearate as a lubricating agent and 2% purified talc as a glidant on 12 station Karnavati tablet press. Three batches of 250 tablets each (average weight of 500 mg and 3–5 mm thickness) containing COMBILOSE 05, COMBILOSE 10, and COMBILOSE 15 were compressed by direct compression method using 10-mm D tooling with a turret speed of 4.5–5.5 RPM. The compressed tablets were evaluated for compact density (CD), Tensile Strength (TS) and bonding index, solid fraction (SF), and porosity (Table 2) (Olorunsola et al., 2017; Sandhan and Derle, 2019; Tye et al., 2005).
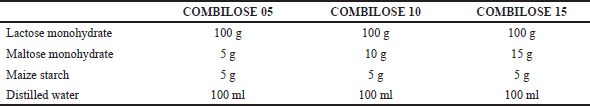 | Table 1. Formulation of COMBILOSE as co-processed excipient. [Click here to view] |
Determination of dilution potential
The dilution potential of the developed COMBILOSE was determined using paracetamol as a model for a poorly compressible drug. The formulation blends containing COMBILOSE 05, COMBILOSE 10, and COMBILOSE 15 were prepared separately with an increasing ratio of paracetamol (Table 3). The prepared blends were mixed with 2% of purified talc and 2% of magnesium stearate as a processing aid and were compressed as a tablet with an average weight of 500 mg using a direct compression method on 12 station Karnavati tablet press. TS of compressed tablets were calculated for each formulation blend using the measured diameter, thickness, and diametrical crushing strength of tablets. TS of compressed tablets were plotted against respective percentage drug content (Gohel et al., 2012; Haruna et al., 2020).
Determination of lubricant sensitivity
The effect of an increased proportion of magnesium stearate on the percentage friability of the COMBILOSE tablet was evaluated to determine the lubricant sensitivity. Tablets of COMBILOSE with an average weight of 500 mg were compressed using increasing proportions (0%–5%) of magnesium stearate and 2% of purified talc. The percentage friability of tablets from each batch was analyzed against the proportion of magnesium stearate (Daraghmeh et al., 2010; Haruna et al., 2020).
RESULT AND DISCUSSION
Flow properties
The developed excipient COMBILOSE exhibited fair to excellent flow properties. This may possibly be due to uniformity in the size of micro-fine granules. The results are tabulated in Table 4. The decrease in flowability was observed with an increase in the concentration of maltose. This could be due to the tendency of maltose to form aggregate by absorbing moisture (Crouter and Briens, 2014; Lamy et al., 2019). COMBILOSE 05 with 5% of maltose exhibited better flow behavior compared to that of COMBILOSE 10 and COMBILOSE 15.
Moisture sorption capacity and swelling behavior
Moisture content is one of the critical parameters which affects the process of compression. (Patel et al., 2006). About 4%–5% of moisture content in filler binder is usually considered ideal for direct compression (Tomar et al., 2017). The moisture sorption capacity of excipients affects the TS of tablets during storage (Malamataris et al., 1991). Hence, it could be one of the parameters of critical consideration for direct compressible excipient.
High moisture content and moisture sorption capacity were observed for COMBILOSE containing a higher proportion of maltose. This may be due to the solubility behavior and hygroscopic nature of maltose. The aqueous solubility of maltose also affected results of swelling capacity of the excipient formulation. In aqueous medium, maize starch could swell due to pre-gelatinization during the co-drying step of excipient development. However, negative results for the percentage swelling index were observed, which could be due to the solubilization of maltose. The results are presented in Table 5.
 | Table 2. Evaluation of mechanical properties of COMBILOSE. [Click here to view] |
 | Table 3. Evaluation of dilution potential. [Click here to view] |
True density and porosity
Higher true density with a low porosity was observed for COMBILOSE containing a higher proportion of maltose. This could be due to the filling up of voids of agglomerates of maize starch with soluble entities of maltose. The results of the study are presented in Table 6.
CD, TS, and bonding index
The developed excipients COMBILOSE were assessed for tabletability using various evaluation parameters such as CD, porosity, TS, and bonding index (Table 7). The tablets prepared with COMBILOSE containing an increased proportion of maltose exhibited a higher bonding index and CD. The increase in TS of tablets could be due to the pre-gelatinization of maize starch during the excipient development stage (Odeku et al., 2008). In addition to this, loss of water of crystallization also contributes for better fragmentation of COMBILOSE on application of compression force. The SF was found to be increased with a decreased porosity.
Dilution potential and lubricant sensitivity
Results of dilution potential and lubricant sensitivity of COMBILOSE showed that an increase in the percentage of maltose in COMBILOSE provides a better dilution potential; however, the same exhibited high sensitivity for hydrophobic lubricants such as magnesium stearate. The results of the study are tabulated in Tables 8 and 9. The tablets of paracetamol with COMBILOSE 15 exhibited better dilution potential. Tablets with COMBILOSE 5 and COMBILOSE 10 showed acceptable TS at a lower proportion of paracetamol; however, capping was observed for tablets with a higher proportion of paracetamol (Table 8). This could be possibly due to high post-ejection elastic recovery. High lubricant sensitivity was observed for COMBILOSE containing a higher proportion of maltose, which could be due to poor fragmentation during compression (Table 9).
TS for COMBILOSE containing 0%, 20%, 25%, and 30% of maltose were determined mathematically from equations obtained from the graph of TS of COMBILOSE 05, 10, and 15 against respective percentage drug content (Tables 8 and 10, Fig. 1). Effects of an incremental increase in maltose content of COMBILOSE on TS were derived mathematically (Table 11, Figs. 2 and 3). The results indicated that maltose monohydrate in 10% concentration in COMBILOSE substantially improved the dilution potential when compared to other concentrations.
 | Table 4. Flow properties of developed excipient formulations (n = 3*). [Click here to view] |
 | Table 5. Moisture sorption capacity and swelling behavior of developed excipient formulations (n = 3*). [Click here to view] |
 | Table 6. True density and porosity of developed excipient formulations (n = 3*). [Click here to view] |
 | Table 7. Evaluation of CD, TS, and bonding index (n = 3*). [Click here to view] |
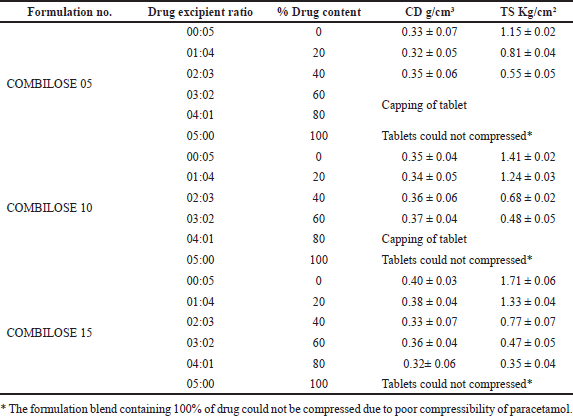 | Table 8. Determination of dilution potential (n = 3*). [Click here to view] |
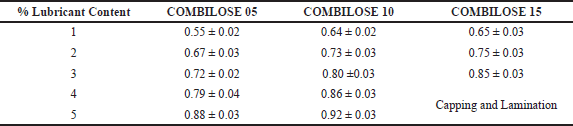 | Table 9. Determination of lubricant sensitivity (n = 3*). [Click here to view] |
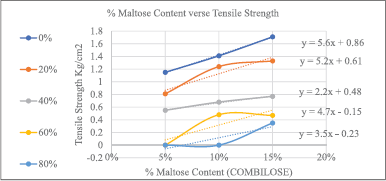 | Figure 1. Graph of % maltose content in COMBILOSE versus TS. [Click here to view] |
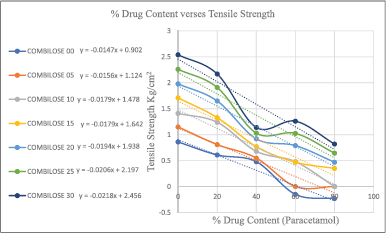 | Figure 2. Graph of % drug content versus TS. [Click here to view] |
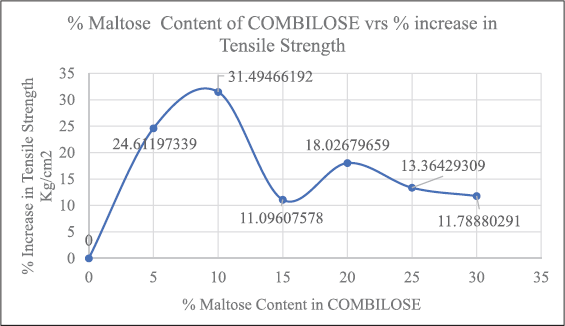 | Figure 3. Increase in TS per 5% increase in maltose content of COMBILOSE. [Click here to view] |
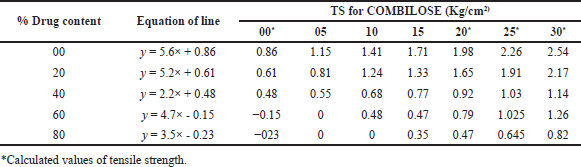 | Table 10. Determination of tensile strength. [Click here to view] |
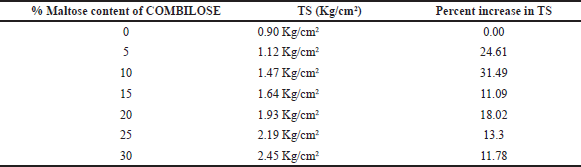 | Table 11. Effect of incremental increase in maltose content of COMBILOSE on TS. [Click here to view] |
CONCLUSION
COMBILOSE was developed as a novel lactose-based co-processed excipient by co-drying of an aqueous dispersion of lactose monohydrate with maize starch and maltose monohydrate. The aqueous dispersions of lactose monohydrate, maltose monohydrate, and maize starch were prepared in varying ratios and refrigerated for 12 hours. Frozen dispersions were pre-heated at 60°C and then dried at 50°C using a tray dryer. The composites that were obtained were size reduced and sifted to obtain micro-fine granules.
The developed excipient, COMBILOSE, showed better flowability, which could be due to uniformity in the size of microfine granules. An increase in CD and bonding index was observed, which could be due to the development of an increased number of contact points between the particles of composite because of better fragmentation of composite excipient. Pre-gelatinization of maize starch during drying could be another reason for the improved TS of the tablets. COMBILOSE with an increased proportion of high compressible saccharide exhibited good compressibility with better dilution potential. This could be attributed to the formation of porous agglomerates of maize starch with lactose. However, COMBILOSE containing a higher proportion of maltose exhibited high lubricant sensitivity and leads to capping and lamination of tablets. This may be due to poor fragmentation and higher elastic recovery. In conclusion, the results of the study revealed that the coprocessing of lactose monohydrate using 10% of maltose monohydrate can improve dilution potential. The compressibility of the excipient can be improved by co-treatment with a plastically deforming form of starch developed by pre-gelatinization.
ACKNOWLEDGMENTS
The authors are thankful to Wallace Pharmaceuticals Pvt Ltd, Goa for providing paracetamol as a gift sample.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report
CONFLICT OF INTEREST
The authors have no conflicts of interest regarding this investigation.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akram M, Naqvi SBS, Gauhar S. Development of co-processed micro granules for direct compression. Int J Pharm Pharm Sci, 2011; 3(Suppl 2):64–9.
Chang CK, Alvarez-Nunez FA, Rinella JV Jr, Magnusson LE, Sueda K. Roller compaction, granulation and capsule product dissolution of drug formulations containing a lactose or mannitol filler, starch, and talc. AAPS PharmSciTech, 2008; 9(2):597–604. CrossRef
Crouter A, Briens L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech, 2014; 15:65–74. CrossRef
Daraghmeh N, Rashid I, Al Omari MM, Leharne SA, Chowdhry BZ, Badwan A. Preparation and characterization of a novel co-processed excipient of chitin and crystalline mannitol. AAPS PharmSciTech, 2010; 11(4):1558–71. CrossRef
Deshkar D, Gupta RN, Jayaram Kumar K. Studies on effect of co-processing on palmyrah and maize starch mixtures using DOE approach. Int J Biol Macromol, 2019; 122:417–24. CrossRef
Dominik M, Vraníková B, Sva?inová P, Elbl J, Pavloková S, Prudilová BB, Šklubalová Z, Franc A. Comparison of flow and compression properties of four lactose-based co-processed excipients: Cellactose® 80, CombiLac®, MicroceLac® 100, and StarLac®. Pharmaceutics, 2021; 13(9):1486. CrossRef
Fuentes-González KI, Villafuerte-Robles L. Powder flowability as a functionality parameter of the excipient GalenIQ 720. Int J Pharm Pharm Sci, 2014; 1:66–74.
Gohel MC, Jogani PD, Bariya SH. Development of agglomerated directly compressible diluent consisting of brittle and ductile materials. Pharm Dev Technol, 2003; 8(2):143–51. CrossRef
Gohel MC, Patel TM, Parikh RK, Parejiya PB, Barot BS, Ramkishan A. Exploration of novel co-processed multifunctional diluent for the development of tablet dosage form. Indian J Pharm Sci, 2012; 74(5):381–6. CrossRef
Haruna F, Apeji YE, Oparaeche C, Oyi AR, Gamlen M. Compaction and tableting properties of composite particles of microcrystalline cellulose and crospovidone engineered for direct compression. Futur J Pharm Sci, 2020; 6(35):9. CrossRef
Hasan MM, Chowdhury SS, Lina SM, Bhoumik NC, Ashab I. Comparative evaluation of Zea mays (L.) and Ipomoea batatas (L.) as a pharmaceutical excipient. IOSR-JPBS, 2012; 3:31–6. CrossRef
Hauschild K, Picker KM. Evaluation of a new coprocessed compound based on lactose and maize starch for tablet formulation. AAPS J, 2004; 6(2):27–38. CrossRef
Kudo Y, Yasuda M, Matsusaka S. Effect of particle size distribution on flowability of granulated lactose. Adv Powder Technol, 2020; 31(1):121–7. CrossRef
Lamy B, Serrano DR, O’connell P, Couet W, Marchand S, Healy AM, Tewes F. Use of leucine to improve aerodynamic properties of ciprofloxacin-loaded maltose microparticles for inhalation. EJPR, 2019; 1(1):2–11. CrossRef
Malamataris S, Goidas P, Dimitriou A. Moisture sorption and tensile strength of some tableted direct compression excipients. Int J Pharm, 1991; 68(1–3):51–60. CrossRef
Mizumoto T, Masuda Y, Yamamoto T, Yonemochi E, Terada K. Formulation design of a novel fast-disintegrating tablet. Int J Pharm, 2005; 306(1–2):83–90. CrossRef
Moondra S, Maheshwari R, Taneja N, Tekade M, Tekade RK. Bulk level properties and its role in formulation development and processing. In: Tekadle RK (ed.). Advances in pharmaceutical product development and research, dosage form design parameters. vol. II. Academic Press, London, UK, pp 221–56, 2018. CrossRef
Odeku OA, Schmid W, Picker-Freyer KM. Material and tablet properties of pregelatinized (thermally modified) Dioscorea starches. Eur J Pharm Biopharm, 2008; 70(1):357–71. CrossRef
Olorunsola EO, Akpan GA, Adikwu MU. Evaluation of chitosan-microcrystalline cellulose blends as direct compression excipients. J Drug Deliv, 2017; 2017:8. CrossRef
Olowosulu AK, Oyi A, Isah AB, Ibrahim MA. Formulation and evaluation of novel coprocessed excipients of maize starch and acacia gum (StarAc) for direct compression tabletting. IJPRI, 2011; 2:39–45.
Patel P, Telange D, Sharma N. Comparison of different granulation techniques for lactose monohydrate. Int J Pharm Sci Drug Res, 2011; 3(3):222–5.
Patel S, Kaushal AM, Bansal AK. Compression physics in the formulation development of tablets. Crit Rev Ther Drug Carrier Syst, 2006; 23(1):1–65 CrossRef
Rani U, Begum N. Overview of co processed excipients used to improve tabletting performance. JADD, 2014; 1(6):8.
Sandhan SB, Derle DV. A review on functionality assessment of multifunctional excipients. IJPSR, 2019; 10(9):4078–89.
Schwarz E, Fichtner V, Luhn O, Häusler O. Case study: properties of a co-processed compound versus the physical blend based on lactose and starch. Available via https://www.roquette.com/media-center/resources/pharma-poster-properties-of-a-co-processed-compound-vs-blend-lactose-starch (Accessed 08 May 2022).
Tomar M, Kumar SA, Raj SA. Effect of moisture content of exicipient (microcrystalline cellulose) on direct compressible solid dosage forms. IJPSR, 2017; 8(1):282.
Tye CK, Sun CC, Amidon GE. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J Pharm Sci, 2005; 94(3):465–72. CrossRef
Ugoeze KC, Idris MEJ. Development of co-processed powders containing lactose, Mucuna flagellipes seed gum and Ipomoea batatas tuber starch. Int J Appl Biol Pharm, 2020; 11(4):256–75.