INTRODUCTION
It has been more than 2 years since the outset of the COVID-19 pandemic. While the emerging virus affected older populations during the first wave of the pandemic, new highly contagious variants emerged with higher infection rates among younger populations with increased severity. Increasing numbers of children are infected, with minors accounting for 11.5% of all COVID-19 cases in the United States (Y?lmaz and Sahin, 2021). Clinical signs in children are becoming more common, indicating that the disease has developed (She et al., 2020).
According to Centers for Disease Control and Prevention (CDC) reports, vaccination against COVID-19 is the most effective method to prevent infection and development into severe illness (CDC, 2022). Many countries have started immunizing adolescents against COVID-19. However, immunization rates are much lower than those for the adult population. This, along with the elevated infection rates among children, advocates the need for accelerating vaccination rates among children. This is highly important, especially in countries where the majority of the population consists of younger individuals (Sah et al., 2021). To acquire effective herd immunity, 70% of the population should be vaccinated, including children (Sah et al., 2021). This is important, especially since Pfizer-BioNTech indicated that its two-dose COVID-19 vaccination was safe and had a robust antibody response in children aged 5 to 11 (Xu et al., 2022).
Since the children have the right to decide whether to be vaccinated, it is critical to understand their willingness to receive the COVID-19 vaccine and the barriers to and promoters of COVID-19 vaccination. In the COVID-19 vaccination, the most significant restriction mentioned by parents was the vaccine’s safety and effectiveness (Goldman et al., 2020). However, children could have different views that could facilitate the vaccination program in this group (Adler et al., 2019). Children are now seen to be active in the construction and determination of their own social lives (Waksler, 1986), the lives of those around them, and the societies in which they live(Norozi and Moen, 2016). However, in the context of care management, the agency of the child remains relatively unsearched. The focus is shifting from pursuing evidence directly from children rather than other information sources, such as their parents (Docherty and Sandelowski, 1999).
In Jordan, around 30% of the total population is younger than 11 years old. However, the immunization rate in this age group still seems very slow, and the majority of children have not been vaccinated. As of October 2022, Jordan reported 1.75 million COVID-19 cases and a total of 14,122 deaths. Among the population residing in Jordan, a total of 10.1 million vaccine doses were given, with 44.7% of the total population reported being fully vaccinated (JHU, 2022). Several previous studies have reported a low rate of adults’ willingness to receive COVID-19 vaccines (Abu-Farha et al., 2021b; Qunaibi et al., 2021a; Qunaibi et al., 2021b), while only one explanatory cross-sectional study reported a very low proportion of Jordanian parents (25.4%) willing to vaccinate their 5–12 years old children against SARS-CoV-2 (Alsulaiman et al., 2022). In fact, the hesitancy of a significant proportion of Jordanian parents to administer the COVID-19 vaccine raises concern about attaining herd immunity, especially since children are a potential source for carrying and disseminating the virus, which in turn would endanger its effective control (Chou et al., 2022a). To our knowledge, this study is the first of its kind to study children’s willingness to take the COVID-19 vaccine.
MATERIALS AND METHODS
A structured interview was used in the current qualitative study to assess children’s willingness to take the COVID-19 vaccine. Study sites for this project were classrooms within different primary and secondary schools in Jordan. Individual school names were not included to retain confidentiality. The schools were chosen to represent all the different types of schools in Jordan (one public, one private, and one nonprofit school). In this study, 54 students participated in the different focus groups. Students were from different age groups: 6–8-year-old pupils (first, second, and third grades), 9–11-year-old pupils (fourth, fifth, and sixth grades), and 12–14-year-old pupils (seventh, eighth, and ninth grades). The sample size recruited in this study (n = 54) was enough to reach saturation, as the general recommendation for in-depth interviews is to have a sample size of 20–30 to reach saturation (Boddy, 2016; Creswell and Poth, 2016).
Via personal communication with school head teachers, through the study’s principal investigator, permission was sought to carry out focus group sessions with groups of children within the age groups detailed above. Having gained this permission, letters were prepared for each school to be sent to parents/guardians of children detailing the research that was to be performed and seeking parental/guardian permission for their child to take part. These letters were sent out by the schools to the parents/guardians of children within a particular year group selected by the head teacher. As well as parent/guardian consent for their child to participate in the study, the written assent of all children was also sought. Children for whom written consent and assent was obtained were invited to attend a focus group discussion. The timing of the focus group discussions was determined by the head teacher, for example, during normal class time, over the lunch break, or immediately after school.
All the participating schools in the study allowed the researcher to use one of their teaching rooms to carry out the focus group discussions. The room was made ready by the researcher before requesting the children’s presence. A table, with several chairs surrounding it, was placed in the middle of the room, and a recording device was placed in the middle of that table. During the focus group discussions, notes were taken by the facilitator to help identify particular participants when analyzing the transcripts.
A detailed study guide was used to ensure that all focus groups were undertaken in a relatively uniform manner. The “facilitator” (author) was introduced to each group of pupils by one of their teachers. The teacher told the group briefly about the topic to be discussed but did not remain in the room during the focus group to keep the student answers confidential and to avoid any influence on the discussion. The teachers were briefed after the discussions so that they would be prepared to answer questions that the children may raise with them as a result of the research study. The facilitator put the children at their ease by first giving some details about himself, and then the group was told that the discussion was going to be audio-recorded to help the facilitator remember the views that had been expressed.
The facilitator put the children at ease by first giving some details about themselves and then asking each child to say who they are and whether they have any knowledge about COVID-19 and the vaccination program. The language used was adjusted as appropriate to the different age groups. Following this, the questions in the study guide were asked, leaving time for discussion around any related subjects raised by the participants. As before, the language used was modified, as appropriate, to take account of the age of the children involved. However, the meaning was kept constant. Each focus group was planned to last no longer than 45 minutes, and indeed most focus groups took a shorter time (30–40 minutes). The focus group interviews were conducted in April 2022.
All discussions were audio-recorded and transcribed in full. A thematic analysis was performed using QSR NVivo® computer software to allow a complex organization, indexing, sorting, and retrieval of data. The further analysis consisted of refining the concepts which had been identified, grouping similar statements into themes, and presenting a series of hierarchical tree structures whereby themes and categories were identified as they “emerge” from the data from each of the age groups of children External validation of the analysis was performed by a separate investigator. Finally, the demographic characteristics of the study participants were presented as frequency and percentages.
The study received ethical approval from the IRB at the Kind Abdulla University Hospital, Jordan University of Science and Technology, and was funded by the Jordan University of Science and Technology (REF: 20210592).
RESULTS
A total of 54 children from three schools in the main cities of Jordan participated in a total of nine focus groups. Students were recruited from three different groups, as seen in Figure 1 (19 from a public school, 18 from a private school, and 17 from a nonprofit school). Moreover, students were from different age groups (17 were 6–8 years old, 18 were 9–11 years old, and 19 were 12–14 years old). Among the participating students, around half of them were females (n = 28, 51.9%). Also, more than two-thirds of children taking part in the study (n = 38, 70.4%) indicated that they were willing to take the COVID-19 vaccine, and the majority of children (n = 51, 94.5%) were aware that both their parents took the COVID-19 vaccine. Further details of children who attended the focus groups are presented in Table 1.
Three overarching themes were identified in the analysis: benefits, motivators, hesitancy, and barriers to taking the COVID-19 vaccine. A summary of themes generated from this study is presented in Table 2. Participating children who were willing to take the vaccine discussed that the vaccine was beneficial as it could protect them from being infected with COVID-19 and reduce the severity of the disease had they been infected with the virus. Moreover, participating children were motivated to take the vaccine because of their families’ support and positive experience with the vaccine. Participants were also influenced by the awareness created by news in the media about giving the vaccine to children in other countries, which encouraged them to take the vaccine.
However, participating children who were hesitant to take the vaccine discussed that the vaccine could be harmful, mainly because they thought that the vaccine was developed in a short period and was not tested thoroughly, especially in their age group, and that the vaccine could have side effects that could harm them. This thought was mainly generated because some of the children’s parents suffered from the vaccine’s side effects and because of reports on news and social media. Some participants feared the vaccine as it was delivered in an injection, and they indicated that they do not favor this delivery method. Had the vaccine been delivered as pills, they would be more willing to take it. Other children who were hesitant to take the vaccine indicated that they think that the vaccine is not effective enough as it does not protect people from being infected with COVID-19, and its efficacy could protect individuals for a short period. Participating children reported knowledge of people who took the vaccine and were infected, and this highly affected the hesitancy to take the vaccine. Furthermore, many participants believed that they were protected and that there is no need to take the vaccine as the virus does not infect children and is more common among adults, not being aware of the recent developments in the virus that makes it more contagious among this age group.
DISCUSSION
As the SARS-CoV-2 continues to circulate and mutate, the COVID-19 pandemic is evolving into different stages, while the emphasis is being shifted from a disease of adults to a disease of the young. The younger generation is now the main source of SARS-COV-2 spread (Cui et al., 2021; Li et al., 2020). As such, in November 2021, the CDC issued recommendations to vaccinate children aged 5 years and older (CDC, 2021b). However, the pace of vaccination in this age group is slow worldwide (Zou and Cao, 2021). While several studies have recently provided data on children’s COVID-19 vaccination uptake and hesitancy, most of them were self-administered questionnaires confined to parents’ opinions (Di Giuseppe G et al., 2022; Fazel et al., 2021; Rane et al., 2022; Temsah et al., 2021). Nonetheless, these studies were susceptible to social desirability and recall bias. At the time of writing this paper, this was the first qualitative study that was conducted among children aged 6–14 years, which has reflected participants’ spontaneous, honest, and genuine responses.
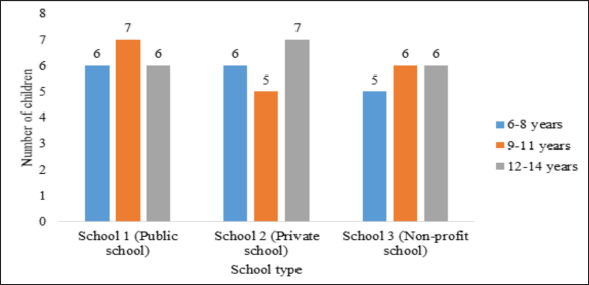 | Figure 1. Number of children who attended the focus groups based on their age groups and school type. [Click here to view] |
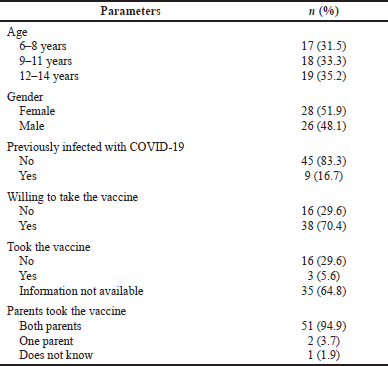 | Table 1. Characteristics of participating children in the focus groups (n = 54). [Click here to view] |
To optimize the control of the COVID-19 pandemic and fasten the return to pre-pandemic activity, health policymakers should promote the uptake of the COVID-19 vaccine among the population (Abu-Farha et al., 2021a; Murewanhema et al., 2021; Velavan et al., 2020). However, to achieve this, effective herd immunity should be attained. Recruiting children in the early vaccination trials might expose them to health risks, but delaying their enrollment might deprive them of being immunized against the virus, hence interrupting the adequate and effective control of the pandemic (Mintz et al., 2021; Velavan et al., 2020).
Although only 30% of children are reluctant to take the COVID-19 vaccine, it is still considered a number of appreciable concern, as it may affect the vaccination rate in this age group to a lower rate and, in turn, affect the mitigation of COVID-19 infections and transmission. It is worth mentioning that recent data issued by the American Academy of Pediatrics revealed that the high transmissibility rates of the highly contagious Omicron variant were mainly attributed to children, which were recently considered potential spreaders and dangerous carriers of COVID-19 and new emerging variables. Researchers from Massachusetts General Hospital showed important findings that infants, toddlers, children, and adolescents are equally capable of carrying a very high level of SARS-COV2 virus in their respiratory system even when asymptomatic (Chou et al., 2022b).
However, among the children and adolescents who are hesitant to receive the COVID-19 vaccine, the main reasons are attributed to safety issues. This finding could be interpreted by the fact that antivaccine activists target vulnerable parents on social media that are easily influenced by vaccine misinformation. A recent study has found that 68% of parents that use social media reported using it for health information (Frey et al., 2021). These parents are the main target of antivaccine activists who are more skilled at tackling messages that impact children’s safety and cause serious harm.
Nonetheless, the other proportion of hesitant children was mainly related to speculations and uncertainty related to the efficacy of COVID-19 vaccines. This hypothesis was particularly stronger among children, as breakthrough cases were observed even in previously infected individuals who were fully vaccinated. Thus, this could have influenced their perception of the COVID-19 vaccine. Some children believed that the COVID-19 vaccine would not provide any additional protection as it does not infect children. This underestimated perception requires specific attention and highlights the urge for initiatives to be taken to increase public awareness about pediatric COVID-19 and implement broader testing programs in children. This vulnerable group is an essential component in beating the pandemic, and a thorough investigation of how they are affected and interact with others would add a stop to the outbreak. These initiatives are extremely important since recent studies have found that children are potential spreaders, as the true incidence of COVID-19 among them is unknown due to prioritization of testing among adults and severe cases, not forgetting that also COVID-19 symptoms duration is shorter, and symptoms burden is lower among this age group.
This study will serve as an opportunity to alert policymakers in optimizing the role of pediatricians and other healthcare providers in building parents’ trustworthiness in the vaccine since they are a trusted source of information on COVID-19. These influencers can use social media platforms to provide scientific information in addressing parents’ concerns and doubts about pediatric COVID-19 vaccination. Furthermore, the solution may also lie in working with schools to empower children to feel confident. The CDC recommended action steps that schools should take to increase the uptake of the COVID-19 vaccine and improve health literacy among students and their parents (CDC, 2021a).
This study was proactive in investigating the children’s willingness to receive the COVID-19 vaccine using a qualitative method approach, reflecting their genuine responses. Nonetheless, several limitations should be pointed out. First, this study was conducted among students of three schools only, which might induce selection bias and thus limit the generalizability of results to the general population. However, the selected schools have a diverse student body with different backgrounds, cultures, and financial statuses. Second, several potential confounding variables that influence the participants’ willingness to receive the vaccine were not controlled during the study’s conduction; these include parents’ educational and employment status, age, cultural background, exposure to media, and children’s knowledge and perception toward COVID-19 and its severity. Nonetheless, this is an exploratory qualitative study that aimed to identify the children’s spontaneous responses and did not intend to thoroughly investigate the determinants of children’s willingness. Third, at the time of the study’s conduction, the Jordanian Government did not mandate vaccination for children of the targeted age group. Hence, the decision to vaccinate belongs to their parents. As such, it would have been more interesting to investigate and compare the parents’ and their children’s willingness to receive the COVID-19 vaccine.
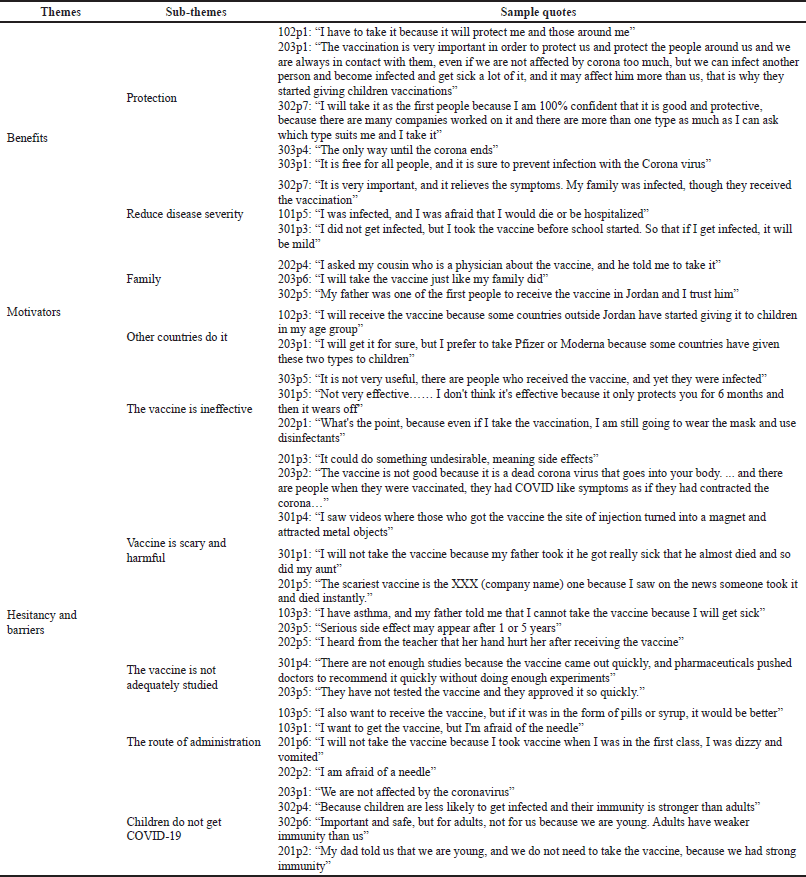 | Table 2. A summary of themes and subthemes generated from the focus groups. [Click here to view] |
CONCLUSION
This study shows a clear willingness among children to take the COVID-19 vaccine. Such outcomes should be tested further on a wider scale to deliver future recommendations to include younger children in the vaccination program. Disease prevention, severity reduction, parental support, and media awareness were the main predictors of vaccination among children.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The study was funded by the Deanship of Research, Jordan University of Science and Technology.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study received ethical approval from the IRB at the Kind Abdulla University Hospital, Jordan University of Science and Technology, and was funded by the Jordan University of Science and Technology (REF: 20210592).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abu-Farha R, Mukattash T, Itani R, Karout S, Khojah HM, Al-Mahmood AA, Alzoubi KH. Willingness of Middle Eastern public to receive COVID-19 vaccines. Saudi Pharm J, 2021a; 29(7):734–9. CrossRef
Abu-Farha R, Mukattash T, Itani R, Karout S, Khojah HMJ, Al-Mahmood AA, Alzoubi KH. Willingness of Middle Eastern public to receive COVID-19 vaccines. Saudi Pharm J, 2021b; 29(7):734–9. CrossRef
Adler K, Salanterä S, Zumstein-Shaha M. Focus group interviews in child, youth, and parent research: an integrative literature review. Int J Qual Meth, 2019; 18:1609406919887274. CrossRef
Alsulaiman JW, Mazin M, Al-Shatanawi TN, Kheirallah KA, Allouh MZ. Parental willingness to vaccinate their children against SARS-CoV-2 in Jordan: an explanatory cross-sectional study. Risk Manag Healthc Policy, 2022; 15:955–67. CrossRef
Boddy CRJQMRAIJ. Sample size for qualitative research, 2016. CrossRef
CDC. Centers for Disease Control and Prevention. 6 ways schools can promote COVID-19 vaccines. CDC, 2021a.
CDC. Centers for Disease Control and Prevention. CDC recommends pediatric COVID-19 vaccine for children 5 to 11 years. CDC, 2021b.
CDC. Centers for Disease Control and Prevention. Benefits of getting a COVID-19 vaccine. CDC, 2022.
Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol, 2022a; 23(2):177–85. CrossRef
Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol, 2022b; 1–9. CrossRef
Creswell JW, Poth CN. Qualitative inquiry and research design: choosing among five approaches. Sage Publications, Thousand Oaks, CA, 2016.
Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, Zhang J, Dong C, Na R, Zheng L. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol, 2021; 93(2):1057–69. CrossRef
Di Giuseppe G, Pelullo CP, Volgare AS, Napolitano F, Pavia M. Parents’ willingness to vaccinate their children with COVID-19 vaccine: results of a survey in Italy. J Adolesc Health, 2022; 70(4):550–8. CrossRef
Docherty S, Sandelowski M. Focus on qualitative methods: interviewing children. Res Nurs Health, 1999; 22(2):177–85. CrossRef
Fazel M, Puntis S, White SR, Townsend A, Mansfield KL, Viner R, Herring J, Pollard AJ, Freeman D. Willingness of children and adolescents to have a COVID-19 vaccination: results of a large whole schools survey in England. E Clin Med, 2021; 40:101144. CrossRef
Frey EFJ, Bonfiglioli C, Brunner M, Frawley J. Parents’ use of social media as a health information source for their children: a scoping review. Acad Pediatr, 2021; 22(4):526–39. CrossRef
Goldman RD, Yan TD, Seiler M, Cotanda CP, Brown JC, Klein EJ, Hoeffe J, Gelernter R, Hall JE, Davis AL. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine, 2020; 38(48):7668–73. CrossRef
JHU. Corona virus resource center. Johns Hopkins University of Medicine, Baltimore, MD, 2022.
Li X, Xu W, Dozier M, He Y, Kirolos A, Lang Z, Song P, Theodoratou E. The role of children in the transmission of SARS-CoV2: updated rapid review. J Global Health, 2020; 10(2):021101. CrossRef
Mintz K, Jardas E, Shah S, Grady C, Danis M, Wendler D. Enrolling minors in COVID-19 vaccine trials. Pediatrics, 2021; 147(3):e2020040717. CrossRef
Murewanhema G, Mukwenha S, Dzinamarira T, Mukandavire Z, Cuadros D, Madziva R, Chingombe I, Mapingure M, Herrera H, Musuka G. Optimising COVID-19 Vaccination policy to mitigate SARS-CoV-2 transmission within schools in Zimbabwe. Vaccines, 2021; 9(12):1481. CrossRef
Norozi SA, Moen T. Childhood as a social construction. J Educ Soc Res, 2016; 6(2):75.
Qunaibi E, Basheti I, Soudy M, Sultan I. Hesitancy of Arab Healthcare workers towards COVID-19 vaccination: a large-scale multinational study. Vaccines, 2021a; 9:446. CrossRef
Qunaibi EA, Helmy M, Basheti I, Sultan I. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. Elife, 2021b; 10:e68038. CrossRef
Rane MS, Robertson MM, Westmoreland DA, Teasdale CA, Grov C, Nash D. Intention to vaccinate children against COVID-19 among vaccinated and unvaccinated US parents. JAMA Pediatr, 2022; 176(2):201–3. CrossRef
Sah P, Vilches TN, Moghadas SM, Fitzpatrick MC, Singer BH, Hotez PJ, Galvani AP. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. E Clin Med, 2021; 35:100865. CrossRef
She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol, 2020; 92(7):747–54. CrossRef
Temsah MH, Alhuzaimi AN, Aljamaan F, Bahkali F, Al-Eyadhy A, Alrabiaah A, Alhboob A, Bashiri FA, Alshaer A, Temsah O, Bassrawi R, Alshahrani F, Chaiah Y, Alaraj A, Assiri RA, Jamal A, Batais MA, Saddik B, Halwani R, Alzamil F, Memish ZA, Barry M, Al-Subaie S, Al-Tawfiq JA, Alhasan K. Parental attitudes and hesitancy about COVID-19 versus routine childhood vaccinations: a national survey. Front Public Health, 2021; 9:752323. CrossRef
Velavan TP, Pollard AJ, Kremsner PG. Herd immunity and vaccination of children for COVID-19. Int J Infect Dis, 2020; 98:14–5. CrossRef
Waksler FC. Studying children: phenomenological insights. Hum Stud, 1986; 9:71–82. CrossRef
Xu W, Tang J, Chen C, Wang C, Wen W, Cheng Y, Zhou M, Wu Q, Zhang X, Feng Z. Safety and efficacy of the COVID-19 vaccine in children and/or adolescents: a meta-analysis. J Infect, 2022; 84(5):722–46. CrossRef
Y?lmaz M, Sahin MK. Parents’ willingness and attitudes concerning the COVID-19 vaccine: a cross-sectional study. Int J Clin Pract, 2021; 75(9):e14364. CrossRef
Zou X, Cao B. COVID-19 vaccines for children younger than 12 years: are we ready? Lancet Infect Dis, 2021; 21(12):1614–5. CrossRef