INTRODUCTION
Glaucoma is the world’s most prevalent eye disease and the leading cause of permanent blindness (Sun and Zhou, 2018). It is characterized by “tunnel vision,” which is caused by the purposeful degeneration of retinal ganglion cells. High intraocular pressure is one of the most dangerous characteristics of glaucoma Intra-ocular pressure (IOP) (Soltau and Zimmerman, 2002). Glaucoma affects more than 67 million people globally, according to the World Health Organization (Kaur et al., 2011). Since the 1970s, there has been a concerted attempt to improve ocular therapeutic delivery. Various ways were tried at the beginning, which may be split into two categories, such as controlled-release medication administration and bioavailability enhancement (Mohsen et al., 2020). The bioavailability enhancement category includes viscosity enhancers (Patton and Robinson, 1975), gels (Zignani et al., 1995), penetration enhancers (Kaur et al., 2000), and prodrugs (Lee and Robinson, 1986). Controlled-release ocular medication delivery devices might replace traditional drug release strategies such as ocusert (Saettone and Salminen, 1995), in-situ gel (Cohen et al., 1997), implant systems (Kunou et al.,1995), and collagen shields (Friedberg et al., 1991).
The traditional way of treating eye illness is no longer appropriate for the modern age of medicine (Calvo et al., 1997; Grass and Robinson, 1988; Kreuter, 1993). Scientists and industry researchers have been more interested in pharmaceutical semisolid dosage forms, particularly emulgels systems, during the last decade. The main advantages of the drug delivery system were consistency and homogeneous behavior. The said drug delivery system is a biphasic dosage form, in the form of an o/w emulsion system in which an aqueous base is a gel, and called emulsion gel or emulgels. As the interior phase of colloidal systems contains an aqueous base and is mixed with a lipophilic material, it has explored the cosmeceutical industry for a long period of time (Marquardt and Sucker, 1998). In the modern era, emulgel has been investigated for controlled delivery, stability, and storage purposes in the pharmaceutical, dermatological, and food industries respectively. The invention of emulgels has been greatly aided in recent years by the discovery of new polymers with various functionalities such as increased gelling capacity, emulsifying agents, and thickening agents. This improved the viscosity of the aqueous phase and lowered surface or interfacial tension, resulting in the stability of emulsions and lotions (Gupta et al., 2010). Finally, their rheological qualities may be readily modified by selecting the right gelling agent and an oily component.
An eye drop is an ideal alternative for topical treatment of ocular illnesses in conventional dose form, especially in certain circumstances when the drug’s localized action (e.g., the cornea and/or anterior chamber) is required. However, a barrier such as lacrimal secretion and blinking reflex, along with corneal impermeability, make drug retention time so short that frequent administration is necessary for highly efficient therapy (Wei et al., 2002). The drug retention period is so brief due to the self-protecting barrier, which causes frequent very effective treatment, which leads to several side effects with toxicity. To reduce the toxicity and overcome the situation the work was projected. This study’s goal is to create acetazolamide (ACZ)-loaded in-situ emulgel systems using Corn Oil (CO), Pectin, Gellan Gum (GG), and surfactants (tween 80& span 80).
ACZ, a carbonic anhydrase inhibitor, is used to treat open-angle glaucoma symptoms and postpone the development of blindness, and lower IOP before surgery (Khaw and Cordeiro, 2000). Among the presently available treatments for treating open-angle glaucoma, ACZ remains the most effective and commonly used Carbonic anhydrase inhibitors (CAIs) (CAIs) (Kaur et al., 2002). ACZ works by blocking the carbonic anhydrase enzyme (CAE), which is involved in the transfer of CO2 from the tissues to the lungs and stimulates the generation of aqueous humor through ciliary mechanisms. To achieve the desired drop in IOP, high oral dosages of ACZ should be taken due to the widespread diffusion of CAE throughout the body (Kaur et al., 2002). The high ACZ dosages cause systemic negative effects (Epstein and Graant, 1977; Gamm, 1984) such as diuresis, renal failure, vomiting, metabolic acidosis, and central nervous system depression. As a result, oral ACZ is despised, and several researchers have proposed that oral ACZ be replaced by topical ACZ to avoid systemic adverse effects (Kaur et al., 2002).
The requirement of polysaccharides in the development of pharmaceutical preparation has been explored for the last two decades. GG is one of such anionic polysaccharides (Prajapati et al., 2013) which brings a special attraction to the development of in-situ formulations (Balasubramaniam et al., 2003; Carlfors et al., 1998; Fernández-Ferreiro et al., 2015; Paulsson et al., 1999; Rozier et al., 1997; Rupenthal et al., 2011a, 2011b). The GG is water-soluble and obtained from the Pseudomonas elodea, and it shows anionic properties due to the presence of the D-glucose and one of each residue of D-glucuronic acid and L-rhamnose. Researchers use GG for its heat and acid resistance capacity and with a wide range of gel textures (Chen and Chen, 2007). GG is transparent and can bind with synthetic and natural polymers (Zia et al., 2018).
Pectin an effective polysaccharide is a byproduct material of agriculture and food industries, used for the production of an edible film, because not only of its biocompatibility and biodegradability but also its non-toxicity nature (Lin et al., 2020). Pectin draws attention toward the food and pharmaceutical industry because of its semi-soluble nature, that is, it retains some water (Marcus, 2013).
From ancient days, edible oil is used for cooking purposes and CO draws attention to the drug delivery system in the last few years. The main aim of chosen CO is due to the presence of essential fatty acids and vitamin E, and the pleasant sensory properties, which make it a suitable ingredient for delivery carriers (Krstonoši? et al., 2009).
In this work, a series of emulgels were developed using various amounts of an aqueous solution of GG and pectin with CO to achieve the goal of combining the beneficial qualities of all the ingredients. The produced emulgel will have a better texture, drug solubility, and drug absorption over caprine (goat) ocular tissue. The formulations were characterized using FTIR, gelation temperature, gelation time, hemocompatibility, and pH. The release study was done with the use of a semipermeable membrane barrier, and ACZ from the synthesized emulgels was evaluated in vitro. To know the efficacy of the emulgel as ocular delivery, it will be tested in vivo eye irritation test and ex vivo corneal permeation test.
MATERIALS AND METHODS
Materials
CO was purchased from Grainotch Industries Limited (Maharashtra, India). Pectin and GG were purchased from Suvidhinath Lab (Baroda, Gujarat). ACZ was gifted from Nakoda Chemicals Ltd, (Hyderabad, India, 99.91% pure), Tween 80 and span 80 (Hi-media Pvt. Ltd, Mumbai), and other chemical reagents including NaOH, K2HPO4 were purchased from Suvidhinath Laboratories Pvt. Ltd. (Vadodara, India). Double distilled water and preservative water were used during the experimental study.
Preparation of in-situ gel
Preparation of pectin solution
About 500 mg of pectin was weighed and slowly added to the 99.5 g of the double distilled water, which contain 0.1% of methylparaben. The mixture was homogenized using a magnetic stirrer (500 rpm) to form a pectin sol.
Preparation GG solution
About 500 mg of GG was weighed and slowly added to the 99.5 g of the double distilled water, which contain 0.1% of methylparaben. The mixture was homogenized using a magnetic stirrer (500 rpm) to form a GG sol.
Preparation of in-situ emulsion
In-situ emulsions of different compositions were prepared by using CO containing dissolved ACZ. The composition of the CO within the in-situ emulsion varied from 10%–50% (w/w). For the preparation of in-situ emulsion pectin, GG was weighed and added to the homogenizer tube. This was followed by the addition of the emulsifier (Tween 80 and span 80 in the ratio of 1:1). In all the formulations, the concentration of the emulsifier was kept constant at 0.2% (w/w). At last calculated amount of the CO containing ACZ was added to the homogenizer tube. The mixture was then homogenized at 3,000 rpm for 15 minutes at room temperature. The formulations were then transferred into falcon tubes and kept under refrigeration (5°C); the composition of the prepared formulations has been tabulated in Table 1.
Characterization of the prepared acz in-situ gels
Visual appearance, homogeneity, and clarity
The prepared in-situ gel formulation was evaluated visually. For the color and homogeneity study, we take the help of a white and dark background (Neeraja et al., 2014).
Determination of gelation time and gelling capacity
A drop of the formulation was put into a watch glass holding 2 ml of newly manufactured artificial tear fluid at a temperature of 37°C (680 mg NaCl, 200 mg NaHCO3, 8 mg sodium chloride dehydrate, and volume make with water to 1 l); the gelation time and gelling capacity of the prepared in-situ forming emulsion were determined. The time it took to produce the gel and the time it took to dissolve it was observed to estimate the gelling capacity grade which was determined as follows:
− There was no gelation
+ The gel formed after a few minutes and quickly dissolved
++ The gel formed instantly and lasted for a few hours
+++ The gel formed quickly and remained stable for a long period.
pH and isotonicity of prepared formulations
At room temperature, the pH of the produced formulations was measured with a calibrated pH meter (L1617 Elico Instrument Mumbai, India). Placing the pH meter probe in contact with the samples was used to determine the pH of the in-situ gel (about 1 cm deep). The measurements were done three times to compute the mean ± SD (Maru et al., 2019). The hemolytic technique is used to determine isotonicity. The produced formulations were administered to a few blood drops, which were then compared to hypotonic, hypertonic, and normal saline solutions under a 45× optical microscope (Wo and Zhai, 2021).
Microscopic studies
On a glass slide, the in-situ emulsion gel drop was inserted, then covered with a coverslip. An upright bright-field compound microscope was used to view the microstructure organization of the produced emulgel (Leica Microsystems, model: DM750, GmbH, Wetzlar, Germany).
Fourier transform infrared spectroscopy analysis
Prepared in-situ gels were analyzed by attenuated total reflection infrared (ATR-IR) spectrometer (AlphaE ATR-FTIR, Bruker, USA) in the range of 4,000–4,500 cm−1. This instrument was used to examine the chemical interaction between the in-situ gels (Behera et al., 2013).
Hemocompatibility study
The in-situ gel samples were subjected to a hemocompatibility test to determine the degree of hemolysis (Pal et al., 2007a, 2007b). About 50 ml of normal saline (0.9% w/v; 37°C, 30 minutes) was used to equilibrate in-situ gels. The final volume was made up to 10 ml by adding normal saline after 0.5 ml of the equilibrated sample was diluted with 0.5 ml of diluted blood (produced by diluting 4 ml of citrated blood with 5 ml of normal saline). Positive control was made by mixing 0.5 ml diluted blood with 0.5 ml 0.1N HCl and 9 ml normal saline, whereas negative control was made by mixing 0.5 ml diluted blood with 9.5 ml normal saline. The samples were incubated for 1 hour and then centrifuged at 3,000 rpm for 10 minutes (Model: Remi-R-8C). A spectrophotometer (Shimadzu 1800, Japan) was used to determine the optical density (OD) of the supernatant at 545 nm. The percentage of hemolysis was calculated as per the following formula:
Ex vivo corneal permeation study
Franz’s diffusion cell was used to test the ex-vivo drug permeation of ACZ from the formulations. In the donor compartment, 1 ml of the formulation was deposited, while the receptor compartment was filled with 12 ml of artificial tear solution (ATF, pH 7.4). Corneas were taken from the goat eyeball and cleaned extensively in normal saline. The cornea was then visually checked to see if there were any wrinkles or pores. Between the donor and receptor compounds, the cornea was mounted. The receptor fluid was constantly stirred (300 rpm) at 37°C and the diffusion area was 0.64 cm2. At regular intervals, 1.5 ml of receptor fluid was removed from the receptor compartment and replaced with STF (STF). A spectrophotometer (UV-1900i, Shimadzu Corporation, Japan) at 267 nm was used to evaluate the drug content in the samples (Quereshi et al., 2020).
In vivo glaucoma study
The rabbits were divided into three groups, each with three rabbits, to investigate the effects of topically given ACZ in-situ emulsions. For IOP measurements, the rabbits were confined in a restricted cage. Rabbits were anesthetized with 4% lignocaine HCl, 30 minutes before the experiment began, and initial IOP was measured. Then, a 5% glucose solution was injected through the marginal vein and IOP was recorded. After that the prepared in-situ gel solution was injected into the inferior conjunctival sac in the center, then the lid was closed. The IOP was measured on the cornea using a Schiotz Tonometer (Riester, Germany). The IOP in mmHg units is calculated by converting the scale value into the table value given by the manufacturer.
 | Table 1. Composition of prepared formulations. [Click here to view] |
Ocular irritation test
Visual evaluation research was carried out to evaluate the corneal tolerance of the generated emulsions to that of the corneal tissues. The rabbits used in this investigation were albino rabbits weighing between 1.5 and 2.0 kg. In a nutshell, 0.1 ml of the produced emulsions were administered into the rabbit’s right eye’s ocular sacs. About 0.1 ml saline was administered into the rabbit’s left eye, which functioned as the control eye. Following that, the rabbit’s eyes were checked for evidence of ocular chemosis after 0, 1, 24, 48, and 72 hours. The process was repeated three times (Quereshi et al., 2020).
RESULTS AND DISCUSSION
Preparation of in-situ emulgels
The pictographs of the in-situ gel formulations are shown in Figure 1. The prepared in-situ emulgel is milky white. With the addition of oil to the formulations, the shade of white was found to rise. Light scattering from the interface between the aqueous and oil phases can explain this phenomenon (Quereshi et al., 2020). The drug was dissolved in the oil phase and the final concentration was made to 0.5% w/v.
Determination of gelation time
The viscosity and the gelling capacity are the most important requirement for in-situ forming gelling systems. Gelation time plays a vital role in the in-situ formulations because it affects the release of drugs at the site of action.
The gelation time of the formulations varies from 16 to 54 seconds (Table 2). The drug’s water affinity could be a determining factor in the drug-loaded solution’s sol dynamic rate. The presence of ACZ (a drug that is insoluble in water) had a considerable impact on the sol dynamics because it took longer to slide.
 | Figure 1. Pictographs of in-situ gel (a) PG1, (b) PG2, (c) PG3, (d) PG4, and (e) PG5. [Click here to view] |
Gelling capacity determination
The developed formulations have a “+++” gelling capability since they gelled quickly and remained in the same state for a long time, which is more acceptable to the other formulation because it could be assumed that the release of the drug is to the site of action is for a long period which can reduce the toxicity and the taking the medicaments doses (Table 2).
pH and isotonicity of the prepared gels
The pH of all formulations is measured and found within 5.58 to 5.75 which does not irritate the eye as confirmed by the Draize test.
From the isotonicity observation under a microscope, it was confirmed that the said formulations are isotonic with the blood or/and the lachrymal fluid. Because of the hemolytic test, it was confirmed that the presence of the said preparation in the blood does not change the shape or size of the red blood cell, which shows that the prepared formulation is isotonic to human use (Table 2).
Microscopic study
The microstructure of the in-situ emulsion gel was shown in Figure 2. It was visualized that PG1 shows a low globular size particle whereas the concentration of CO increased with the globular particle was also increased till PG3. After that in PG4, it has been seen that the oil particles were aggregated in a region, and when oil and the hydrophilic solution were the same at that time the particle was not clearly visualized. In all the formulations the particle size was the same and in PG3 it was found to be clumsy.
Fourier transform infrared spectroscopy analysis
The FTIR analysis was carried out to evaluate the interactions between the CO, pectin, GG, and ACZ within the in-situ gel. FTIR spectra of all the formulations (PG1–PG5) are shown in Figure 3. The FTIR spectrum of all the formulations showed broadband in between the wavenumber range of 3,700 cm−1 and 2,980 cm−1. The peak of the broadband was located at the wavenumber of 3,327 cm−1. This peak can be related to hydrogen bonding and −OH stretching vibration. The presence of hydroxyl groups in the glucopyranose ring of GG as well as in pectin could form both inter and intramolecular hydrogen bonds (Gnanasambandam and Proctor, 2000; Narkar et al., 2010). In addition, the different signals were observed at 2,923 cm−1, 2,855 cm−1, 1,739 cm−1, 1,636 cm−1, 1,455 cm−1, and 1,158 cm−1. The sharp peak at 2,923 cm−1and 2,855 cm−1 was attributed to C–H stretching vibrations of methyl groups present in the pectin and GG (Ezati and Rhim, 2020; Narkar et al., 2010). A common peak found at 1,457 cm−1 and 1,162 cm−1(except PG5) was found in all formulations due to the bending vibration of CH2 and the stretching vibration of C-O-C (Zhang et al., 2021). The C=O stretching of the methyl ester (which is generally employed to detect the degree of esterification of pectin) was responsible for the band at 1,739 cm−1 (Shivangi et al., 2021).
Hemocompatibility study
In the presence of the sample leachant, the % hemolysis of chelated human blood (with EDTA) was determined and summarized in Table 2. The result recommended that all the in-situ gel samples were highly hemocompatible (% hemolysis <5%) and may be considered safe and biocompatible (Pal et al., 2007a).
 | Table 2. Physicochemical properties of formulations. [Click here to view] |
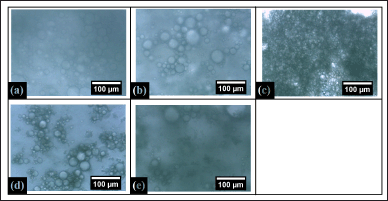 | Figure 2. Micrographs of in-situ gel (a) PG1, (b) PG2, (c) PG3, (d) PG4, and (e) PG5. [Click here to view] |
Ex vivo corneal permeation study
Drug permeation is the transport of drug molecules from the carrier through the biological membrane to the releasing medium. The carrier type(s), its partition coefficient, and its concentration within the delivery system affect the drug permeation. The figure depicts the cumulative percentage drug release (CPDR) profiles obtained from the prepared in-situ forming emulsion (Fig. 4). The permeation of the drug was evaluated for 180 minutes. It was observed that PG1 had the lowest CPDR (1.17% ± 0.05%) and PG5 showed the highest CPDR (1.97% ± 0.11%). The CPDR of the formulations was found in the order of PG5 (1.97% ± 0.11%) >PG4 (1.59% ± 0.09%) >PG3 (1.38% ± 0.07%) >PG2 (1.24% ± 0.03%) and PG1 (1.17% ± 0.05%). The results showed that the CPDR from the formulations was increased with the increase of oil content.
The mechanism of drug diffusion from the films was fitted to the Korsmeyer-Peppas model (KP model) (Qureshi et al., 2021). The diffusion coefficient “K” and the release exponent “n” in the KP model were calculated using Equation (2) and the results are tabulated in Table 3. An increased K value was observed in the increment of oil content within the formulation in PG1 to PG3. The K value of PG4 was the lowest among all formulations. Further, increase in oil content in the formulation, the K value was increased compared to PG4, but lower than PG3. This suggested the fact that the rate of diffusion of the drug was composition-dependent. The diffusional exponent (n) further explained the release mechanism of the drug from a gel base. The drug release mechanism may be Fickian transport (0.45 ≤ n), anomalous (non-Fickian) transport (0.45 ≤ n < 0.89), and/or super case-II transport (n > 0.89) (Dash et al., 2010). The n values of all formulations were greater than 0.45. This suggested the fact that the drug release from all formulations followed anomalous transport. Thus, the KP model explained the drug release from the polymeric gel base is composition-dependent and follows anomalous diffusion. Further, the CPDR profile was fitted to the Peppas-Shalin model (PS model) to examine the influence of both Fickian diffusion and the relaxation of polymers in releasing ACZ from the gel base. The Fickian diffusion constant (kd) and the polymer relaxation constant (kr) were calculated using Equation (2) and tabulated in Table 3. The results of the PS model parameter designated that the mechanism of diffusion from the emulgel was influenced by the polymer relaxation.
F = K.tn (2)
where, F is the solute fraction released; K is the release rate constant; t sampling time and n signifies the diffusion exponent.
F = kd.tm + kr.t2m (3)
Where kd, kr, and m represent the Fickian diffusion constant, polymer relaxation constant (case II), and the diffusion exponent, respectively.
In vivo corneal tolerance
The Draize rabbit eye test technique was used to conduct the ocular irritation investigation. Institutional animal ethical approval was obtained for this investigation by license # 49/IAEC/IPT/19, dated 16/03/2019. The left eye served as the control, and regular saline was administered to it.
The prepared gel sample (0.04 ml) was applied to the right eye. Chemosis, conjunctival redness, and corneal opacity were all seen in the ophthalmic health of both eyes. The eyes were assessed after 0, 1, 24, 48, and 72 hours (Fig. 5). All formulations appeared to be biocompatible based on visual inspection.
Intraocular pressure result
After giving the 5% glucose solution the IOP increases to 18.7 ± 0.5 mm of Hg. When the in-situ emulsion gel was inserted, the IOP was decreased to 9.5 ± 0.15 mm of Hg within 2 hours. From these results, it can be concluded that the prepared formulation was used in ocular drug delivery. The studies were done in triplicate and data are tabulated in Table 4 (Fig. 6).
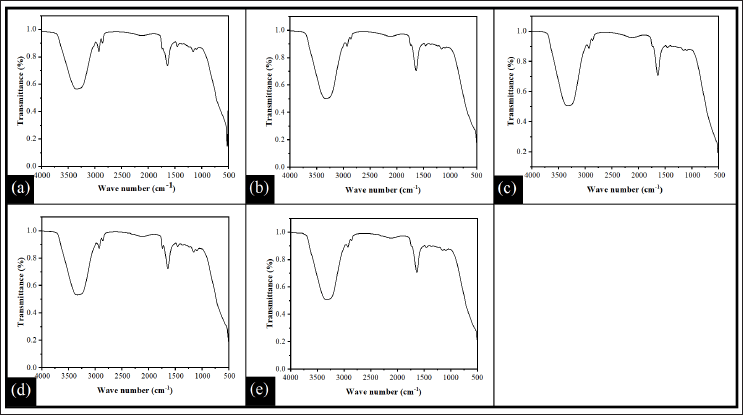 | Figure 3. FTIR spectra of in-situ gel (a) PG1, (b) PG2, (c) PG3, (d) PG4, and (e) PG5. [Click here to view] |
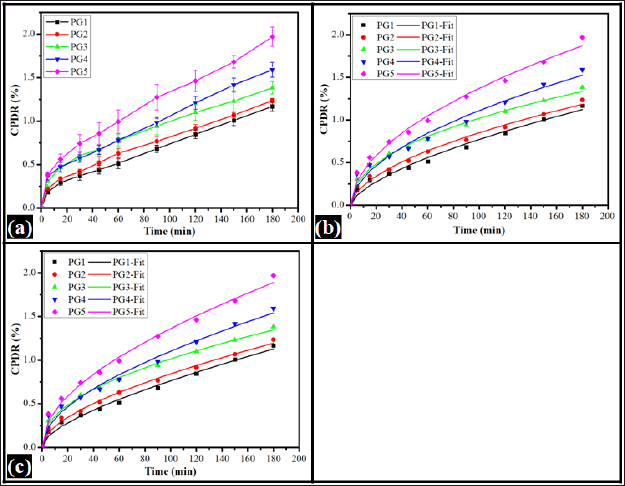 | Figure 4. Drug permeation profile of in-situ emulgels (a) CPDR, (b) Korsmeyer-Peppas model, and (c) PS model. [Click here to view] |
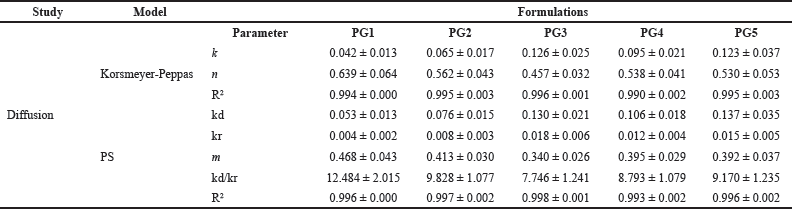 | Table 3. Drug permeation parameters. [Click here to view] |
 | Figure 5. Draize test conducted in rabbit model as per SOP. Above picture of the eye administered with PG5: (a) Left eye administered with normal saline at 0 hours, (a1) Right eye administered with PG5 at 0 hours, (b) Left eye administered with normal saline at 1 hour, (b1) Right eye administered with normal saline at 1 hour, (c) Left eye administered with normal saline at 24 hours, (c1) Right eye administered with PG5 at 24 hours, (d) Left eye administered with normal saline at 48 hours, (d1) Right eye administered with PG5 at 48 hours, (e) Left eye administered with normal saline at 72 hours, (e1) Right eye administered with PG5 at 72 hours. [Click here to view] |
 | Table 4. Intro ocular pressure result. [Click here to view] |
 | Figure 6. Measuring of IOP by using Schiotz tonometer (a) After giving the emulgel formulation. (b) After giving the 5% glucose solution. [Click here to view] |
CONCLUSION
In this study, the results and discussions proved the potential effects of ACZ-incorporated emulgel for ocular disease. The selection of excipients and methodologies adopted to manufacture in-situ emulgel were found to be result-oriented as evidenced by various investigations of this research work. Currently, the regularly used ocular formulations are associated with many unwanted complications. Henceforth, the developed formulation of in-situ emulgel warrants further investigations on the mechanism of action of the drug at the molecular level in various ocular diseases. Furthermore, extensive research work is needed to develop different dosage forms of ACZ incorporated in-situ gel with reduced toxicity and value-added clinical utility.
AUTHOR CONTRIBUTIONS
F.A.: Methodology, Investigation, Formal analysis, Writing – original draft. S.H.: Formal analysis, Data curation, assisted during the in vivo, and ex vivo studies. B.M.: Conceptualization, methodology, supervision, review, editing, and approval of the manuscript. A.B.: Review, editing, and Data curation., Y.G., Formal analysis, Data curation, assisted during the in vivo, and ex vivo studies. B.S.N.: Methodology, formal analysis, data curation. All authors have read and agreed to publish the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
Institutional animal ethical approval was obtained for this investigation by license # 49/IAEC/IPT/19, dated 16/03/2019.
DATA AVAILABILITY
The data presented in this study are available from the corresponding author upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of Gelrite® gellan gum-based ocular delivery system for indomethacin. Acta Pharm, 2003; 53(4):251–62.
Behera B, Sagiri SS, Pal K, Srivastava A. Modulating the physical properties of sunflower oil and sorbitanmonopalmitate-based organogels. J Appl Polym Sci, 2013; 127(6):4910–7. CrossRef
Calvo P, Vila-Jato JL, Alonso MJ. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int J Pharm, 1997; 153(1):41–50. CrossRef
Carlfors J, Edsman K, Petersson R, Jörnving K. Rheological evaluation of Gelrite® in situ gels for ophthalmic use. Eur J Pharm Sci, 1998; 6(2):113–9. CrossRef
Chen MJ, Chen KN. Applications of probiotic encapsulation in dairy products, Encap and cont release tech in food systems, Blackwell Publisher, Hoboken,NJ, pp 83–112, 2007. CrossRef
Cohen S, Lobel E, Trevgoda A, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Release, 1997; 44(2–3):201–8. CrossRef
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm, 2010; 67(3):217–23.
Epstein DL, Grant WM. Carbonic anhydrase inhibitor side effects: serum chemical analysis. Arch Ophthalmol, 1977; 95(8):1378–82. CrossRef
Ezati P, Rhim JW. pH-responsive pectin-based multifunctional films incorporated with curcumin and sulfur nanoparticles. Carbohydr Polym, 2020; 230:115638. CrossRef
Fernández-Ferreiro A, Barcia MG, Gil-Martínez M, Vieites-Prado A, Lema I, Argibay B, Méndez JB, Lamas MJ, Otero-Espinar FJ. In vitro and in vivo ocular safety and eye surface permanence determination by direct and magnetic resonance imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur J Pharm Biopharm, 2015; 94:342–51. CrossRef
Friedberg ML, Pleyer U, Mondino BJ. Device drug delivery to the eye: collagen shield’s, iontophoresis, and pumps. Ophthalmology, 1991; 98(5):725–32. CrossRef
Gamm E. Origination of side effects of acetazolamide. Glaucoma, 1984; 6:60–3.
Gnanasambandam R, Proctor A. Determination of pectin degree of esterification by diffuse reflectance fourier transform infrared spectroscopy. Food Chem, 2000; 68(3):327–32. CrossRef
Grass GM, Robinson JR. Mechanisms of corneal drug penetration II: ultrastructural analysis of potential pathways for drug movement. J Pharm Sci, 1988; 77(1):15–23. CrossRef
Gupta A, Mishra AK, Singh AK, Gupta V, Bansal P. Formulation and evaluation of topical gel of diclofenac sodium using different polymers. Drug Invent Today, 2010; 2(5):250–3.
Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm, 2000; 199(2):119–27. CrossRef
Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm, 2002; 248(1–2):1–4. CrossRef
Kaur IP, Singh H, Kakkar S. Newer therapeutic vistas for antiglaucoma medicines. Crit Rev Ther, 2011; 28(2):165–202. CrossRef
Khaw PT, Cordeiro MF. Towards better treatment of glaucoma: recent advances could have a major impact on preventing damage worldwide. BMJ, 2000; 320(7250):1619–20. CrossRef
Kreuter J. Particulates (nanoparticles and microparticles). Drugs Pharma Sci, 1993; 58:275–87.
Krstonoši? V, Doki? L, Doki? P, Dap?evi? T. Effects of xanthan gum on physicochemical properties and stability of corn oil-in-water emulsions stabilized by polyoxyethylene (20) sorbitan monooleate. Food Hydrocoll, 2009; 23(8):2212–8. CrossRef
Kunou N, Ogura Y, Hashizoe M, Honda Y, Hyon SH, Ikada Y. Controlled intraocular delivery of ganciclovir with use of biodegradable scleral implant in rabbits. J Control Release, 1995; 37(1–2):143–50. CrossRef
Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J OculPharmacol Ther, 1986; 2(1):67–108. CrossRef
Lin D, Zheng Y, Wang X, Huang Y, Ni L, Chen X, Wu Z, Huang C, Yi Q, Li J, Qin W, Zhang Q, Chen H, Wu D. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int J Biol Macromol, 2020; 165:1241–9. CrossRef
Marcus JB. Food science basics: healthy cooking and baking demystified: the science behind healthy foods, cooking and baking. Academic Press, San Diego, CA, pp 51–97, 2013. CrossRef
Marquardt D, Sucker H. Oil-in-water-emulsion gels: determination and mathematical treatment of flow properties. Eur J Pharm Biopharm, 1998; 46(1):115–24. CrossRef
Maru S, Ongarora DS, Njoroge RW. Formulation and in vitro evaluation of a mucoadhesive metronidazole dental gel for oral application. East Cent Afr J Pharm Sci, 2019; 22(2):52–6.
Mohsen AM, Salama A, Kassem AA. Development of acetazolamide loaded bilosomes for improved ocular delivery: preparation, characterization and in vivo evaluation. J Drug Deliv Sci Technol, 2020; 59:101910. CrossRef
Narkar M, Sher P, Pawar A. Stomach-specific controlled release gellan beads of acid-soluble drug prepared by ionotropic gelation method. Aaps Pharm Sci Tech, 2010; 11(1):267–77. CrossRef
Neeraja P, Amaleshwari M, Ravali G. Formulation and evaluation of nifedipine multiple emulsions. Int J Pharm Chem Biol Sci, 2014; 4(3): 673-680
Pal K, Banthia AK, Majumdar DK. Biomedical evaluation of polyvinyl alcohol–gelatin esterified hydrogel for wound dressing. J Mater Sci Mater Med, 2007a; 18(9):1889–94. CrossRef
Pal K, Banthia AK, Majumdar DK. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. Aaps Pharm Sci Tech, 2007b; 8(1):142–6. CrossRef
Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci, 1975; 64(8):1312–6. CrossRef
Paulsson M, Hägerström H, Edsman K. Rheological studies of the gelation of deacetylated gellan gum (Gelrite®) in physiological conditions. Eur J Pharm Sci, 1999; 9(1):99–105. CrossRef
Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr Polym, 2013; 93(2):670–8. CrossRef
Qureshi D, Behera KP, Mohanty D, Mahapatra SK, Verma S, Sukyai P, Banerjee I, Pal SK, Mohanty B, Kim D, Pal K. Synthesis of novel poly (vinyl alcohol)/tamarind gum/bentonite-based composite films for drug delivery applications. Colloids Surf A PhysicochemEng Asp, 2021; 613:126043. CrossRef
Quereshi D, Dhal S, Das D, Mohanty B, Anis A, Shaikh H, Hanh Nguyen TT, Kim D, Sarkar P, Pal K. Neem seed oil and gum arabic-based oil-in-water emulsions as potential ocular drug delivery system. J Dispers Sci Technol, 2020; 41(13):1911–24. CrossRef
Rozier A, Mazuel C, Grove J, Plazonnet B. Functionality testing of gellan gum, a polymeric excipient material for ophthalmic dosage forms. Int J Pharm, 1997; 153(2):191–8. CrossRef
Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: physicochemical characterisation and in vitro release. Int J Pharm, 2011a; 411(1–2):69–77. CrossRef
Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: precorneal retention and in vivo pharmacodynamic study. Int J Pharm, 2011b; 411(1–2):78–85. CrossRef
Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Deliv Rev, 1995; 16(1):95–106. CrossRef
Shivangi S, Dorairaj D, Negi PS, Shetty NP. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active component. Food Hydrocoll, 2021; 121:107046. CrossRef
Soltau JB, Zimmerman TJ. Changing paradigms in the medical treatment of glaucoma. Surv Ophthalmol, 2002; 47:S2–5. CrossRef
Sun J, Zhou Z. A novel ocular delivery of brinzolamide based on gellan gum: in vitro and in vivo evaluation. Drug Des DevelTher, 2018; 12:383. CrossRef
Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release, 2002; 83(1):65–74.
Wo N, Zhai J. Combinatorial therapeutic drug delivery of riboflavin and dexamethasone for the treatment of keratoconus affected corneas of mice: ex vivo permeation and hemolytic toxicity. Micro Nano Lett, 2021; 16(10):492–9. CrossRef
Zhang X, Chen X, Gong Y, Li Z, Guo Y, Yu D, Pan M. Emulsion gels stabilized by soybean protein isolate and pectin: effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason Sonochem, 2021; 79:105756. CrossRef
Zia KM, Tabasum S, Khan MF, Akram N, Akhter N, Noreen A, Zuber M. Recent trends on gellan gum blends with natural and synthetic polymers: a review. Int J Biol Macromol, 2018; 109:1068–87. CrossRef
Zignani M, Tabatabay C, Gurny R. Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv Drug Deliv Rev, 1995; 16(1):51–60. CrossRef