INTRODUCTION
Microbial infections are life-threatening diseases; they weaken the immune system and deteriorate most disease conditions. The burden of infections due to antimicrobial resistance, especially in Africa and Asia, is alarming. According to the European Center for Disease Prevention and Control, about 33,000 people die annually from infections (Bamigboye et al., 2021; Ejidike et al., 2019; El-Saied et al., 2017). Antibiotic-resistant bacteria are one of the underlying conditions that have been increasing the coronavirus disease-2019 virus mortality rate to date, with which over 5 million people have been affected according to the World Health Organization (2021) (Feldman and Anderson, 2021; Wainwrighr, 1998). Most approved antibiotics are inefficient in treating bacterial infections due to their ability to form biofilms, regularly change strains, and develop complex structures with other pathogens (Roy et al., 2018). Oxidative stress may deteriorate the health of patients already infected with bacteria or fungi, resulting in more severe conditions; these challenges call for potential novel drugs that will combat the challenges of infections and oxidative stress (Bamigboye et al., 2021; Dikio et al., 2017).
Antioxidants are substances that can prevent or slow damage to cells caused by free radicals. Free radicals are unstable molecules/atoms with unpaired electrons; they can be from either endogenous or exogenous sources (Ejidike, 2018; Rahman et al., 2015). The accumulation of free radicals has been strongly associated with several health conditions such as cancer, diabetes, cardiovascular diseases, and neurodegenerative diseases (Gilgun-Sherki et al., 2002; Islam et al., 2013; Naskar et al., 2011). Thus, there is a need for an antioxidant to neutralize the effects of free radicals. Research has shown that antioxidants can reduce the risk of chronic diseases, including cancer and cardiovascular disease (Gilgun-Sherki et al., 2002; Naskar et al., 2011). Although the human biological system has antioxidant defense mechanisms that scavenge free radicals, they are not sufficient. Therefore, there is a need for an external antioxidant.
Schiff base ligands have received attention recently due to structural flexibility and a wide range of biological applications. Adole et al. (2020) reported that 2-(2-hydrazineyl)thiazole derivatives showed antibacterial, antifungal, and antioxidant activities. Similarly, reports have shown that ligands exhibit a wide range of activities which include antimicrobial, anti-inflammatory, antimalarial, and antipyretic among other properties, and the presence of the imine group in Schiff base ligands has been shown to impact their biological activities (Bringmann et al., 2004; Ejidike et al., 2019, 2021; El-Behery and El-Twigry, 2007; Pathade et al., 2021; Przybylski et al., 2009; Shinde et al., 2021a, 2021b). Transition metal complexes containing Schiff base ligands have been used in the treatment of several pathogenic infections and have been shown to have great antioxidant properties; these complexes scavenge free radicals that are causative factors in cardiovascular diseases and cancer. Studies have shown that the metal complexes of different Schiff base ligands have higher activities than the ligands alone (Ejidike and Ajibade, 2017; Fonkui et al., 2018; Omar et al., 2006). Pure metallic forms of copper (Cu), cobalt (Co), nickel (Ni), and zirconium (Zr) have been documented to exhibit antibacterial properties (Bonaccorso et al., 2020; Khanna et al., 2019; Reiss et al., 2014). The chelating behavior of the Schiff base ligand upon complexation with metals is responsible for both increased antimicrobial activity and antioxidant properties of the complexes (Santos-Sánchez et al., 2019). Thus, Schiff base metal complexes exhibit dual-mode actions as antioxidants and antimicrobials (Adole et al., 2020; Ejidike, 2018; Ejidike et al., 2021).
In this study, we report the antibacterial and antioxidant activities of the 4,4’-{ethane-1,2-diylbis[nitrilo(Z)methylylidene]}bis(2-methoxyphenol) ligand (SV) Schiff base complexes of Cu(II), Co(II), Ni(II), Zr(IV), VO(IV), and UO2(II) against two Gram-positive [Staphylococcus aureus (ATCC-25923) and Enterococcus faecalis (ATCC-29212)] and two Gram-negative [Klebsiella pneumonia (ATCC-13883) and Pseudomonas aeruginosa (ATCC-15442)] bacteria. It is believed that the presence of these metal ions with broad antimicrobial properties will improve the efficiency of the complexes as potential antibacterial and antioxidant agents when compared to the free Schiff base ligand alone.
MATERIALS AND METHODS
The chemicals and reagents were of analytical grade; they were purchased from Sigma-Aldrich and used without further purification. The cultured organisms were collected from the Biotechnology Laboratory, Vanderbijlpark, South Africa. A Gallenkamp melting point apparatus was used for the determination of the melting point. The percentage composition of elements (C, H, and N) present in the complexes was determined using a PerkinElmer 2400 CHN Elemental Analyzer (PerkinElmer, Waltham, MA). The functional groups present in the compounds were recorded on a Shimadzu FTIR-8400S Fourier Transform Infrared (FTIR) Spectrophotometer. The ultraviolet-visible spectra of the compounds were recorded on a Jenway 6405 UV-vis spectrophotometer within the range of 200–800 nm. The molar conductance was obtained using a Jenway 4510 Conductivity Meter with a cell constant of 1.42.
Synthesis of Schiff base ligands
The ligands were synthesized as reported by Raman et al. (2001) and Ejidike and Ajibade (2015). Briefly, ethane-1.2-diamine (1.25 ml, 0.02 mol) dissolved in 20 ml of ethanol was added slowly to a 50 ml ethanol solution containing 4-hydroxy-3-methoxy benzaldehyde (6.086 g, 0.04 mol) while stirring. The resulting light yellow mixture was refluxed for 4 hours at 60°C. The resulting mixture of the bidentate ligand was washed with cold ethanol and dried in a desiccator (yield = 6.6447 g, 78.78%).
Synthesis of metal Schiff base complexes
The metal complexes of the Schiff base ligand were prepared by the addition of 0.75 mmol of each metal ion (VO(acetate)2, UO2(NO3)2·6H2O, CoCl2·6H2O, CuCl2·2H2O, ZrClO2·8H2O, and NiCl2·6H2O) dissolved in 20 ml of ethanol to the hot ethanolic solution (20 ml) of the bidentate ligand (1.5 mmol, 0.7895 g) at a ratio of 1:2 while stirring. The colors changed immediately. The mixture was buffered with 0.21 ml of triethylamine. The resulting mixtures were then refluxed for 3–4 hours at 70°C and allowed to cool. The resulting precipitated solids were filtered, washed, and dried in a desiccator.
Antimicrobial activities
The selected strains of microbes were maintained in the Biotechnology Laboratory, Vanderbijlpark, South Africa. They consisted of two Gram-positive [S. aureus (ATCC-25923) and E. faecalis (ATCC-29212)] and two Gram-negative [K. pneumoniae (ATCC-13883) and P. aeruginosa (ATCC-15442)] bacteria. The antimicrobial activities of the complexes were evaluated using the agar diffusion method as described by Bamigboye et al. (2021). Seven grams of nutrient agar was weighed and dissolved in 250 ml of distilled water in a conical flask. Cotton wool and aluminum foil were used to cover the content of the flask before autoclaving. The microbes were inoculated on top of the prepared agar, which was solidified in the Petri dish. About 5 mm of a sterilized cork borer was used to drill holes within the plates, followed by the addition of 25 μg/ml of each metal complex solution to the prepared agar plate. The plates were allowed to stand for 30 minutes to ensure proper penetration of the test compounds before incubation at 37°C for 24 hours. Zones of inhibition (mm) were measured and recorded.
Antioxidant assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging activity evaluation is a standard assay used in antioxidant activity studies. The antioxidant activity of the Schiff base ligand [SV] and Schiff base bidentate metal complexes were obtained using the DPPH reagent as previously reported (Ejidike and Ajibade, 2017). The complexes were dissolved in 1 ml of dimethylformamide (DMF) to make various concentrations (100, 200, 300, 400, and 500 μg/ml) which were mixed thoroughly with 1 ml of methanol and allowed to interact in the dark for about 30 minutes. Ascorbic acid and gallic acid were used as standards for control. The absorbance of the solutions and the control was measured at 517 nm using a spectrophotometer. Ligands and metal complexes contain several hydrogen atoms that can be donated. The donating ability of the hydrogen atoms in the complexes was determined by the decolorization of the DPPH reagent. DPPH produces a violet/purple color in a methanol solution, which changes to a yellow color in the presence of antioxidants. The concentration of the tested complex required to inhibit 50% of the DPPH free radical known as the IC50 value of the samples was calculated using the inhibition curve. The lower the absorbance of the reaction mixture, the higher the free radical inhibitory activity (Koleva et al., 2021). The analysis of all the tests was carried out in triplicate to obtain mean ± SD. The following equation was used to obtain the percentage of the scavenging activity:
RESULTS AND DISCUSSION
Chemistry of the complexes
The proposed structures of the synthesized ligands (SV) and their corresponding metal Schiff base complexes represented as CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV are shown in Figure 1. The compounds were characterized using spectroscopic methods: UV-visible, FTIR, and elemental analysis. The synthesized complexes were of good yield, which ranged from 67.84% to 90.30%, as shown in Table 1; NiSV had the least yield, while UO2SV had the most yield; the complexes were stable at room temperature to air and are colored, and the coloration is due to partial filling of the d-orbital of the metal ion (Ejidike, 2018). The elemental analysis data of the complexes is presented in Table 1; the calculated value is in good agreement with the experimental values; it is evident that the complexes are in the ratio of 1:2 (M:SV), and these suggest a successful synthesis of the bidentate metal Schiff base complexes.
The physicochemical properties of the parent ligands and their corresponding metal complexes are shown in Table 1. The melting point of the metal complexes, which is in the range of 172–182, is higher than that of the ligand alone whose value ranged from 167 to 168. The increase in the melting point is due to the presence of metal in the bidentate complex. Their conductivity ranged from 12.30 to 18.26 μScm−1; the conductance value, which is below 40 μScm−1, is consistent with the nonelectrolyte nature of complexes in solution (Ejidike and Ajibade, 2017; Ejidike et al., 2019).
Infrared spectra
The FTIR bands of the compounds are presented in Table 2. The IR spectrum of the ligands (SV) shows bands in the regions 3468–3220 cm−1 which can be assigned to υ(OH) frequency; these bands are also observed in the metal complexes due to the free phenolic OH group present in the compounds (Ejidike and Ajibade, 2015; Ejidike et al., 2021). A broad peak between 3,104 and 2,858 cm−1 assigned to the υOCH3 stretch observed in the ligand shifted to a higher or lower frequency in the complexes. The strong υOCH3 bond might be a result of intramolecular hydrogen bonding between the OH group and the O atom of the methoxy group which is in the ortho position. Bands corresponding to υ(C = N) (azomethine) appeared at 1,633 and 1,603 cm−1 upon the successful formation of the ligand. This band shifted to a higher wavenumber to the extent of 17–55 cm−1 on the formation of the metal complexes, an indication that the nitrogen of the azomethine groups is responsible for the coordination of the ligand to the metal atoms (Alias et al., 2014; Ejidike, 2018; Malik et al., 2011). Other relevant peaks in the Schiff base ligand at about 1,516, 1,288, and 883 cm−1 are attributed to υ(C = C), υ(C = O), and δ(C = O), respectively, and are presented in Table 2. The bands are common for all the complexes, and the data are consistent with what has been documented in the literature (Ejidike and Ajibade, 2017; Köse and Necefo?lu, 2008; Nandiyanto et al., 2019). In the IR spectra of the VOSV, ZrOSV, UrO2SV, CuSV, CoSV, and NiSV complexes, new bands at 620, 621, 642, 625, 645, and 670 cm−1 were assigned to υ(M = N) stretching vibrations (Ejidike and Ajibade, 2015; Ejidike et al., 2019; Fierro et al., 2011). The presence of an intense peak around 983 cm−1 is characteristic of the υ(V = O) vibration (Raman et al., 2001), while in the uranyl complex (UrO2SV) the spectrum showed a band in the region of 905 cm−1 assigned to the antisymmetric υ(0 = U = O) vibration (El-Behery and El-Twigry, 2007). The bands at 962 cm−1 are assigned to the υ(Zr = O) vibration of the complexes (ZrOSV) (Ashoor and Ediab, 2014). These trends found in all the complexes are consistent with what has been documented in the literature (Köse and Necefo?lu, 2008; Nandiyanto et al., 2019). The new υ(M = N) bands confirmed the successful formation of the Schiff base metal complexes. From the FTIR spectra, the synthesis of the 4,4’-{ethane-1,2-diylbis[nitrilo(Z)methylylidene]}bis(2-methoxyphenol) ligand (SV) and its metal complexes represented as CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV was coordinated through two nitrogen atoms of the azomethine groups. The FTIR spectra data confirmed the coordination of the imino nitrogen atoms to the Co, Cu, Ni, ZrO, VO, and UO2 ions. Hence, the absorptions expected for all the complexes are present and explained following previous literature (Ashoor and Ediab, 2014; Bamigboye et al., 2021; Dikio et al., 2017; Ejidike and Ajibade, 2015; Nandiyanto et al., 2019; Raman et al., 2001).
UV-visible absorption
The electronic spectra of the SV ligands and metal Schiff base complexes were recorded in 10−3M DMF solutions and are shown in Figure 2. The synthesized complexes (CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV) showed characteristic λmax when compared to the ligand alone, and this also confirmed successful coordination. Three bands were observed in the spectrum of the ligand (SV) at about 295, 315, and 405 nm. The peaks at about 295 and 315 nm are due to aromatic π-π* and imino π-π* transitions, and the band at 405 nm is attributed to the n→π* (Bamigboye et al., 2021; Dikio et al., 2017; Ejidike and Ajibade, 2015; Raman et al., 2001). These peaks maintained their wavelength or underwent a hypochromic shift to higher frequencies in coordination with metal. The electronic spectrum of the CoSV complex shows a charge-transfer (CT) band at 470 nm and d-d absorption at the visible region around 535 nm of less intensity indicating a distorted tetrahedral environment of the ligand around the Co ion due to 4A2(F) → 4T1(P) transition (Ejidike and Ajibade, 2017; Raman et al., 2001). The copper complex (CuSV) in the visible region exhibited two absorption bands at 440 and 565 nm, which can attribute to 2B1g → 2E1g and 2B1g → 2A1g transition, respectively, associated with metals having d-orbitals of a square planar copper(II) complex (Bamigboye et al., 2021; Ejidike and Ajibade, 2017; Ejidike, 2018; Raman et al., 2001). The electronic spectra of the diamagnetic uranyl complex, UO2SV (Fig. 2), show two absorption bands alongside the ligand bands. The first band was observed at around 475 nm attributable to the CT transition from equatorial donor atoms of the SV ligand to the uranium ion, and the second band was observed at about 670 nm, characteristic of electronic transitions from the apical oxygen atom to the f-orbitals of the uranyl atom (El-Behery and El-Twigry, 2007). The ZrOSV complex exhibited absorption peaks that were observed at frequencies 295, 330, 410, and 525 nm. The first two peaks are ascribed to π→π*, the one at 410 nm to n→π* type transitions, and the one at 525 nm to a CT transition; this identification is similar to those reported in the literature (Ashoor and Ediab, 2014). Electronic absorption spectral data of the VOSV complex showed intraligand CT transition observed around 391 nm, while two absorption bands around 415 and 510 nm are attributed to 2B2 → 1E and 2B2 → 1A1 transitions of the VO complex in a square pyramidal environment (Raman et al., 2001). In the NiSV complex absorption spectrum, a CT transition L → M was observed around 390 nm, while two absorption bands around 425 and 560 nm are attributed to 1A1g → 1A2g and 1A1g → 1B1g transitions of the Ni(II) complex in a square planar environment (Ejidike and Ajibade, 2017; Ejidike et al., 2019; Fierro et al., 2011; Raman et al., 2001).
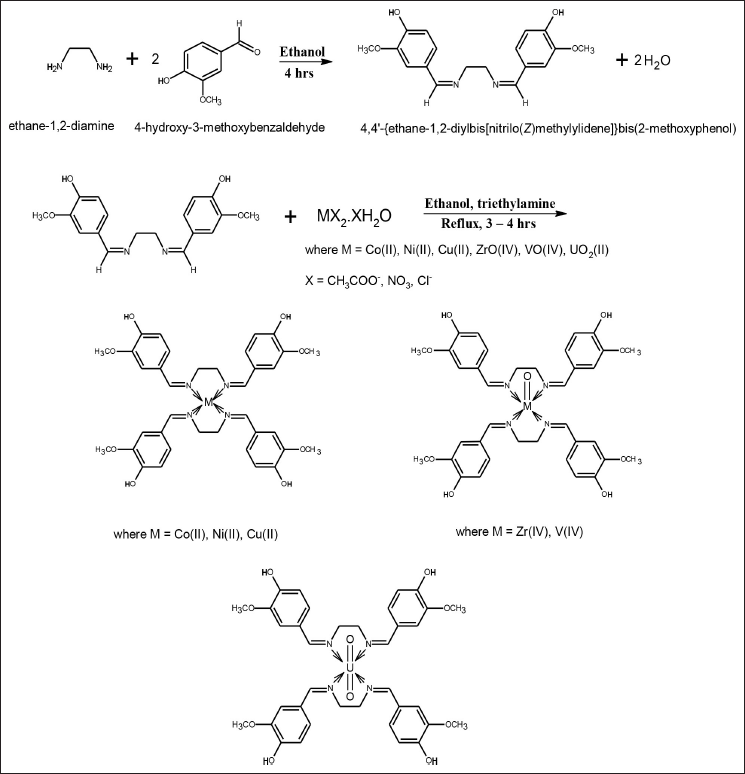 | Figure 1. Schematic pathways for the synthesis of the ligand (SV) and its metal complexes. [Click here to view] |
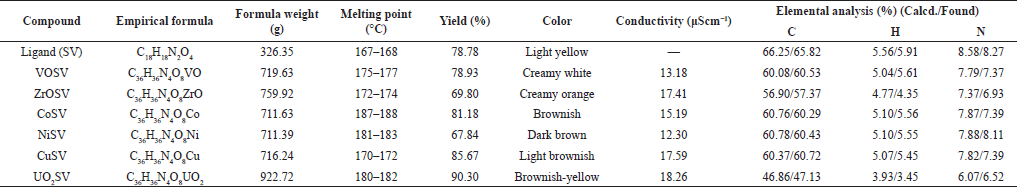 | Table 1. Analytical properties of the ligand (SV) and metal Schiff base complexes. [Click here to view] |
Antioxidant properties
The DPPH free radical scavenging method was adopted to test the antioxidant activities of Schiff base ligand (SV) and metal complexes represented as CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV. The represented metal complexes showed lower antioxidant potential than standard ascorbic or gallic acid, as shown in Table 3. The synthesized complexes showed IC50 (μg/ml) DPPH radical scavenging activities ranging from 3.24 ± 1.37 to 6.44 ± 0.44 with lower inhibition than those of the standard agents: ascorbic and gallic acid with IC50 (μg/ml) values of 1.22 ± 0.84 and 2.02 ± 0.42, respectively (p < 0.05). The scavenging ability of the synthesized metal Schiff base ligand on DPPH may be a result of the hydrogen-donating ability of the compound. The complexes contain some free hydroxyl groups that can form hydrogen bonding with DPPH, thus reducing the violet color of the DPPH solution to yellow DPPH (Ejidike et al., 2021; Rahman et al., 2015). Free radicals cause oxidative damage due to their ability to initiate a chain reaction, while antioxidants neutralize oxidative damage by donating electrons to free radicals, thus acting as reducing agents that neutralize the damage before initiation (Halliwell, 2012; Herrling et al., 2008). This study corroborates a report in the literature (Ejidike and Ajibade, 2017), which showed that the Co(II), Ni(II), Cu(II), and Zn(II) complexes of the 4,4’-{ethane-1,2-diylbis[nitrilo(1E)eth-1-yl-1-ylidene]}dibenzene-1,3-diol metal Schiff base complexes exhibited higher antioxidants properties than the free ligand.
Antimicrobial studies
Microbial infections are life-threatening diseases that affect both the health and the economy of various nations. The ingestion of food and water contaminated with both Gram-positive and Gram-negative bacteria can result in so many illnesses, such as sepsis, typhoid, fever, and diarrhea. The antimicrobial activities of the parent ligand represented as (SV) and its synthesized metal Schiff base complexes represented as CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV were tested against S. aureus (ATCC-25923), E. faecalis (ATCC-29212), K. pneumonia (ATCC-13883), and P. aeruginosa (ATCC-15442), and their activities are enlisted in Table 4. The Schiff base complexes containing metals exhibited strong to moderate inhibitory effects compared to their parent-free ligands, in both Gram-positive and Gram-negative bacteria (Fig. 3). All the complexes were potent against E. faecalis compared to the parent ligand which did not show any activity. The higher activities of the Schiff base metal complexes are a result of the chelating behavior upon complexation. Metals partially share their positive charges with the donor atoms in the ligand; this results in electron delocalization over the metal complex ring. Thus, the metal’s complex lipophilic nature increases its permeation capability and makes it efficiently go through the microorganisms’ lipid layer (Bamigboye et al., 2021; More et al., 2017; Omar et al., 2006).
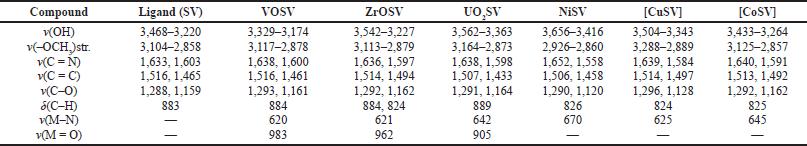 | Table 2. FTIR data of the ligand (SV) and the metal Schiff base complexes. [Click here to view] |
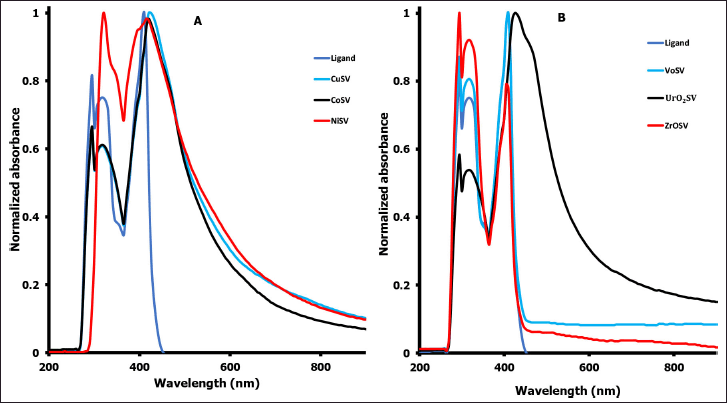 | Figure 2. UV-visible spectra of the ligands and their complexes. [Click here to view] |
The zirconium metal Schiff base ligand complex (ZrOSV) showed high antimicrobial properties with zones of inhibition of 15, 16, 17, and 17 mm against S. aureus, P. aeruginosa, E. faecalis, and K. pneumonia, respectively, as compared to their parent ligands, as shown in Table 4. The high antimicrobial activities of the ZrOSV complex may be due to an increase in the lipophilic nature of the complex after coordination with the ligand. A similar pattern of increase in antimicrobial activities due to improving lipophilic nature of Zr after complexation was also shown by Mahind et al. (2013), who tested the activities of the Zr(IV) Schiff base complex against S. aureus and other bacteria, Escherichia coli, Salmonella typhi, and Bacillus subtilis, and recorded zones of inhibition which were not seen in the parent ligand. The antimicrobial activities of other Schiff base metal complexes containing Ni, UO2, and Co ions were higher than the ligand alone based on the enhanced lipophilic nature of the ligand in coordination with metals (David et al., 2005; Liu et al., 2013; More et al., 2017; Omar et al., 2006).
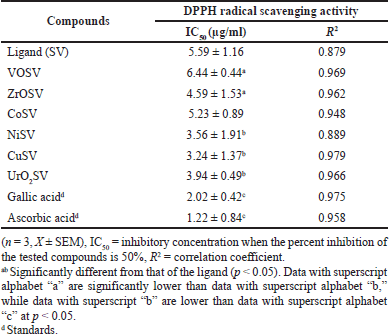 | Table 3. DPPH scavenging capacities (IC50, μg/ml) of standard drugs, SV Schiff base, and its metal complexes. [Click here to view] |
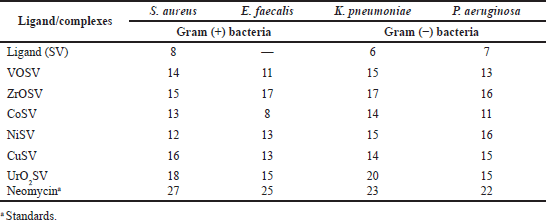 | Table 4. Antimicrobial activities (zone of inhibition, mm) of the ligand (SV) and its metal complexes. [Click here to view] |
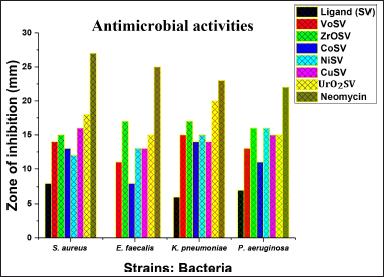 | Figure 3. Antimicrobial potentials of the ligand (SV) and metal complexes. [Click here to view] |
The copper-containing Schiff base complex showed comparative high zones of inhibition of 16, 15, 13, and 14 mm in the four tested strains of bacteria. The antimicrobial activities of copper may be due to the redox cycling reactions between Cu(II) and Cu(I) oxidation states which results in the formation of reactive radical species. Under aerobic conditions, the reactive radical species produces free hydroxyl radicals that react within the cell including membrane lipids, proteins, and nucleic acids to generate different dangerous species and products. This reaction results in the damage of the bacterial DNA (?ongrádyová et al., 2014). The result is consistent with what was reported by Duncan and White (2012) in the literature, who showed that the copper salicylate ligand exhibited higher antibacterial activities against E. coli than the free ligand.
The nickel-containing complex showed a moderately high inhibitory effect against P. aeruginosa with a 16 mm zone of inhibition compared to the CoSV and VOSV complexes, which showed antimicrobial activity with 11 and 13 mm zones of inhibition, respectively. Nickel metal has been shown to have characteristics of antimicrobial properties in medicinal inorganic chemistry because of its potential to penetrate microbial cells and destroy the microorganism by inactivating their enzymes (Chohan and Kausar, 2000; Ejidike et al., 2019).
The antimicrobial activities confirmed that the complexes possess better medicinal therapeutic potential with a wide spectrum against the activities of organisms than their free ligands, hence their resourcefulness in the preparation of antibiotics or chemotherapeutic agents.
CONCLUSION
The synthesis of the 4,4’-{ethane-1,2-diylbis[nitrilo(Z)methylylidene]}bis(2-methoxyphenol) (SV) ligand and its metal complexes represented as CoSV, CuSV, NiSV, ZrOSV, VOSV, and UO2SV was successful. The complexes were physicochemically characterized by elemental analysis, melting point, UV spectra, conductivity measurement, and FTIR. Antimicrobial analysis of the SV ligand and its metal complexes was evaluated against bacterial strains such as S. aureus, E. faecalis, K. pneumoniae, and P. aeruginosa; the bacteria were highly susceptible to the metal complexes showing moderate to strong activities that were higher than the parent ligands. The antimicrobial results indicate the complexes could be used alone or in combination as alternative antibiotics. Similarly, the pronounced antioxidant properties of the complexes also indicate that they could be used as free radical scavengers.
FUNDING
The authors gratefully acknowledge the National Research Foundation, South Africa (Grant No. 120790), and the Directorate of Research, University of South Africa, Florida Campus, South Africa.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest in this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adole VA, More RA, Jagdale BS, Pawar TB, Chobe SS. Efficient synthesis, antibacterial, antifungal, antioxidant and cytotoxicity study of 2-(2-hydrazineyl) thiazole derivatives. Chem Select, 2020; 5(9):2778–86. CrossRef
Alias M, Kassum H, Shakir C. Synthesis, physical characterization and biological evaluation of Schiff base M(II) complexes. JAAUBAS, 2014; 15:28–34. CrossRef
Ashoor SE, Ediab IM. Synthesis, investigation, DFT study and biological activity of zirconium(IV) complexes. Int J Chem Mol Nucl Mater Metall Eng, 2014; 8(2):161–5.
Bamigboye OM, Ejidike IP, Lawal M, Nnabuike GG, Clayton HS. Mixed amodiaquine—acetylsalicylic acid metal complexes: characterization and antimicrobial potentials. Sci Technol Asia, 2021; 26(1):53–63.
Bonaccorso C, Marzo T, La Mendola D. Biological applications of thiocarbohydrazones and their metal complexes: a perspective review. Pharmaceuticals, 2020; 13(1):4. CrossRef
Bringmann G, Dreyer M, Faber JH, Dalsgaard PW, Jaroszewski JW, Ndangalasi H, Mbago F, Brun R, Christensen SB. Ancistrotanzanine C and related 5,1- and 7,3-coupled naphthylisoquinoline alkaloids from Ancistrocladus tanzaniensis. J Nat Prod, 2004; 67(5):743–8. CrossRef
Chohan ZH, Kausar S. Synthesis, characterization, and biological properties of tridentate NNO, NNS and NNN donor thiazole-derived furanyl, thiophenyl and pyrrolyl Schiff bases and their Co(II), Cu(II), Ni(II) and Zn(II) metal chelates. Met Based Drugs, 2000; 7:17–22. CrossRef
?ongrádyová A, Jomová K, Kucková L, Kožíšek J, Monco? J, Valko M. Antimicrobial activity of copper(II) complexes. J Microbiol Biotech Food Sci, 2014; 3:67–70.
David S, Barros V, Cruz C. In vitro effect of free and complexed Indium(III) against Mycobacterium tuberculosis. FEMS Microbiol Lett, 2005; 251:119–24. CrossRef
Dikio CW, Ejidike IP, Mtunzi FM, Klink MJ, Dikio E. Hydrazide Schiff bases of acetylacetonate metal complexes: synthesis, spectroscopic and biological studies. Int J Pharm Pharm Sci, 2017; 9(12):257–67. CrossRef
Duncan C, White AR. Copper complexes as therapeutic agents. Metallomics, 2012; 4:127–38. CrossRef
Ejidike IP, Ajibade PA. Synthesis, characterization, in vitro antioxidant and anticancer studies of ruthenium(III) complexes of symmetric and asymmetric tetradentate Schiff bases. J Coord Chem, 2015; 68(14):2552–64. CrossRef
Ejidike IP, Ajibade PA. Synthesis, spectroscopic, antibacterial and free radical scavenging studies of Cu(II), Ni(II), Zn(II) and Co(II) complexes of 4,4’-{ethane-1,2- diylbis[nitrilo(1E)eth-1-yl-1-ylidene]} dibenzene 1,3-diol Schiff base. J Pharm Sci Res, 2017; 9(7):593–600.
Ejidike IP, Bamigboye OM, Clayton HS. Spectral, in vitro antiradical and antimicrobial assessment of copper complexes containing tridentate Schiff base derived from dihydroxybenzene functionality with diaminoethylene bridge. Spectrosc Lett, 2021; 54(3):212–30. CrossRef
Ejidike IP, Mtunzi FM, Klink MJ. Spectroscopic, XRD, in vitro anti-oxidant, antifungal and antibacterial studies of heterocyclic Schiff base Nickel(II) complexes bearing anions. In: Ramasami P, Gupta Bhowon M, Jhaumeer LS, Li K, Wah H (eds.). Chemistry for a clean and healthy planet. ICPAC-2018, Springer, Cham, Switzerland, 2019, pp 283–305. CrossRef
Ejidike IP. Cu(II) complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino] ethyl}ethanimidoyl]benzene-1,3-diol Schiff base: synthesis, a spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules, 2018; 23(7):1581–99. CrossRef
El-Behery M, El-Twigry H. Synthesis, magnetic, spectral, and antimicrobial studies of Cu(II), Ni(II), Co(II), Fe(III), and UO2(II) complexes of a new Schiff base hydrazine derived from 7-chloro-4-hydrazinoquinoline. Spectrochim Acta A Mol Biomol Spectrosc, 2007; 66:28–36. CrossRef
El-Saied FA, Al-Hakimi AN, Wahba MA, Shakdofa MME. Preparation, characterization and antimicrobial activities of N’-((3-(hydroxyimino) butan-2-ylidene)-2 (phenylamino) acetohydrazide and its metal complexes. Egypt J Chem, 2017; 60(1):1–24.
Feldman C, Anderson R, The role of co-infections an secondary infections in patients with COVID-19. Pneumonia, 2021; 13:5. CrossRef
Fierro CM, Smith PD, Horton PN, Hursthouse MB, Light ME. Synthesis and structures of mono and binuclear nickel(II) thiolate complexes of a dicompartmental pseudo-macrocycle with N(imine)2S2 and N(oxime)2S2 metal-binding sites. Inorg Chim Acta, 2011; 368:257–62. CrossRef
Fonkui TY, Ikhile MI, Ndinteh DT, Njobeh PB. Microbial activity of some heterocyclic Schiff bases and metal complexes: a review. Trop J Pharm Res, 2018; 17(12):2507–18. CrossRef
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev, 2002; 54:271–84. CrossRef
Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev, 2012; 70(5): 257–65. CrossRef
Herrling T, Jung K, Fuchs J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim Acta A Mol Biomol Spectrosc, 2008; 69:1429–35. CrossRef
Islam S, Nasrin N, Khan MA, Hossain ASMS, Islam F, Khandokhar P, Mollah MNH, Rashid M, Sadik G, Rahman AA, Alam AHMK. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement Altern Med, 2013; 13:142–52. CrossRef
Khanna S, Singh P, Kau H, Joon A. Synthesis, characterization and antimicrobial investigation of mixed ligand complexes of Cobalt(II) and Nickel(II) with P-dimethylaminobenzaldehyde thiosemicarbazone. MJChem, 2019; 21(3):100–9.
Koleva V, Yotova I, Dragoeva A, Enchev D. Synthesis, cytotoxicity, and promising anticancer potential of novel β-amino- and β-iminophosphonates. J Appl Pharm Sci, 2021; 11:080–8.
Köse DA, Necefo?lu H. Synthesis and characterization of bis(nicotinamide) m-hydroxybenzoate complexes of Co(II), Ni(II), Cu(II) and Zn(II). J Therm Anal Calorim, 2008; 93(2):509–14. CrossRef
Liu LX, Wax XQ, Yan JM, Li Y, Sun CJ, Chen W, Zhou B, Zhang HB, Yang XD. Synthesis and antitumor activities of novel dibenzo[b,d]furan-imidazole hybrid compounds. Eur J Med Chem, 2013; 66:423–37. CrossRef
Mahind LH, Waghmode SA, Nawale A, Mane VB, Dagade SP. Evaluation of antimicrobial activities of zirconium (IV) complex. J Pharm Biol Sci, 2013; 5:102–5. CrossRef
Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Med Chem Commun, 2018; 9(3):409–36. CrossRef
More G, Raut D, Aruna K, Bootwala S. Synthesis, spectroscopic characterization and antimicrobial activity evaluation of new tridentate Schiff bases and their Co(II) complexes. J Saudi Chem Soc, 2017; 21:954–64. CrossRef
Nandiyanto ABD, Oktiani R, Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol, 2019; 4:97–118. CrossRef
Naskar S, Mazumder UK, Pramanik G, Bala A, Haldar PK, Islam A, Gupta M. Comparative in vitro antioxidant activity of different parts of Cocos nucifera (Linn.) on reactive oxygen and nitrogen species. Int J Pharm Pharm Sci, 2011; 3:104–7.
Omar MM, Mohamed GG, Hindy AMM. Transition metal complexes of heterocyclic Schiff base biological activity, spectroscopic and thermal characterization, J Therm Anal Calorim, 2006; 86(2):315–25. CrossRef
Pathade SS, Adole VA, Jagdale BS. PEG-400 mediated synthesis, computational, antibacterial and antifungal studies of fluorinated pyrazolines. Curr Res Green Sustain Chem, 2021; 4:100172. CrossRef
Przybylski P, Huczynski AW, Pyta KK, Brzezinski B, Bartl F. Biological properties of Schiff bases and azo derivatives of phenols. Curr Org Chem, 2009; 13:124–48. CrossRef
Rahman M, Islam, Biswas BM, Alam K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes, 2015; 8:621–30. CrossRef
Raman N, Raja YP, Kulandaisamy A. Synthesis and characterization of Cu(II), Ni(II), Mn(II), Zn(II) and VO(II) Schiff base complexes derived from ophenylenediamine and acetoacetanilide. J Chem Sci, 2001; 113(3):183–9. CrossRef
Reiss A, Chifiriuc MC, Amzoiu E, Spînu CI. Transition metal(II) complexes with cefotaxime-derived Schiff base: synthesis, characterization, and antimicrobial studies. Bioinorg Chem Appl, 2014; 2014:926287. CrossRef
Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanism of action. Virulence, 2018; 9:522–54. CrossRef
Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. Antioxidants, 2019; 10:1–29.
Shinde RA, Adole VA, Jagdale BS, Pawar TB. Superfast synthesis, antibacterial and antifungal studies of halo-aryl and heterocyclic tagged 2, 3-dihydro-1H-inden-1-one candidates. Monatsh Chem, 2021; 2(6):649–58. CrossRef
Shinde RA, Adole VA, Jagdale BS. Synthesis, computational, antibacterial and antifungal investigation of two tri-fluorinated chalcones of 1-(2, 3-Dihydrobenzo [b][1, 4] dioxin-6-yl) ethan-1-one. Polycycl Aromat Comp, 2021:1–18. CrossRef
Wainwrighr M. Photodynamic antimicrobial chemotherapy. J Antimicrob Chemother, 1998; 42:13–28. CrossRef
World Health Organization. Estimating the burden of foodborne diseases. 2021. Available via https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (Accessed 4 October 2021).