INTRODUCTION
When a wound occurs, the body initiates a wound healing response involving numerous cell types, mediators, cytokines, and the vascular system. Neutrophils and macrophages produce transforming growth factor-beta (TGF-β1) and platelet-derived growth factor (PDGF), which activates fibroblasts to become myofibroblasts and initiates the fibrosis process (Andrews et al., 2016). PDGF is a growth factor secreted by platelets, macrophages, endothelial cells, keratinocytes, and fibroblasts (Gao et al., 2005). PDGF regulates injury response and induces cellular responses by binding to receptor tyrosine kinases, dimerizing their receptors, and triggering multiple signaling pathways after phosphorylation of the receptor has begun. In response to injury, PDGF receptors (PDGF-α and PDGF-β) are upregulated as a wound healing response. However, uncontrolled PDGF signaling may play a role in hypertrophic scarring as well as keloids (Barrientos et al., 2008; Shih et al., 2010).
Keloids are skin tumors that occur as a result of aberrant wound healing processes. Although the pathophysiology of keloids is not fully understood, fibroblasts are known to have a role in keloid development (Eming et al., 2014). Fibroblasts and myofibroblasts can cause disturbances in the composition of collagen and elastin in the extracellular matrix (ECM) during an abnormal remodeling phase of tissue repair and regeneration (Cohen et al., 2017). Recent research has revealed that keloid fibroblasts have distinct actin filament stiffness and force generation compared to normal fibroblasts, which may define keloid expansion beyond the initial wound size (Hsu et al., 2018). Keloid fibroblasts are also known to overexpress a number of growth factors, e.g., the vascular endothelial growth factor, TGF-β1, TGF-β2, and PDGF-α. Additionally, keloid fibroblasts have a higher number of growth factor receptors and react more strongly to growth factors like TGF and PDGF (Wolfram et al., 2009).
The most widely used treatment for keloid scars is the intralesional injection of triamcinolone acetonide (TA) due to its anti-inflammatory properties (Morelli Coppola et al., 2018). Corticosteroids can suppress myofibroblast activation, thereby reducing collagen in the ECM, reducing inflammation, and also decreasing endogenous growth factors. Despite the benefits, intralesional corticosteroid injections can have some negative effects, such as fat atrophy, hyper-/hypopigmentation, skin necrosis, or even Cushing’s syndrome (Roques and Téot, 2008; Nie et al., 2010). Due to its limitations in treating keloids, efforts to cure keloids are still ongoing, among others by using natural compounds for alternative keloid therapy.
Gambir [Uncaria gambir (Hunter) Roxb.] is an Indonesian plant that contains flavonoids, especially catechins (40%–80% of the dry extract weight), procyanidin B1, procyanidin B3, and gambirin (1%), and (-)-epicatechin (1.5%). Gambir is reported to have antibacterial, antiseptic, antioxidant, and anti-inflammatory properties (Dewi et al., 2018; Oswari et al., 2019; Melia et al., 2015; Ningsih and Rahayuningsih, 2019). In animal models of liver fibrosis using white rats (Rattus norvegicus L.) induced by the hepatotoxic compound carbon tetrachloride (CCl4), the gambir extract showed potency as a hepatoprotector (Fahrudin et al., 2015). Through the anti-inflammatory activity of gambir, a study showed that gambir could prevent and improve bleomycin-induced pulmonary fibrosis (Desdiani, 2019). However, the use of natural products has faced many obstacles, such as insufficient data on molecular analysis. Therefore, molecular docking studies are needed to evaluate the interaction between phytochemical compounds and molecular targets as the basis for drug development.
Structure-based drug design (SBDD) is the process of designing and optimizing a lead molecule based on structural information collected from the computational method. It uses information from protein target structures to locate the active region of a protein that binds with chemical substances. By SBDD, many three-dimensional (3D) structural data of targets of biological molecules, such as enzymes, proteins, or receptors, have been found (Lamb and Jorgensen, 1997). Also, SBDD can visualize the binding of ligands to the target and predict the affinity of ligands (Wang et al., 2018). Molecular docking is one of the most widely utilized SBDD approaches. Molecular docking methods investigate the ligand conformation used inside macromolecular target binding sites. This method calculates the free energy of ligand-receptor binding by analyzing crucial occurrences in the process of intermolecular recognition (Ferreira et al., 2015). This study used in silico examination of the molecular docking approach to determine the binding affinity, and the interaction between compounds in the gambir plant with the amino acid at its binding site was analyzed to see whether it may be used as an herbal medicine for additional keloid therapy. In vitro studies were also conducted to determine the concentration of the gambir extract in inhibiting the growth of keloid fibroblast cells.
The aim of this study is to determine the potency of the compounds in gambir [U. gambir (Hunter) Roxb.] to inhibit the proliferation of keloid fibroblasts using an in silico study with molecular docking and an in vitro assay compared to TA as the positive control.
MATERIAL AND METHODS
Computational method
The two- and 3D structures of the target ligands were created on MarvinSketch. The molecular docking, interface residues, and 3D visualization of the ligand-receptor complex were studied using MOE 2020. These tools overall require at least 8 GB of RAM, a 2.80 GHz processor, and 6.2 GB of free disk space.
Preparation of receptor
The receptor of PDGF-α [Protein Data Bank (PDB) ID: 3MJK] was downloaded from www.rcsb.org in .pdb format; then, the chain receptor was separated from the ligand. The preparation of protein includes protonating in 3D, minimizing energy, and removing water.
Preparation of ligand
Compounds found in gambir [U. gambir (Hunter) Roxb.] were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov) in .sdf format and then converted to .pdb format using MarvinSketch. The reported target compounds are catechin, gambirin A, gambirin A2, gambirin B1, gambirin B2, gambirin C, procyanidin B1, procyanidin B2, epigallocatechin gallate, and (+)-epicatechin (Desdiani et al., 2020, Saad et al., 2020).
Validation of docking method, docking analysis
The PDGF-α protein obtained from the PDB and each target’s compounds were docked using MOE 2020 with TA as the drug control. The possible active sites of the protein were predicted in MOE 2020 as well as visualizing the interacting residues of the ligand-receptor complex.
Absorption, distribution, metabolism, and excretion (ADME) prediction
Drug-likeness and ADME prediction of the target ligands were performed using SwissADME (https://www.swissadme.ch).
MTT assay of keloid fibroblast proliferation
The inhibition of keloid fibroblast proliferation by the gambir [U. gambir (Hunter) Roxb.] ethanolic extract was determined by the cytotoxicity activity using an MTT assay at various concentrations. Keloid fibroblast cell cultures that had been incubated were taken; then, media Dulbecco’s Modified Eagle Medium (DMEM) cultures in Petri dishes were discarded. Cell cultures were washed with Phosphate Buffer Saline (PBS) once; then, 2 ml of trypsin Etylenediaminetetraacetic acid (EDTA) was added. A media DMEM culture was added later. Cell cultures were divided into 96 microplates as much as 3–5 × 103, incubated at 37°C for 24 hours. The well in column 1 was used as a control medium. The well in column 2 was used as the control cell. Meanwhile, the wells in columns 3–7 were given samples that had concentrations of 12.5, 25, 50, and 100 μl. After that, the microplate was incubated at 37°C for 24 hours. MTT dissolved in PBS 5 mg/ml was also added to the microplate at 25 μl and then incubated for 4 hours. Alpha culture media MEM in the microplate was discarded; next, an amount of 100 μl Dimethyl Sulfoxide (DMSO) was added to each well. The absorbance of formazan was read in human gingival fibroblast cells’ spectrophotometry with an active Enzyme Linked Immunosorbent Assay (ELISA) reader on a 595-nm wavelength. The percentage of living cells was calculated using the following formula:
where % living cells is the percentage of living cells after treatment,
OD treatment is the formazan OD value (optical density) of each sample after testing,
Media OD is the average formazan OD value (optical density) of each control medium,
Cell OD is the formazan OD value (optical density) on the mean of the control cells.
RESULTS AND DISCUSSION
PDGFs and their receptors (PDGFRs) are prototypical growth factors and receptor tyrosine kinases with important developmental activities. Shim et al. (2010) discovered that the prodomain sequences of PDGFs (PDB ID: 3MJK) contain a conserved area that can stay noncovalently connected with the mature cystine-knot growth factor domain after processing. With this conserved, the hydrophobic attachment mode was seen in the structure of the PDGF-α or propeptide complex.
The structure of the complex between PDGF-β and PDGFR-β’s first three immunoglobulins’ (Ig) domains also shows two PDGF-β protomers clamping PDGFR-β at its dimerization seam. The PDGFRs and PDGF propeptide occupy overlapping locations on mature PDGFs justifying the need for propeptides by PDGFs to cover functionally essential hydrophobic surfaces during its secretion. When PDGF-β binds to a receptor, it undergoes a larger-scale structural organization and rearrangement, in which the disorders L1 loop of PDGF-β adopts a highly specialized conformation to make hydrophobic contacts with the third Ig domain of PDGF-β. Calorimetric evidence also demonstrates that, while needed for activation, the membrane-proximal homotypic PDGF-α interaction harms ligand binding. The structural and biochemical data together offer insight into PDGF-PDGFR signaling as well as strategies for PDGF-antagonism (Shim et al., 2010). The active site of PDGF includes its amino acids as follows: Ile38, His39, Ser40, Ile41, Leu44, Glu90, Ala91, Val92, Pro93, Ala94, Cys132, Cys133, Asn134, Thr135, and Ser136 (Fig. 2).
Drug-likeness and ADME prediction
Drug-likeness rules are a set of guidelines for the structural properties of compounds that can be used to quickly calculate a molecule’s drug-like properties. These guidelines are not absolute, and they are not intended to form strict cut-off values for determining which property values are drug-like and which are not. Drug development increasingly involves assessing ADME earlier in the discovery process, at a time when the number of compounds under consideration is large but its access to physical samples is limited (Daina et al., 2017). The Lipinski and drug’s ADME with SwissADME were used to analyze the chemical constituent’s properties (Fig. 1 and Table 1).
Of the 12 bioactive compounds in the gambir extract, seven compounds have good solubility and five compounds are poorly soluble. Several approaches have been developed to predict the necessary physicochemical properties for drug-like pharmacokinetics. According to the studies combining molecular weight (MW) and polar surface area (PSA), the majority of recently marketed orally bioavailable drugs contain MW <500 and total polar surface area (TPSA) <120 Å (Ertl et al., 2008). Because ligand recognition by any biomolecule is dependent on 3D orientation and electrostatic interaction, ligand preparation has a significant impact on docking results. This demonstrates the importance of ligand conformation as well as ligand preparation. Concerning tautomers, the question of which tautomer to use or whether to use all possible tautomers remains unsolved (Elokely and Doerksen, 2013). LogP from 12 bioactive gambir compounds showed an acceptance value (<5) with a range of 0.83–3.95. The role of lipophilicity in drug discovery and design is a critical one. Lipophilicity is a key physicochemical property that plays a crucial role in determining absorption, distribution, metabolism, excretion, and toxicity properties and the overall suitability of drug candidates (Waring, 2010).
Docking of substance with PDGF
This research predicted 12 bioactive compounds as ligands from the gambir extract, and these have interactions with PDGF as a target with the molecular docking approach. Molecular docking can predict an optimized orientation of the ligand on its target. The result from molecular docking is shown in Table 2.
Those three compounds, gambirin A1, procyanidin B2, and neooxygambirtannine, have their best affinity scores of −12.8324, −13.4987, and −14.5446 and pKi values of 8.962, 10.218, and 9.129, respectively. Gambirin A1 has two hydrogen bond interactions with Glu135 and Cys96. Procyanidin B2 has three hydrogen bond interactions with Ile38, Asn134, and Cys96 (Fig. 3). Neooxygambirtannine has three hydrogen bond interactions with Glu135, Arg131, and Cys96. Amino acids K/E/D/D(Lys/Glu/Asp/Asp) signature is found in all functional protein kinases and plays an important role in protein kinase catalysis (Knighton et al., 1991).
This molecular docking shows that the gambir extract consists of 12 compounds that have Gibbs energy relativity active compared to the positive control TA. This method is more advantageous in that it is more compatible with accepting ligand flexibility. Furthermore, assessing the molecular recognition between ligand and target is more realistic. However, due to a large amount of energy dissipating for each conformation, this approach takes longer to estimate the optimal docked conformer. Recently, fast optimization methods and grid-based tools have dominated and revolutionized this limitation in order to make simulation approaches more user-friendly (Scoichet et al., 2022). Lead generation optimization molecular docking can predict a ligand’s optimal orientation on its target. It can predict various ligand binding modes in the target molecule’s groove. This can be applied to the development of more potent, selective, and efficient drug candidates (Gschwend et al., 1996; Scoichet et al., 2022). Hit identifications docking in combination with the scoring function can be used to evaluate large databases for finding out potent drug candidates in silico, which can target the molecule of interest (Ferreira et al., 2015).
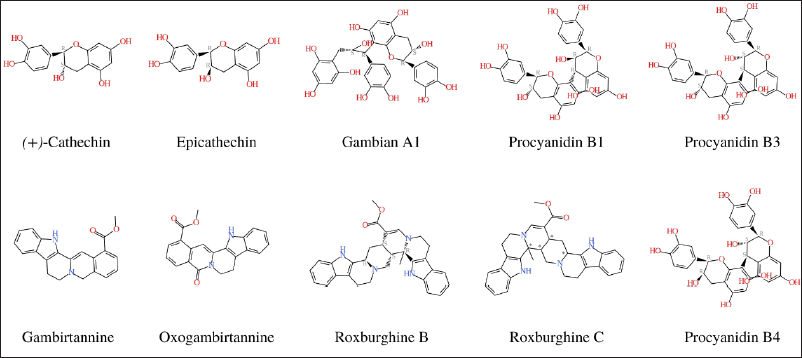 | Figure 1. Chemical properties of gambir extract. [Click here to view] |
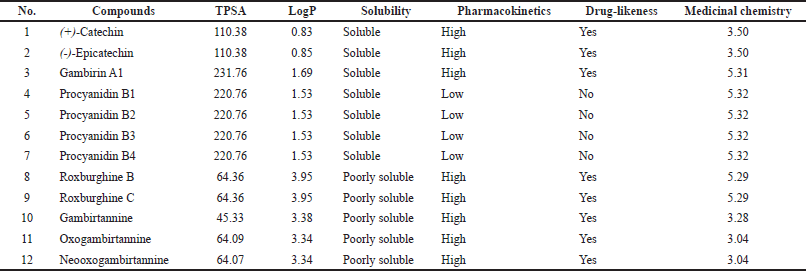 | Table 1. ADME parameters, pharmacokinetics properties, drug-like nature, and medicinal chemistry chemical compounds of gambir extract. [Click here to view] |
MTT assay
The cytotoxicity activity of the gambir [U. gambir (Hunter) Roxb.] ethanolic extract was tested on primary keloid fibroblasts using the MTT assay at various concentrations. The ethanolic extract of U. gambir reduced cell viability in the treated groups with an IC50 of 22.40 and 0.071 g/ml. In vitro research revealed that the U. gambir ethanolic extract inhibited growth. According to Li et al. (2021), they tested the effect of pinocembrin on TGF-1-induced proliferation in vitro, and the results showed that cell viability and numbers were greatly raised after 24 hours of growth, and cell viability and numbers were even higher after stimulation with TGF-1 (Li et al., 2021). Pinocembrin decreased TGF-1 cell viability, indicating that pinocembrin could inhibit the growth of activated skin fibroblasts (Li et al., 2021).
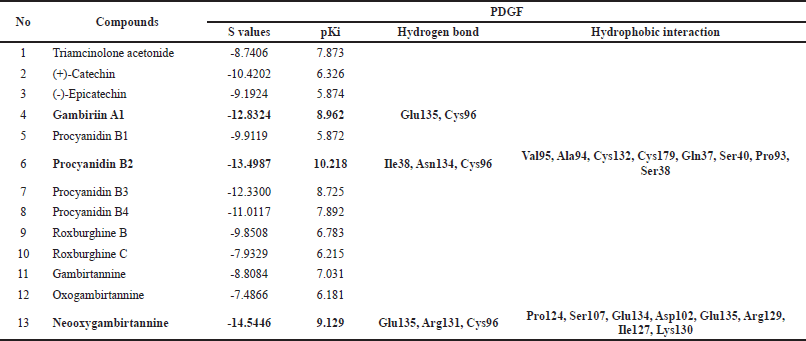 | Table 2. The results of molecular docking PDGF with compounds from gambir extract and drug control (Triamcinolone acetonide) [Click here to view] |
 | Figure 2. Active site of PDGF [Click here to view] |
The histopathologic hallmark of dermatologic illness is skin fibrosis, which is defined by excessive proliferation of skin fibroblasts and deposition of collagen. Keloids are one of the most difficult skin fibrotic illnesses to cure, and they frequently recur (Al-Attar et al., 2006). Our body’s first response is constriction of the injured blood vessels and activation of platelets, which not only help form a hemostatic plug but also cause the secretion of several wound healing mediators like PDGF and TGF-β, which start the inflammatory response (Trace et al., 2016). The results of antikeloid action on keloid fibroblast cells are shown in Table 3. Antikeloid therapy is used to control the proliferation of fibroblasts that are involved in the formation of the ECM, which includes collagen. Gallin et al. (1978) treated fibroblasts with DNA, RNA, and protein synthesis.
 | Figure 3. The complex of PDGF with compounds (A) procyanidin B2 and (B) neooxygambirtannine. [Click here to view] |
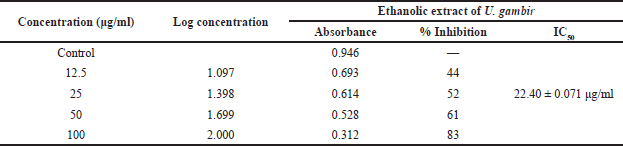 | Table 3. The IC50 value of an ethanolic extract of U. gambir (Hunter) Roxb. with primary fibroblast keloid cells. [Click here to view] |
Gallin et al. (1978), found that the cells’ response to PDGF is dependent on continuous protein synthesis in fibroblasts treated with inhibitors of DNA, RNA, and protein synthesis. Fibroblasts differ from leukocytes in this aspect (Gallin et al., 1978). Uncaria gambir (Hunter) Roxb.’s ethanolic extract was found to have an inhibitory effect with an IC50 of 22.40 and 0.071 g/ml. When compared to the positive control TA, a literature search showed that 12 bioactive chemicals as ligands from the extract of U. gambir (Hunter) Roxb. have interactions with PDGF as a target with Gibbs energy relativity active (Desdiani et al., 2020). According to the results from in silico and in vitro tests, the ethanol extract of U. gambir (Hunter) Roxb. is potent for antikeloid therapy by regulating the proliferation of fibroblasts as they play a role in the development of protein synthesis.
CONCLUSION
This study identified potential bioactive compounds as inhibitors of PDGF, such as gambirin A1, procyanidin B2, and neooxygambirtannine. These compounds might be isolated to perform further research in vitro or in vivo. Furthermore, the inhibitory concentration might be utilized as a reference value for protein analysis or other studies to understand more about the molecular mechanism of gambir extract suppression against keloid fibroblast cells.
ACKNOWLEDGMENTS
This research has been supported by Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) No. NKB-140/UN2.RST/HKP.05.00/2021, through the Ministry of Research and Technology of the Republic Indonesia/National Research and Innovation Agency of the Republic Indonesia.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
This study was supported by Ministry of Research and Technology of Indonesia [PDUPT Grant No: NKB-140/UN2.RST/HKP.05.00/2021.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg, 2006; 117(1):286–300; doi:10.1097/01.prs.0000195073.73580.46 CrossRef
Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: The paradigm of skin fibrosis — Pathomechanisms and treatment. Matrix Biol, 2016; 51:37–46; doi:10.1016/j.matbio.2016.01.013 CrossRef
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing: Growth factors and cytokines in wound healing. Wound Repair Regen, 2008; 16(5):585–601; doi:10.1111/j.1524-475X.2008.00410.x CrossRef
Cohen BE, Geronemus RG, McDaniel DH, Brauer JA. The role of elastic fibers in scar formation and treatment. Dermatol Surg, 2017; 43(1):S19–24; doi:10.1097/dss.0000000000000840 CrossRef
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017; 7:42717; doi:10.1038/srep42717 CrossRef
Desdiani D. Fibropreventive and fibrolysis effects of gambir (Uncaria gambir (Hunter) roxb.) in the lung and pleural rat of fibrosis models. Chest, 2019; 155(4):207A; doi:10.1016/j.chest.2019.02.377 CrossRef
Desdiani D, Rengganis I, Djauzi S, Setiyono A, Sadikin M, Jusman SWA, Siregar NC, Eyanoer PC, Fadilah F. Vitro Assay and Study Interaction of Uncaria gambir. Pharmacogn J, 2020; 12(6):1232–40. CrossRef
Dewi, S. R. P., Pratiwi, A., & Teodorus, T. The effect of gambier extracts (Uncaria gambir [Roxb.]) as antiseptic on gingival wound in rats. ODONTO Dental Journal, 2018; 5(1):80–88; doi: 10.30659/odj.5.1.%p. CrossRef
Elokely KM, Doerksen RJ. Docking challenge: protein sampling and molecular docking performance. J Chem Infor Model, 2013; 53(8):1934–45; doi:10.1021/ci400040d CrossRef
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med, 2014; 6(265):265sr6; doi:10.1126/scitranslmed.3009337 CrossRef
Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem, 2000; 43(20):3714–7; doi:10.1021/jm000942e CrossRef
Fahrudin F, Solihin DD, Kusumorini N, Ningsih S. Isolasi efektivitas ekstrak gambir (Uncaria gambir (Hunter) Roxb.) sebagai hepatoprotektor pada tikus (Rattus norvegicus L.) yang diinduksi CCl4. J Ilmu Kefarm Indo, 2015; 13(2):115–22.
Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules, 2015; 20(7):13384–421; doi:10.3390/molecules200713384 CrossRef
Gallin JI, Wright DG, Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest, 1978; 62(6):1364–74. CrossRef
Gao Z, Sasaoka T, Fujimori T, Oya T, Ishii Y, Sabit H, Kawaguchi M, Kurotaki Y, Naito M, Wada T, Ishizawa S, Kobayashi M, Nabeshima Y, Sasahara M. Deletion of the PDGFR-beta gene affects key fibroblast functions important for wound healing. J Biol Chem, 2005; 280(10):9375–89; doi:10.1074/jbc.M413081200 CrossRef
Gschwend DA, Good AC, Kuntz ID. Molecular docking towards drug discovery. J Mol Recognit, 1996; 9(2), 175–86; doi:10.1002/(sici)1099-1352(199603)9:2<175::aid-jmr260>3.0.co;2-d CrossRef
Hsu CK, Lin HH, Harn HI, Hughes MW, Tang MJ, Yang CC. Mechanical forces in skin disorders. J Dermatol Sci, 2018; 90(3):232–40; doi:10.1016/j.jdermsci.2018.03.004 CrossRef
Knighton DR, Zheng JH, Ten Eyck LF, Ashford VA, Xuong NH, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science, 1991; 253(5018):407–14; doi:10.1126/science.1862342 CrossRef
Lamb ML, Jorgensen WL. Computational approaches to molecular recognition. Curr Opin Chem Biol, 1997; 1(4):449–57; doi:10.1016/s1367-5931(97)80038-5 CrossRef
Li X, Zhai Y, Xi B, Ma W, Zhang J, Ma X, Miao Y, Zhao Y, Ning W, Zhou H, Yang C. Pinocembrin ameliorates skin fibrosis via inhibiting TGF-β1 signaling pathway. Biomolecules, 2021; 11(8):1240; doi:10.3390/biom11081240 CrossRef
Melia S, Novia D, Juliyarsi I. Antioxidant and antimicrobial activities of gambir (Uncaria gambir roxb) extracts and their application in rendang. Pak J Nutr, 2015; 14(12):938–41; doi:10.3923/pjn.2015.938.941 CrossRef
Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol, 2018; 11:387–96. CrossRef
Nie Z, Bayat A, Behzad F, Rhodes LE. Positive response of a recurrent keloid scar to topical methyl aminolevulinate-photodynamic therapy: MAL-PDT for keloid. Photodermatol Photoimmunol Photomed, 2010; 26(6):330–2; doi:10.1111/j.1600-0781.2010.00539.x CrossRef
Ningsih E, Rahayuningsih S. Extraction, isolation, characterization and antioxidant activity assay of catechin gambir (Uncaria gambir (Hunter). Roxb. Al-Kimia, 2019; 7(2):177–88. CrossRef
Oswari L, Hidayat R, Fatmawati F, Hayati L, Alisa BS. Gambir extract (Uncaria gambir) decreases inflammatory response and increases gastric mucosal integrity in Wistar rats - model gastritis. Open Access Maced J Med Sci, 2019; 7(19):3149–52; doi:10.3889/oamjms.2019.758 CrossRef
Roques C, Téot L. The use of corticosteroids to treat keloids: a review. Int J Low Extrem Wounds, 2008; 7(3):137–45; doi:10.1177/1534734608320786 CrossRef
Saad MF, Goh HH, Rajikan R, Yusof TR, Baharum SN, Bunawan H. Uncaria gambir (W. Hunter) Roxb: From phytochemical composition to pharmacological importance. Trop J Pharm Res, 2020; 19(8):1767–73; doi:10.4314/tjpr.v19i8.28 CrossRef
Shoichet BK, McGovern SL, Wei B, Irwin JJ. Lead discovery using molecular docking. Curr Opin Chem Biol, 2002; 6(4):439–46. CrossRef
Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen, 2010; 18(2):139–53; doi:10.1111/j.1524-475X.2009.00553.x
Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A, 2010; 107(25):11307–12; doi:10.1073/pnas.1000806107 CrossRef
Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and hypertrophic scars: A spectrum of clinical challenges Am J Clin Dermatol, 2016; 17(3):201–23; doi:10.1007/s40257-016-0175-7 CrossRef
Wang X, Song K, Li L, Chen L. Structure-based drug design strategies and challenges. Curr Top Med Chem, 2018; 18(12):998–1006; doi:10.2174/1568026618666180813152921 CrossRef
Waring MJ. Lipophilicity in drug discovery. Expert Opin Drug Discov, 2010; 5(3):235–48; doi:10.1517/17460441003605098 CrossRef
Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids: a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg, 2009;35:171–81. CrossRef