INTRODUCTION
Hyperlipidemia is characterized by an elevated triglyceride (TG) levels and free fatty acid content, a decrease in high-density lipoprotein cholesterol (HDL-C) levels, and a normal or slightly elevated low-density lipoprotein cholesterol (LDL-C) level in circulation (Sheik et al., 2022). Lipid metabolism is essential to maintain blood lipid levels and cardiovascular disease (CVD) is caused by dysfunction in lipid metabolism (Chen et al., 2014). The cholesterol is absorbed in the intestine and transported in the form of chylomicrons to the liver as it is the predominant organ in maintaining the lipid homeostasis (Mansbach and Gorelick, 2007). The cholesterol absorption pathway has been recognized as an important pharmacological intervention for hyperlipidemia due to its close relationship with lipid metabolism. The intestinal absorption of cholesterol can, therefore, be reduced to prevent hyperlipidemia (El-Tantawy and Temraz, 2019)
To treat hyperlipidemia, lifestyle modifications are required, including a low-fat diet, weight loss, exercise, and pharmacotherapy. Despite being first-line drugs, statins do not universally correct metabolic abnormalities and are associated with several side effects if taken for a long period. Hence, there is a need to develop natural hypolipidemic drugs for preventing and treating hyperlipidemia (Watts and Karpe, 2011). Nutraceuticals have been used long back by the people, but it is only recently that scientific and medical evidence began supporting their potential for effectiveness (Dillard and German, 2000). Hippocrates stated, “Let food be thy medicine and medicine be thy food,” describing the significance of nutraceuticals as substances that can be considered foods or parts of foods, attributing to the therapeutic purpose including disease prevention and treatment (Elkhalifa et al., 2021). The International Lipid Expert Panel has approved these therapies (Ji et al., 2019). Studies on food consumption trends and ecological studies provide early evidence of the role of diet on CVD (McGill, 1979). Due to their pathophysiological role, nutraceuticals may block intestinal cholesterol absorption, inhibit liver cholesterol synthesis, and enhance LDL-C excretion. It is possible that these agents may be of considerable help to patients with low-to-moderate hyperlipidemia, as well as in those with statin-related side effects (Sanidas and Grassos, 2020). Some of the nutraceuticals which are inspected for hypolipidemic activity include Tetracarpidium conophorum, commonly known as African walnut, and Buccholzia coriacea, popular as wonderful kola (Oluchi Nwaichi et al., 2017), the plant named Capparis spinosa L. (Mollica et al., 2017), and lyophilized beetroot powder (Sarfaraz et al., 2021).
Numerous studies show synergistic and pleiotropic effects of highly bioactive components on CVD risk factors as outweighing their individual benefits (Ramprasath et al., 2015), and hence there is a need for extensive research on supplements containing a mixture of nutraceuticals as the compounds present in the mixture may have the enhanced effect by interacting with each other (Santana-Gálvez et al., 2019). Developing a nutraceutical diet as an adjunctive strategy to treat hyperlipidemia may reduce the adverse side effects of pharmacotherapy medications and also reduce healthcare expenses (Johnston et al., 2017). Therefore, following identification and authentication by the nutritionist, the study selected five ingredients for preparation of the nutraceutical diet, including flaxseeds, foxtail millet, moong, barley, and fish oil, which were tested for the antihyperlipidemic activity both individually and in combination on the Triton X-100 hyperlipidemic rat model.
MATERIALS AND METHODS
Reagents and chemicals
The reagents and chemicals were purchased from Sigma-Aldrich, India, and they include 96% absolute ethanol (CN64-17-5), potassium mercuric iodide (CN7783-33-7), 20% alcoholic α-naphthol (CN90-15-3), concentrated sulfuric acid (CN77664-93-9), potassium sodium tartrate tetrahydrate (CN6381-59-5), copper sulfate (CN7758-98-7), sodium hydroxide (CN1310-73-2), ferric chloride (CN7705-08-0), lead acetate (CN 6080-56-4), chloroform (CN67-66-3), sodium bicarbonate (CN 144-55-8), glacial acetic acid (CN64-19-7), and acetic anhydride (CN108-24-7).
Raw materials to prepare the nutraceutical diet
Raw materials such as barley (Hordeum vulgare), foxtail millet (Setaria italic), flaxseeds (Linum usitatissimum), and moong (Vigna radiata) were purchased at a local store in Manipal, India, and Seacod® cod-liver oil capsules were purchased at a local pharmacy in Manipal, India.
Preparation of the nutraceutical diet
The diet was prepared according to AIN-93 purified diets for laboratory rodents (Reeves, 1997), with slight modifications according to the Indian food composition tables (Longvah et al., 2017) and Indian Council of Medical Research dietary guidelines for Indians (Manual, 2011). The raw ingredients used were identified and authenticated by the Department of Nutrition, Welcomgroup Graduate School of Hotel Administration in Manipal, Karnataka, India. The concentration of each nutraceutical being effective to reduce hyperlipidemia is selected based on the literature, and the composition of the diet is designed. An electronic balance was used to accurately weigh and mix feed ingredients in a clean bowl. The ingredients were washed, air-dried, fried on a smaller flame with a temperature of 100°C for 10 minutes, cooled, and ground into powder. The diets were made into square confectionaries (Table 1).
Experimental animals
Adult male Wistar rats (150–200 g, 12 weeks old) were obtained from the Central Animal Research Facility, Manipal, Karnataka, India. All rats were housed under identical conditions in an aseptic facility and given free access to water and food. The study was approved by the Institutional Animal Care and Use Committee of the Manipal Academy of Higher Education (approval no.: IAEC/KMC/66/2019).
Experimental design
Step 1: Grouping of animals
The Wistar rats were randomly allocated into 11 groups (n = 6):
1. Normal controls (NC) fed with basal diet.
2. Hyperlipidemic controls (HC) fed with basal diet.
3. Hyperlipidemic rats (HFS) fed with flaxseeds.
4. Hyperlipidemic rats (HFM) fed with foxtail millet.
5. Hyperlipidemic rats (HM) fed with moong.
6. Hyperlipidemic rats (HB) fed with barley.
7. Hyperlipidemic rats (HFO) fed with fish oil.
Step 2: The formulation is fed orally (15 g/day) to each rat in their respective cages for 15 days.
Step 3: The hyperlipidemia was induced in overnight fasted Wistar rats by administration of Triton X-100 ((100 mg/kg BW) dissolved in physiological saline (Parwin et al., 2019), followed by blood withdrawal within 24 hours by puncturing the retro-orbital sinus. The serum was used to analyze the lipid profile.
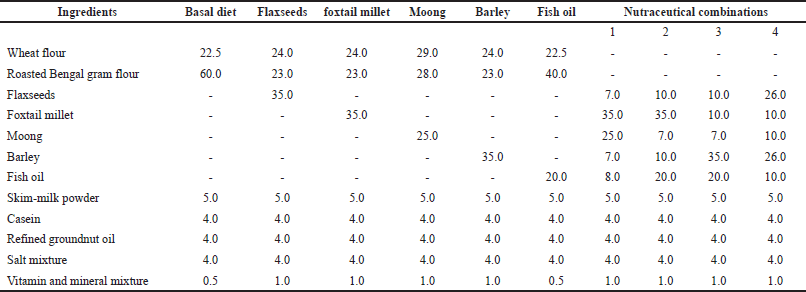 | Table 1. Composition of feed formulations (in g). [Click here to view] |
Step 4: The nutraceutical combinations are formulated based on the results obtained after the individual nutraceutical effect on hyperlipidemia in Wistar rats.
Step 5: The nutraceutical combination is fed for 15 days to the below grouped rats (15 g/day, orally):
8. Hyperlipidemic rats (HC1) fed with nutraceutical combination 1.
9. Hyperlipidemic rats (HC2) fed with nutraceutical combination 2.
10. Hyperlipidemic rats (HC3) fed with nutraceutical combination 3.
11. Hyperlipidemic rats (HC4) fed with nutraceutical combination 4.
Step 6: Inducing hyperlipidemia similar to that of step 3 in the above groups. The serum was again used to analyze the lipid profile.
Biochemical analysis
Total cholesterol (TC), TG, and HDL-C were analyzed using the commercial kits available from Coral Clinical Systems, India, and the levels were determined using the semiautomatic biochemistry analyzer (Medsource Ozone Biomedicals Pvt. Ltd.). LDL-C and very low-density lipoprotein cholesterol (VLDL-C) concentrations were calculated using the Friedewald formula.
Extraction of the nutraceutical diet for phytochemical analysis
An extraction was obtained by using the hot Soxhlet method for 72 hours with 200 g of dried nutraceutical powdered diet soaked in 500 ml 96% absolute ethanol. A portion of the filtrate was collected inside an amber bottle for phytochemical analysis based on the amount of filtrate obtained.
In vitro phytochemical analysis
The analysis was carried out to test for alkaloids (Mayer’s test), steroids (Libermann–Burchard test and Salkowski’s test), phenols and tannins (ferric chloride test, lead acetate test, and alkaline reagent test), saponins (foam test and sodium bicarbonate test), flavonoids (Shinoda’s test and sodium hydroxide test), carbohydrates (Benedicts test), proteins (Biuret test), and cardiac glycosides (Killer Kilani’s test) (Usunomena and Ngozi, 2016).
In vitro proximate composition analysis
The standard AOAC procedure was used to estimate the moisture, protein, fat, and ash content. Moisture was estimated gravimetrically using a hot air oven by heating at 105°C. Protein was estimated using total nitrogen by the Kjeldahl digestion and distillation. Fat was estimated using the Soxhlet apparatus using petroleum ether and ash by combustion using Muffle furnace at 550°C. The following formulae was used to calculate the total energy: Energy (kcal) = 4× (g protein+ g carbohydrate) + 9× (g fat) (Sharif et al., 2017).
Statistical analysis
Statistical analysis was carried out using Jamovi 2.0 and data are expressed as mean ± standard deviation (SD). One-way analysis of variances (Welch’s method) with subsequent post-hoc comparison with the help of the Games Howell test was used. P-value < 0.05 was considered statistically significant.
RESULTS
The effect of individual nutraceuticals on the lipid profile levels in hyperlipidemic rats
TC, TG, LDL-C, and VLDL-C levels were significantly increased (p < 0.001) in the HC rats except the levels of HDL-C when compared to the NC rats. The treatment with flaxseeds reduced TC, TG, LDL-C, and VLDL-C significantly (p < 0.001) and increased the HDL-C (p < 0.05) when compared to the HC rats. Similarly, treatment with barley reduced TC (p < 0.05), TG (p < 0.001), LDL-C (p < 0.05), and VLDL-C (p < 0.001), and improved the HDL-C (p < 0.05) when compared to the HC rats. Treatment with Foxtail millet reduced TC (p ≤ 0.05), TG (p < 0.05), LDL-C (p < 0.05), and VLDL-C (p < 0.05) when compared to the HC rats. The rats receiving moong reduced TC (p < 0.001), TG (p < 0.001), LDL-C (p < 0.01), and VLDL-C (p < 0.001). Similarly, the rats receiving fish oil significantly reduced TC (p ≤ 0.05), TG (p < 0.001), VLDL-C (p < 0.01), and LDL-C (p < 0.001) when compared to the HC rats. There was no significance observed in the levels of HDL-C in rats receiving foxtail millet, moong, and fish oil. Flaxseeds and barley appeared to have an effect on each of the five components of the lipid profile, as indicated by the results shown in Figure 1).
The effect of nutraceutical combinations on the lipid profile levels in hyperlipidemic rats
Table 2 presents the levels of the serum lipid profiles in hyperlipidemic rats given the nutraceutical combination. Nutraceutical combinations 1, 2, and 4 were not effective in either improving the HDL-C or reducing the other lipid profile when compared to the HC rats. Rather, it was observed that nutraceutical combination 4 significantly increased TC (p < 0.05) and LDL-C (p ≤ 0.05). Nutraceutical combination 3, rich in barley, effectively reduced TC (p < 0.01), TG (p < 0.001), LDL-C (p < 0.001), and VLDL-C (p < 0.001), and improved the HDL-C (p < 0.05) when compared to the HC rats.
In vitro phytochemical analysis
Table 3 presents the qualitative phytochemical analysis of the nutraceutical combination rich in barley. Phytoconstituents such as alkaloids, carbohydrates, proteins, steroids, and cardiac glycosides were present. However, tannins, saponins, phenols, and flavonoids were absent.
In vitro proximate composition analysis
The proximate analysis revealed that the barley-rich nutraceutical combination is a rich source of carbohydrate (46.34 g), protein (10.87 g), ash (6.07 g), and fat (5.2 g). It contained a substantial amount of fiber (1.18 g), and the total energy value was calculated to be 275.64 kcal/100 g of dry matter (Table 4).
DISCUSSION
Hyperlipidemia is the key contributing factor to the development of CVD (Tietge, 2014) and it results from the abnormality of cholesterol homeostasis. Through 3-hydroxy-3-methylglutaryl-CoA reductase, the liver plays a vital role in cholesterol homeostasis (Li, 2014). CVD can be prevented by reducing serum lipids. The consumption of a healthy diet has improved serum lipid profiles, decreased bad cholesterol (LDL-C) levels, and increased good (HDL-C) cholesterol levels (Chen et al., 2014). Hence, the present study investigated to develop a nutraceutical combination that can act as an adjuvant in pharmacotherapy to prevent hyperlipidemia.
In the present study, the hyperlipidemia in rats is manifested by intraperitoneally inducing Triton X-100 which through the process of emulsification increases intestinal lipid absorption, hepatic cholesterol synthesis, inhibits lipoprotein lipase, and blocks the extrahepatic tissues from absorbing lipoproteins from circulation, leading to an increase in blood lipid concentrations (Abdelgadir et al., 2020).Thus, the level of lipid profile is significantly increased (p < 0.001) with a significant decrease in HDL-C (p < 0.001) in the HC rats when compared to the NC. Treatment with flaxseeds and barley significantly decreased TC, TG, LDL-C, and VLDL-C (p < 0.001) and increased the HDL-C (p < 0.05); this can be attributed to the presence of bioactive cardioprotective component α-linolenic acid (ALA) (57%), the essential omega-3 fatty acid and linoleic acid(LA) (16%), the essential omega-6 fatty acid in flaxseeds (Anurag et al., 2020), and β-glucan in barley (Bacchetti et al., 2015). Similarly, individual nutraceuticals, such as foxtail millet, moong, and fish oil, significantly reduced the lipid profile (p < 0.05) but they did not improve the HDL-C levels. The lipid lowering effect of foxtail millet is accredited to the improvement in insulin sensitivity and improvement in cholesterol metabolism by increasing the adiponectin concentration (Choi et al., 2005). Moong, during the process of digestion, forms propionate, short-chain fatty acid involved in regulating the metabolic pathway and lowering TC and TG (Ganesan and Xu, 2018). Fish oil has a cardioprotective bioactive component, n-3 polyunsaturated fatty acid (Owen et al., 2004).
The bioactive components present in the nutraceuticals mentioned above may also be present in other foods. However, they do not take into account the possibility of other compounds interacting and even interfering with their activity. Furthermore, people usually eat a wide variety of foods in different combinations, which can alter the activity of compounds of interest by interacting with the compounds in other foods (Santana-Gálvez et al., 2019). Hence, new research of combining the nutraceuticals to target the multiple factors associated to metabolic syndrome is emerging (Efferth and Koch, 2011). In support, the current study focused on developing effective nutraceutical combinations. Among the four different nutraceutical combinations studied, the one rich in barley significantly reduced the lipid profile (p < 0.001) and improved the HDL-C (p < 0.05). The desired effect can occur due to the interactions between the nutraceuticals, and the type of interaction can be assessed by calculating the combination index (CI). CI determines the type of interaction. There is an additive effect if CI = 1, a synergistic effect if CI < 1, and an antagonistic effect if CI > 1. The CI for the mixtures of more than two nutraceuticals can be calculated using the formula:
where (CI)n represents the CI for an n-nutraceutical combination, (C)j is the individual concentration of each component that produces effect y, and (Cy)j is the individual concentration of each component that produces effect y in the mixture (Santana-Gálvez et al., 2019). The CI of the effective barley-rich nutraceutical combination according to the above formulae is found to <1, which indicates the synergistic effect of the combination to prevent hyperlipidemia. Additionally, the barley-rich nutraceutical combination is found to be rich in β-glucan which may increase the viscosity of the gut content, increase excretion of bile acids and cholesterol in feces, and decrease the levels of TC, TG, and LDL-C with a moderate increase in the levels of HDL-C (Dongowski et al., 2002; Zeng et al., 2020). Systemic HDL-C levels and CVD risk have an inverse relationship and the increase in HDL-C levels in the present study indicates the favorable effect of the nutraceutical combination in cholesterol efflux capacity with a strategy to counteract cholesterol accumulation (Adorni et al., 2017; Nicholls and Nelson, 2019).
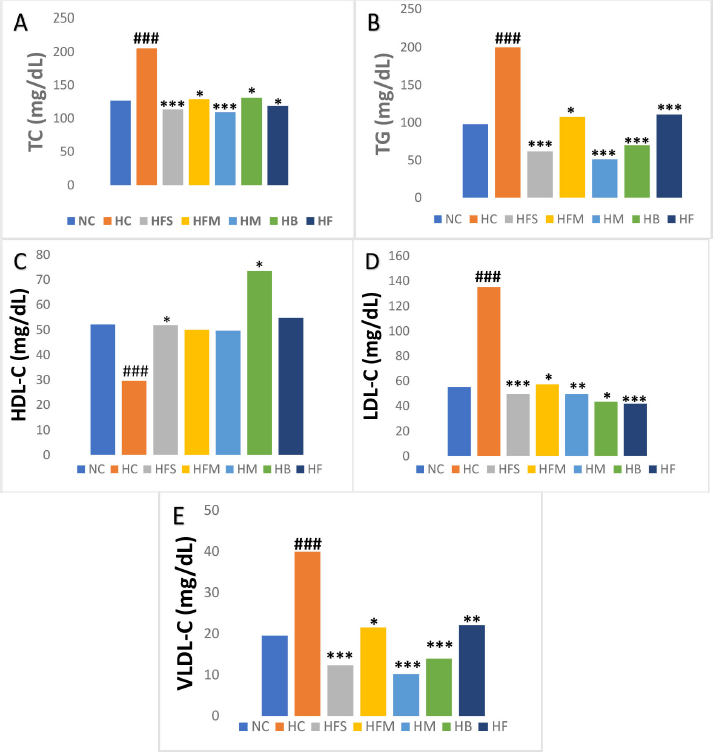 | Figure 1. The effect of individual nutraceuticals on the lipid profile (mg/dl) levels in hyperlipidemic rats. The results are expressed as mean ± SD (n = 6). ###p-value < 0.001 versus NC; *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001 versus HC.Note: NC, Normal control given basal diet; HC, Hyperlipidemic controls given basal diet; HFS, Hyperlipidemic rats given flaxseeds; HFM, Hyperlipidemic rats given with foxtail millet; HM, Hyperlipidemic rats given moong; HB, Hyperlipidemic rats given barley; HFO Hyperlipidemic rats given fish oil. [Click here to view] |
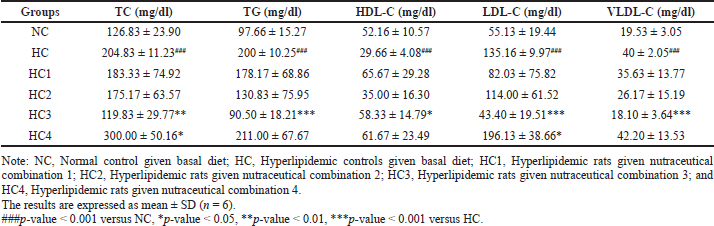 | Table 2. Effect of the nutraceutical combinations on the lipid profile (mg/dl) levels in hyperlipidemic rats. [Click here to view] |
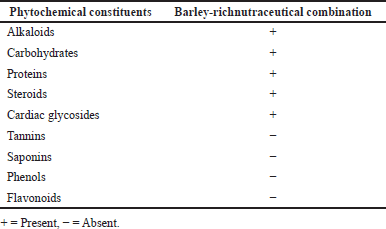 | Table 3. Qualitative analysis of the phytochemical constituents in the experimental diet. [Click here to view] |
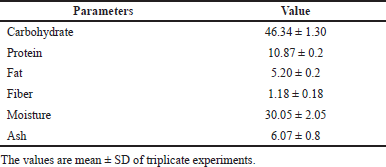 | Table 4. Proximate composition of experimental diet (unit: g/100 g). [Click here to view] |
CONCLUSION
Nutraceuticals are naturally occurring compounds found in foods that can lower the incidence and mortality of CVDs by possessing both preventative and therapeutic properties. The study of nutraceutical blends is an emerging field since only a few nutraceuticals have been tested together of the thousands known. In the conclusion of this investigation, preliminary scientific data on the nutraceutical value (phytochemical and proximate composition) of the nutraceutical combination was presented, as well as its efficacy in reducing the lipid profile synergistically, with the exception that HDL-C levels increased, making it a possible therapeutic strategy to prevent primary hyperlipidemia. Furthermore, the effectiveness of the tested nutraceutical combination over a long term needs to be evaluated in future studies.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHORS’ CONTRIBUTIONS
Samreen M. Sheik and Revathi P. Shenoy significantly contributed to the concept and design; analysis and interpretation of data was done by Samreen M. Sheik, Mamatha B.S., Vani Lakshmi R., and Revathi P. Shenoy; draft was written by Samreen M. Sheik and critical revision of the manuscript was done by all the authors. Final approval was obtained for publication by all authors in the journal.
ETHICAL APPROVALS
The study was approved by the Institutional Animal Care and Use Committee of the Manipal Academy of Higher Education (approval no.: IAEC/KMC/66/2019).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelgadir AA, Hassan HM, Eltaher AM, Khnsaa Mohammed GA, Lamya Mohammed AA, Hago TB. Hypolipidemic effect of cinnamon (Cinnamomum zeylanicum) bark ethanolic extract on triton X-100 induced hyperlipidemia in albino rats. Med Aromat Plants (Los Angeles), 2020; 9(351):2167–412. CrossRef
Adorni MP, Ferri N, Marchianò S, Trimarco V, Rozza F, Izzo R, Bernini F, Zimetti F. Effect of a novel nutraceutical combination on serum lipoprotein functional profile and circulating PCSK9. Ther Clin Risk Manag, 2017; 13:1555. CrossRef
Anurag AP, Prakruthi M, Mahesh MS. Flaxseeds (Linum usitatissimmum): nutritional composition and health benefits. IP J Nutr Metab Health Sci, 2020; 3(2):35–40. CrossRef
Bacchetti T, Tullii D, Masciangelo S, Gesuita R, Skrami E, Brugè F, Silvestri S, Orlando P, Tiano L, Ferretti G. Effect of a barley-vegetable soup on plasma carotenoids and biomarkers of cardiovascular disease. J Clin Biochem Nutr, 2015; 57(1):66–73. CrossRef
Chen G, Wang H, Zhang X, Yang ST. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit Rev Food Sci Nutr, 2014; 54(9):1180–201. CrossRef
Choi YY, Osada K, Ito Y, Nagasawa T, Choi MR, Nishizawa N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci Biotechnol Biochem, 2005; 69(1):31–7. CrossRef
Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agricult, 2000; 80(12):1744–56. CrossRef
Dongowski G, Huth M, Gebhardt E, Flamme W. Dietary fiber-rich barley products beneficially affect the intestinal tract of rats. J Nutr, 2002; 132(12):3704–14. CrossRef
Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets, 2011; 12(1):122–32. CrossRef
Elkhalifa AE, Alshammari E, Adnan M, Alcantara JC, Awadelkareem AM, Eltoum NE, Mehmood K, Panda BP, Ashraf SA. Okra (Abelmoschus esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules, 2021; 26(3):696. CrossRef
El-Tantawy WH, Temraz A. Natural products for controlling hyperlipidemia. Arch Physiol Biochem, 2019; 125(2):128–35. CrossRef
Ganesan K, Xu B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci Hum Wellness, 2018; 7(1):11–33. CrossRef
Ji X, Shi S, Liu B, Shan M, Tang D, Zhang W, Zhang Y, Zhang L, Zhang H, Lu C, Wang Y. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed Pharmacother, 2019; 118:109338. CrossRef
Johnston TP, Korolenko TA, Pirro M, Sahebkar A. Preventing cardiovascular heart disease: promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res, 2017; 120:219–25. CrossRef
Li Y, Qin G, Liu J, Mao L, Zhang Z, Shang J. Adipose tissue regulates hepatic cholesterol metabolism via adiponectin. Life Sci, 2014; 118(1):27–33. CrossRef
Longvah T, An?antan? I, Bhaskarachary K, Venkaiah K, Longvah T. Indian food composition tables. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, 2017.
Mansbach CM, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastroint Liver Physiol, 2007; 293(4):G645–50. CrossRef
Manual A. Dietary guidelines for Indians. Nat Inst Nutr, 2011; 2:89–117.
McGill Jr HC. Relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. Am J Clin Nutr, 1979; 32(12):2664–702. CrossRef
Mollica A, Zengin G, Locatelli M, Stefanucci A, Mocan A, Macedonio G, Carradori S, Onaolapo O, Onaolapo A, Adegoke J, Olaniyan M. Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: in vivo and in vitro evaluation of its nutraceutical potential. J Funct Foods, 2017; 35:32–42. CrossRef
Nicholls SJ, Nelson AJ. HDL and cardiovascular disease. Pathology, 2019; 51(2):142–7. CrossRef
Oluchi Nwaichi E, Obinna Osuoha J, Okechukwu Monanu M. Nutraceutical potential of Tetracarpidium conophorum and Buccholzia coriacea in diet-induced hyperlipidemia. J Chem Health Risks, 2017; 7(3). 157–70.
Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish oil dose-and time-response effects on cardiac phospholipid fatty acid composition. Lipids, 2004; 39(10):955. CrossRef
Parwin A, Najmi AK, Ismail MV, Kaundal M, Akhtar M. Protective effects of alendronate in triton X-100-induced hyperlipidemia in rats. Turk J Gastroenterol, 2019; 30(6):557. CrossRef
Ramprasath VR, Thandapilly SJ, Yang S, Abraham A, Jones PJ, Ames N. Effect of consuming novel foods consisting high oleic canola oil, barley β-glucan, and DHA on cardiovascular disease risk in humans: the CONFIDENCE (Canola Oil and Fibre with DHA Enhanced) study–protocol for a randomized controlled trial. Trials, 2015; 16(1):1–10. CrossRef
Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr, 1997; 127(5):838S–41S. CrossRef
Sanidas E, Grassos C. The role of nutraceuticals in the treatment of primary dyslipidemia. Hell J Cardiol, 2020; 61(1):60–2. CrossRef
Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci Technol, 2019; 88:179–93. CrossRef
Sarfaraz S, Rahela I, Rabia M, Muhammad O, Sabiha G, Muhammad S. Rising trend of nutraceuticals: evaluation of lyophilized beetroot powder at different doses for its hypolipidemic effects. Pak J Pharm Sci, 2021; 4:1315–22.
Sharif S, Shahid M, Mushtaq M, Akram S, Rashid A. Wild mushrooms: a potential source of nutritional and antioxidant attributes with acceptable toxicity. Prevent Nutr Food Sci, 2017; 22(2):124.
Sheik SM, Bakthavatchalam P, Shenoy RP, Hadapad BS, Nayak D, Biswas M, Suryakanth VB. Anti-hyperglycemic, anti-hyperlipidemic, and anti-inflammatory effect of the drug Guggulutiktaka ghrita on high-fat diet-induced obese rats. J Ayur Integrat Med, 2022; 13(3):100583. CrossRef
Tietge UJ. Hyperlipidemia and cardiovascular disease: inflammation, dyslipidemia, and atherosclerosis. Curr Opin Lipidol, 2014; 25(1):94–5. CrossRef
Usunomena U, Ngozi OP. Phytochemical analysis and proximate composition of Vernonia amygdalina. Int J Sci World, 2016; 4:11–4. CrossRef
Watts GF, Karpe F. Triglycerides and atherogenic dyslipidaemia: extending treatment beyond statins in the high-risk cardiovascular patient. Heart, 2011; 97(5):350–6. CrossRef
Zeng Y, Pu X, Du J, Yang X, Li X, Mandal M, Nabi S, Yang T, Yang J. Molecular mechanism of functional ingredients in barley to combat human chronic diseases. Oxidat Med Cell Longev, 2020; 2020 :3836172. CrossRef