INTRODUCTION
A gel hand sanitizer is useful for cleaning or eliminating germs on the hands, containing 60% alcohol as an active ingredient. It has various ingredients that quickly kill microorganisms on the skin of the hands. Hand sanitizers are used for practical reasons, easy to carry everywhere, and can be used quickly without the need to use water. They are simply applied to the palms of the hands and spread evenly across the entire surface of the hands. During this pandemic, apart from wearing masks, one must maintain hand hygiene to prevent transmission. Lobster skin containing chitosan can be used as an antibacterial in hand sanitizer preparations (Kaban et al., 2022; Singh et al., 2020).
Chitosan is a derivative of chitin with the structure β-(1-4)-2-amine-2-deoxy-D-glucose, which is the product of chitin deacetylation. Chitosan is a natural polymer with a molecular structure similar to cellulose; the difference is that it is located in the C-2 chain group, where the hydroxy group (OH) at C-2 is replaced by the amine (NH2) (Fernandes et al., 2014). In general, chitosan with a high molecular weight cannot be dissolved in water. Degraded chitosan will have a lower molecular weight so that it can be more soluble in water and has significant differences in its antimicrobial, antitumor, and plant growth activity compared to chitosan with a higher molecular weight (Hien et al., 2012). Chitosan can be obtained from several animal shells such as shrimp, lobster, crab, and snail. Lobster shell waste has been rarely used and not used by the people of the Central Aceh district but can cause environmental pollution if not treated properly. One of the prevention efforts is utilizing lobster shell waste to have high economic value and usability by processing it into chitin and chitosan (Rossi et al., 2000).
Several studies have shown that chitosan’s antibacterial activity can fight the growth of both Gram-positive and Gram-negative bacteria; for example, Kasta (2020) stated that there was an inhibitory activity of chitosan from shrimp shells as an antibacterial against Staphylococcus aureus with the highest inhibitory diameter on the addition of chitosan with a concentration of 0.2% at 20.27 mm/mg chitosan and antibacterial inhibitory activity against Escherichia coli with an inhibitory diameter. The highest was on the addition of chitosan with a concentration of 0.2% at 31.53 mm/mg chitosan, and thus it could be concluded that the most effective concentration of chitosan in inhibiting the growth of E. coli bacteria was 0.50% with an inhibition zone area of 11 mm. Based on the description above, chitosan has antibacterial activity against E. coli and S. aureus (Haro et al., 2020; Wulandari et al., 2022).
MATERIALS AND METHODS
Materials
The materials used are chitosan from a freshwater lobster shell, Aquadest, waste of freshwater lobster shell, acetic acid (Merck), NaOH pa (Merck), HCl pa (Smart Lab), AgNO3 (Merck), indicator phenolphthalein (Merck), Na-CMC, propylene glycol, and methylparaben, nutrient agar (Merck), Mueller-Hinton agar (MHA) (Merck), NaCl (Merck), and McFarland’s solution (Merck).
Sample preparation
Lobster shell waste was washed under running water and dried in the sun. After drying, the sample was mashed and sieved in a 60-mesh sieve to get a powder-like texture.
Demineralization process
The sample was placed in a glass beaker, and a 1.5 M HCl solution was added in the ratio of 1:15 (w/v) between the solvent and the sample. The mixture was then heated at 60°C–70°C for 4 hours while stirring at 50 rpm with a magnetic stirrer. The residue was separated, dried, and then weighed after cooling in a desiccator (Al Shaqsi et al., 2020).
Deproteination process
The demineralization residue was transferred to a new glass beaker, and a 3.5% NaOH solution was added in the ratio of 1:10 (w/v) between the solvent and the sample. The mixture was then heated at 60°C–70°C for 4 hours while stirring at 50 rpm with a magnetic stirrer. After drying, the residue was chilled in a desiccator before being weighed (Paul et al., 2014).
Depigmentation process
Chitin was placed in a glass beaker, followed by the addition of 60% NaOH in the ratio of 1:20 (w/v), and then heated at 100°C–110°C for 4 hours while stirring with a magnetic stirrer at a speed of 50 rpm. After drying, the residue was chilled in a desiccator before being weighed. The chitosan extracted from each sample was then qualitatively characterized (Boarin-Alcalde and Graciano-Fonseca, 2016).
Chitin deacetylation process
Chitin was placed in a glass beaker, followed by the addition of 60% NaOH in the ratio of 1:20 (w/v), and then heated at 100°C–110°C for 4 hours while stirring with a magnetic stirrer at a speed of 50 rpm. After drying, the residue was chilled in a desiccator before being weighed. The chitosan extracted from each sample was then qualitatively characterized (Vallejo-Domínguez et al., 2021).
Chitosan characterization
Examination of the characteristics of chitosan from the shells of freshwater lobster (Cherax quadricarinatus) includes organoleptic properties, yield, water content, and chitosan solubility (Tripathi et al., 2011).
Organoleptic examination
Organoleptic examination was carried out on chitosan from freshwater lobster (C. quadricarinatus) shells by paying attention to shape, smell, and color.
Chitosan yield
The yield of chitin to chitosan was calculated by dividing the weight of chitin by the percentage of chitosan produced (Tripathi et al., 2011) as follows:
where m is the mass of isolated chitosan and w is the weight of chitin.
Determination of ash content
A total of 2 g of Simplicia powder was weighed carefully, put into a porcelain exchange that had been ignited and tempered, and then leveled. The sample was incandescent at a temperature of 600°C until the charcoal was exhausted, cooled, and weighed until a constant weight was obtained, and the ash content was calculated in percent of the material that had been dried in air (Liu, 2019).
Fourier Transform Infrared (FTIR) analysis
The functional groups of the chitosan formed were determined using an FTIR spectrophotometer at wavenumbers ranging from 4,000 to 400 cm−1. Based on the FTIR data, the degree of deacetylation can be determined using the baseline method formulated by Domszy and Robert. The equation used is as follows:
where A1647 is the absorbance at a wavelength of 1,647 cm−1 for the absorption of the NH group, A3410 is the absorbance at a wavelength of 3,410 cm−1 for the absorption of the OH group, and 1.33 is the constant for the degree of complete deacetylation.
Determination of water content
The amount of water in chitosan is one of the most important factors in determining its quality. The water content of chitosan is specified at 10% by Protan Biopolymer as the quality requirement (Abun and Haetami, 2016). The Association of Official Analytical Chemist method of heating can be used to perform the water content test. The sample was weighed in a porcelain cup or watch glass dish with a known weight of up to 0.5 g. The oven was preheated to 100°C–105°C, and the sample was baked for 1–2 hours, depending on the ingredients. Then, it was weighed after cooling in a desiccator for 30 minutes (Sun et al., 2017). The sample was reheated in the oven and chilled in a desiccator, and the process was repeated until the weight was constant. Calculation of water content can be done with the following formula:
where a is the weight of the cup and initial sample (g), b is the weight of the cup and sample after drying (g), and c is the initial sample weight (g).
Chitosan solubility
The solubility of chitosan is one of the parameters that may be used to measure its quality. The higher the chitosan solubility, the higher the quality of the chitosan generated. In this case, chitosan was dissolved in 1% glacial acetic acid at a ratio of 1:100 (g/ml) (Salamat et al., 2019).
Gel preparation formula
The formula for making hand sanitizer gel from freshwater lobster shell chitosan is shown in Table 1.
Gel hand sanitizer preparation procedure
The gel base was made by dissolving chitosan with 100 ml of 1% glacial acetic acid. The carboxy methyl celulose (CMC) was developed in a mortar previously soaked in hot water. After the CMC bloomed, chitosan and methylparaben were added, which had been previously dissolved in hot water and ground quickly and vigorously until a homogeneous and transparent mass was formed; then, glycerin was added and crushed until it became a homogeneous gel (Wang et al., 2021).
Evaluation of gel hand sanitizer preparations
Gel hand sanitizer physical stability test
Physical stability testing of the hand sanitizer gel includes organoleptic tests, which include the texture, color, and odor of the preparations made after being allowed to stand once for 24 hours (Fahreni et al., 2021). The hand sanitizer gel preparation was stored under extreme conditions in a test cabinet called the climatic chamber at a temperature of 40°C ± 2°C and a humidity of 75% ± 5%.
pH test
The pH meter was dipped into the container containing the sample to be tested, and the value was recorded and compared with the standard skin pH, which ranged from 4.5 to 6.5. The pH test was carried out after the preparation was allowed to stand for 24 hours (Futri and Yaturramadhan, 2021).
Homogeneity test
The homogeneity test was measured by weighing a 0.5 g hand sanitizer sample on a transparent watch glass. The watch glass containing the sample was loaded. The preparation was observed to see if it showed a homogeneous arrangement, and no coarse particles were seen. The test was carried out after the preparation was allowed to stand for 24 hours (Futri and Yaturramadhan, 2021).
Spreadability test
The dispersion test was measured by weighing a 0.5 g hand sanitizer sample and placing it on the same glass. The load given started at 50, 100, 150, and 200 g and was given a time span of 1 minute for each additional load. The surface area of the gel was measured. The test was carried out after the preparation was allowed to stand for 24 hours (Futri and Yaturramadhan, 2021).
Irritation test on volunteers
The irritation test of the gel preparation was carried out using the patch test technique, which was carried out by applying the formula to the backs of the volunteers’ hands. This test was carried out on 15 volunteers, and the symptoms arising were observed. If irritation occurs, it would be indicated by a skin reaction after the preparation was applied to the skin. Irritation that occurs shortly after attachment is called primary irritation, whereas if it occurs after a few hours of attachment, it is called secondary irritation (Futri and Yaturramadhan, 2021).
Hand sanitizer gel antibacterial activity test against E. coli and S. aureus
An antibacterial test of freshwater lobster shell chitosan gel hand sanitizer against E. coli and S. aureus was conducted. A sterile swab was inserted into the inocula of E. coli and S. aureus, and the entire surface of the MHA medium was swabbed aseptically for approximately 5 minutes in laminar airflow. The paper disks were soaked with freshwater lobster shell chitosan hand sanitizer with various concentrations for 10 minutes, and then the paper disks were removed with sterile tweezers. The paper disks were placed on MHA media that had been inoculated by bacteria, and the media were placed in an incubator at 37°C for 24 hours. The diameter was measured with a caliper and recorded in mm (Harahap et al., 2021; Nasri et al., 2021).
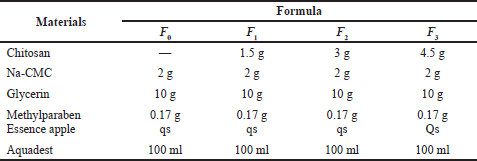 | Table 1. Formula for hand sanitizer gel preparation. [Click here to view] |
RESULTS AND DISCUSSION
Chitosan isolation
The isolation stage of chitin begins with the demineralization process, which is the initial step in removing inorganic salts or mineral content from chitin, particularly CaCO3 present in the sample. The minerals in the sample react with HCl during the demineralization stage, causing the minerals to separate from the lobster shell. The creation of CO2 gas in the form of air bubbles, as well as the dissolution of calcium and phosphate in the sample, as well as fixed and insoluble chitin compounds, characterizes the mineral separation process. In the separation of chitin, this demineralization process is critical. The sample is then taken to the next step after it has been mineral-free (Boarin-Alcalde and Graciano-Fonseca, 2016).
Results of chitosan characterization from freshwater lobster shells
The results shown in Table 2 indicate that the chitosan obtained met the standard values so it can be used for various applications. The resulting chitosan has low water content. The water content of chitosan is influenced by the method and duration of drying, the amount of chitosan being dried, and the surface area of the chitosan being dried. The ash content obtained was 0.93%. This indicates that the chitosan produced met the quality standard for chitosan ash content set by Protan Biopolymer, which is 2%. The results of the isolation of chitin from waste and chitosan from lobster shells are shown in Figure 1.
The solubility of chitosan can be seen in Table 3. The solubility of chitosan in glacial acetic acid is one of the parameters that can be used as a standard for assessing its quality. The higher solubility of chitosan in 1% glacial acetic acid (1 g/100 ml) means that the quality of the chitosan produced is getting better. The resulting chitosan has perfect solubility in 1% glacial acetic acid. Chitosan is a compound that is insoluble in water; slightly soluble in HCl, HNO3, and H3PO4; and insoluble in H2SO4. Chitosan is more soluble in dilute acids such as acetic acid, formic acid, and citric acid. Chitosan is also insoluble in some organic solvents, such as alcohol, acetone, dimethyl formamide, and dimethyl sulfoxide, but chitosan is very soluble in formic acid with a concentration of 0.2%–100% in water solvents.
Yield
In this study, the isolation stage of chitin started with the demineralization stage and continued with the deproteination stage, which lasted for 4 hours at a temperature of 40°C–50°C. This affected the %yield of chitin produced apart from the heating temperature factor and the stirring time. In addition, the influencing factor on the chitin yield is the sequence of stages of chitin isolation. In this study, the sequence of chitin isolation started from demineralization to deproteination. Isolation with the demineralization to deproteination sequence produced more yield than isolation with the demineralization to deproteination sequence. This is because the minerals form a hard barrier on the lobster skin; therefore, moving the minerals first will facilitate the protein removal process so that the %chitin yield is greater. The next step is deacetylation to get chitosan. From this study, the %yield of chitosan obtained was 68.75% (Table 4).
Functional group analysis results using FTIR
The results of the isolation using infrared spectrophotometry can be seen in Figures 2 and 3. The isolation results showed absorption at wavenumbers of 3,750–3,000 (stretching O-H and N-H amines), which was 3,421.72 cm−1 in standard chitosan, while in lobster shell chitosan the wavenumbers were 3,410.15, 3,290.58, and 3,105.39 cm−1. The absorption at wavenumbers 2,400–2,100 cm−1 (stretch-C≡C, ≡CN) was 2,345.44, 2,310.72, and 2,144.84 cm−1 in standard chitosan, while in lobster shell chitosan it was at 2,314.59 cm−1 and 2,129.41 cm−1. Furthermore, the absorption at wavenumbers 1,675–1,500 cm−1 (strain C=C aliphatic, C=O amide, and C=N) was 1,651.07, 1,585.49, and 1,535.34 cm−1, while in lobster shell chitosan the wavenumbers were 1,647.21 and 1,554.63 cm−1. The presence of an absorption band at a wavenumber of 1,068 cm−1 indicates the stretching vibration of the C-O group. Then, the absorption appeared at wavenumbers 1,475–1,300 cm−1 (C-H bending), namely 1,423.47, 1,377.17, and 1,319.31 cm−1, in raw chitosan, while in lobster shell chitosan, it appeared at 1,377.17 and 1,315.45 cm−1. Then, there was absorption at wavenumbers 1,250–1,000 cm−1 (CN stretching vibration), which was 1,145 cm−1 in standard chitosan, while in lobster shell chitosan, it was 1,068.56 and 1,037.70 cm−1. The absorption at wavenumbers 1,470–1,350 cm−1 (CH bending vibration) was 1,377.17 cm−1. Based on the results of the analysis of the chitosan functional group data, the value of the degree of deacetylation can be determined using the formula of Robert and Domzy. Based on the baseline method, the value of the degree of deacetylation is 67.93%. This proves that the conversion of chitin into chitosan has been completed.
 | Table 2. Parameters of chitosan. [Click here to view] |
 | Figure 1. The results of chitin isolated from waste and chitosan from lobster shells. [Click here to view] |
 | Table 3. Solubility of chitosan. [Click here to view] |
 | Table 4. Chitosan yield. [Click here to view] |
 | Figure 2. Results of raw chitosan FTIR spectra. [Click here to view] |
Evaluation results of gel hand sanitizer preparations
The active ingredient used in this gel is chitosan, with additional ingredients consisting of Na-CMC, glycerin, methylparaben, Aquadest, and perfume. In making this gel, four were made: the first formula was made without the addition of chitosan; the second formula was made with the addition of chitosan, with a concentration of 1.5%; the third formula was made with the addition of chitosan, with a concentration of 3%; and the fourth formula was made with the addition of chitosan with a concentration of 4.5%. After the gel (Fig. 2) was formed, a physical evaluation was carried out with testing parameters, including organoleptic observations, homogeneity tests, pH measurements, viscosity tests, and physical stability tests. The gels were stored at room temperature, and observations were made during week 0–3.
Organoleptic test results for gel hand sanitizer preparations
In the results of the organoleptic evaluation of the hand sanitizer gel from lobster shell chitosan, the smell, shape, and color of the gel preparation gave quite good results because there were no visible changes in them starting from week 0 to week 3. The aroma is given the aroma of apples because the essence of apples is added to the preparation. Turbidity in the gel preparation is caused by the addition of chitosan in each hand sanitizer gel preparation. The higher the concentration of the chitosan used, the more cloudy the color of the hand sanitizer gel (Fig. 4) (Kaban et al., 2022).
Test results for homogeneity of gel hand sanitizer preparations
The homogeneity test (Fig. 5) shows that the gel preparation does not form coarse grains. It can be concluded that the hand sanitizer gel preparation is said to be physically homogeneous and the gel ingredients used in the formulation are dissolved and completely mixed.
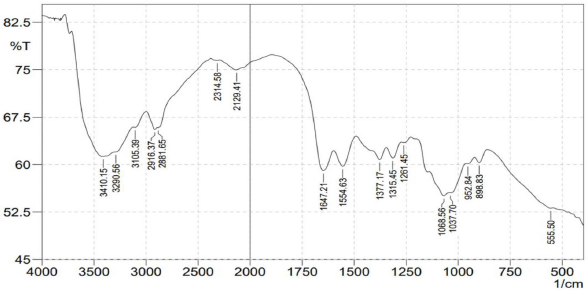 | Figure 3. FTIR spectra results of freshwater lobster shell chitosan. [Click here to view] |
 | Table 5. Testing dispersion. [Click here to view] |
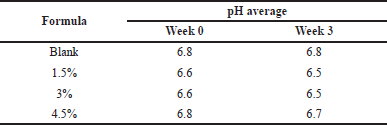 | Table 6. Data of pH measurement results. [Click here to view] |
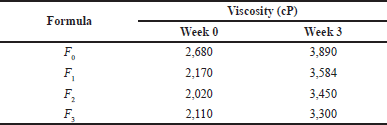 | Table 7. Viscosity test results. [Click here to view] |
Results of testing the spreadability of gel hand sanitizer preparations
The dispersion test on the gel gave results in the form of semiliquid dispersion in each formula (Table 5). The hand sanitizer gel preparation still meets the dispersion requirements, where the application requirements on the skin are between 5 and 7 cm.
Results of pH measurement of gel hand sanitizer preparations
The pH test was carried out to measure the pH (degree of acidity) of the preparation and to determine whether the preparation meets the pH requirements according to the pH of the skin, namely 4–8 (Table 6). The pH observations carried out every week for 3 weeks resulted in a gel that has stable pH, so it is safe to use and does not cause irritation to the skin.
Viscosity test results for gel hand sanitizer preparations
A viscosity test was carried out using a Brookfield spindle L-4 viscometer with a speed of 20 rpm. Viscosity testing was carried out two times, namely at the beginning and after storage for 3 weeks at room temperature. The results of the study showed that the hand sanitizer gel meets the requirements of a good gel (Table 7), which is in the range of 2,000–4,000 cP.
Irritation test results on volunteers
The irritation test on volunteers who observed the irritation test of the hand sanitizer gel from lobster shell chitosan did not show any irritation reactions, such as redness, itching, and crusting on the skin. Therefore, it can be concluded that the hand sanitizer gel preparation is safe to use.
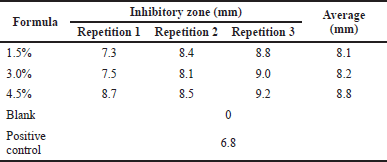 | Table 8. Antibacterial test against E. coli for gel hand sanitizer. [Click here to view] |
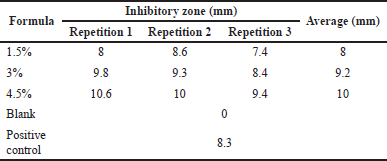 | Table 9. Antibacterial test against S. aureus for gel hand sanitizer. [Click here to view] |
 | Figure 4. Preparation of gel hand sanitizer with chitosan concentrations of 1.5%, 3%, and 4.5%. [Click here to view] |
Gel hand sanitizer antibacterial activity results against E. coli and S. aureus
The results of the antibacterial activity (Tables 8 and 9) showed that the inhibition zone formed from the hand sanitizer gel from freshwater lobster shell chitosan was categorized as having medium inhibition. According to Davis and Stout (1971), the inhibitory zone formed on the media can be categorized into four categories, namely weak inhibition is the inhibition zone of less than 5 mm, medium inhibition is the inhibition zone of 5–10 mm, strong inhibition is the inhibition zone of 10–19 mm, and very strong inhibition is the inhibition zone of values more than 20 mm (Silaban et al., 2022).
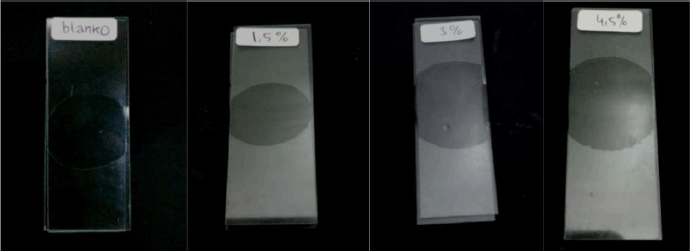 | Figure 5. Homogeneity test. [Click here to view] |
CONCLUSION
Chitosan from freshwater lobster shells was formulated into a stable hand sanitizer gel that does not change for 3 weeks of storage at room temperature. Chitosan from freshwater lobster showed antibacterial activity against E. coli bacteria with resulting inhibition zones of 8.1, 8.2, and 8.8 mm and S. aureus bacteria with resulting inhibition zones of 8, 9.2, and 10 mm, and therefore it is categorized as having moderate inhibition and can be applied in the manufacture of hand sanitizer gel preparations.
AUTHORS’ CONTRIBUTION
Nurhayati and Zulmai Rani carried out research activities, data acquisition, data interpretation, and manuscript preparation. Vera Estefania Kaban and Nasri Nasri prepared the manuscript. Ridwanto and Haris Munandar Nasution conceptualized and designed the research and critically analyzed the manuscript. All authors read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
This research was approved by the Ethics Committee of Universitas Sumatera Utara, No. 509/KEP/USU/2021.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral concerning jurisdictional claims in published institutional affiliation.
REFERENCES
Abun TW, Haetami K. Effect of time processing at steps of bioprocess shrimp waste by three microbes on protein digestibility and metabolizable energy products of native chicken. AgroLife Sci J, 2016; 1(5):209–13.
Al Shaqsi NHK, AL Hoqani HAS, Hossain MA, Al Sibani MA. Optimization of the demineralization process for the extraction of chitin from Omani Portunidae segnis. Biochem Biophys Report, 2020; 23(May):100779. CrossRef
Boarin-Alcalde L, Graciano-Fonseca G. Alkali process for chitin extraction and chitosan production from Nile tilapia (Oreochromis niloticus) scales. Latin Am J Aqua Res, 2016; 44(4):683–8. CrossRef
Davis WW, Stout TR. Disc plate method of microbiological antibiotic assay: II. Novel procedure offering improved accuracy. Appl Microbiol, 1971; 22(4):666–70. CrossRef
Fahreni F, Mardina V, Indriaty I, Ramaidani R. Examination of gel hand sanitizer from mangrove leaves and patchouli oil against Sthapylococcus aureus. Int J Eng, Sci Inform Technol, 2021; 1(4):7–12. CrossRef
Fernandes CE, da Silva Vasconcelos MA, de Almeida Ribeiro M, Sarubbo LA, Andrade SAC, de Melo Filho AB. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem, 2014; 160:67–71. CrossRef
Futri CL, Yaturramadhan H. Formulation of gel handsanitizer ethanol extract salam leaves with carbopol and Na CMC as gelling agent. J Public Health Pharm, 2021; 1(4):72–6.
Harahap U, Dalimunthe A, Hertiani T, Muhammad M, Nasri, Satria D. Antioxidant and antibacterial activities of ethanol extract of Vernonia amygdalina Delile. Leaves. In: AIP conference proceedings, AIP Publishing LLC, Melville, NY, 080011-1–4, 2021. CrossRef
Haro G, Iksen I, Nasri N. Identification, characterization and antibacterial potential of probiotic lactic acid bacteria isolated from naniura (A traditional batak fermented food from carp) against Salmonella typhi. Rasayan J Chem, 2020; 13(1):464–8. CrossRef
Hien NQ, Van Phu D, Duy NN, Lan NTK. Degradation of chitosan in solution by gamma irradiation in the presence of hydrogen peroxide. Carbohydr Polym, 2012; 87(1):935–8. CrossRef
Kaban VE, Nasri N, Dharmawan H, Satria D. Formulasi dan uji efektivitas sabun pencuci tangan dari ekstrak daun pandan (Pandanus amaryllifolius Roxb.) terhadap bakteri Salmonella sp. Herb Med J, 2022; 5(1):8–12.
Kasta G. Antimicrobial activity of ethanol extract of rhizome turmeric (Curcuma longa L.) for growth of Escherichia coli, Staphylococcus aureus and Candida albicans. Asian J Pharm Res Devel, 2020; 8(3):5–8. CrossRef
Liu K. Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. Algal Res, 2019; 40:101486. CrossRef
Nasri N, Harahap U, Silalahi J, Satria D. Antibacterial activity of lactic acid bacteria isolated from Dengke Naniura of Carp (Cyprinus carpio) against diarrhea-causing pathogenic bacteria. Biodivers J Biol Divers, 2021; 22(8):3098–104.
Paul S, Jayan A, Sasikumar CS, Cherian SM. Extraction and purification of chitosan from chitin isolated from sea prawn Fenneropenaeus indicus. Extraction, 2014; 7(4):201–4. CrossRef
Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur J Pharm Sci, 2020; 10(4):251–7. CrossRef
Salamat S, Hadavifar M, Rezaei H. Preparation of nanochitosan-STP from shrimp shell and its application in removing of malachite green from aqueous solutions. J Environ Chem Eng, 2019; 7(5):103328.Singh P, Potlia I, Malhotra S, Dubey H, Chauhan H. Hand sanitizer an alternative to hand washing—a review of literature. J Adv Oral Res, 2020; 11(2):137–42. CrossRef
Silaban S, Nainggolan B, Simorangkir M, Zega TS, Pakpahan PM, Gurning K. Antibacterial activities test and brine shrimp lethality test of Simargaolgaol (Aglaonema modestum Schott ex Engl,) leaves from North Sumatera, Indonesia. Rasayan J Chem, 2022; 15(2):745–50. CrossRef
Sun L, Sun J, Chen L, Niu P, Yang X, Guo Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr Polym, 2017; 163:81–91. CrossRef
Tripathi S, Mehrotra GK, Dutta PK. Chitosan–silver oxide nanocomposite film: preparation and antimicrobial activity. Bull Mater Sci, 2011; 34(1):29–35. CrossRef
Vallejo-Domínguez D, Rubio-Rosas E, Aguila-Almanza E, Hernández-Cocoletzi H, Ramos-Cassellis ME, Luna-Guevara ML, Rambabu K, Manickam S, Munawaroh HSH, Show PL. Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason Sonochem, 2021; 72:105417. CrossRef
Wang M, Bi S, Qin D, Su C, Wang H, Chen X. Quantitative evaluation of the antibacterial effectiveness and efficiency of chitosan considering the effect of neutralization. Carbohydr Polym, 2021; 265:117918. CrossRef
Wulandari IO, Pebriatin BE, Valiana V, Hadisaputra S, Ananto AD, Sabarudin A. Green synthesis of silver nanoparticles coated by water soluble chitosan and its potency as non-alcoholic hand sanitizer formulation. Materials, 2022; 15(13):4641. CrossRef